Ophiobolin sesterterpene compound and preparation and application thereof
A technology of pseudochicin and sesquiterpene, applied in biochemical equipment and methods, microorganisms, organic chemistry, etc., can solve the problems of serious residue, soil and water pollution, refractory degradation, etc., and achieve obvious antibacterial activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
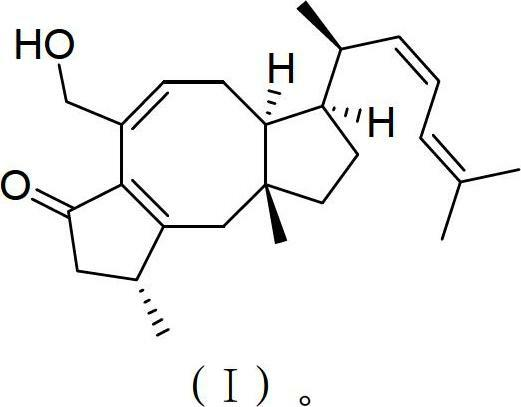
[0020] The sesquiterpene compound of the pseudochicin class derived from seaweed endophytic fungi is represented by the formula (I).
[0021]
Embodiment 2
[0023] The preparation method of the pseudochicin class sesquiterpene compound shown in formula (I):
[0024] Take the fungus Aspergillus ustus cf-42 that grew well on the plate, cut it into small pieces and inoculate it in the PDB liquid medium, put 300mL medium in each 1L Erlenmeyer flask, 50 bottles in total, ferment at room temperature for 35 days, add One-half volume of ethyl acetate in the fermented liquid kills the fungus, is filtered, and the mycelium and the fermented liquid are collected respectively. The PDB liquid medium consists of 200 milliliters of potato juice per liter, 20 grams of glucose, 5 grams of peptone, 3 grams of yeast extract, 500 milliliters of aged sea water, and 300 milliliters of distilled water. Among them, the fungus Aspergillus ustus cf-42 strain was preserved in the China Center for Type Culture Collection CCTCC on November 25, 2011, the preservation number is CCTCC M2011420, the classification name is Aspergillus ustus cf-42, the preservation un
Embodiment 3
[0032] The difference from Example 2 is that
[0033] Take the fungus Aspergillus ustus (Aspergillus ustus) cf-42 strain that grew well on the plate, cut it into small pieces and inoculate it in PDB liquid medium, put 300mL medium in each 1L Erlenmeyer flask, 50 bottles in total, and ferment statically at room temperature for 35 days , adding 1 / 2 volume of ethyl acetate to the fermentation broth to kill the fungi, filtering, and collecting the mycelia and fermentation broth respectively. The PDB liquid medium consists of 200 milliliters of potato juice per liter, 20 grams of glucose, 5 grams of peptone, 3 grams of yeast extract, 500 milliliters of aged sea water, and 300 milliliters of distilled water.
[0034] Collect about 15L of fermented liquid, extract three times with ethyl acetate, concentrate under reduced pressure; extract three times with acetone after the mycelia is pulverized, then extract with ethyl acetate, concentrate under reduced pressure; Thin-layer chromatograp
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap