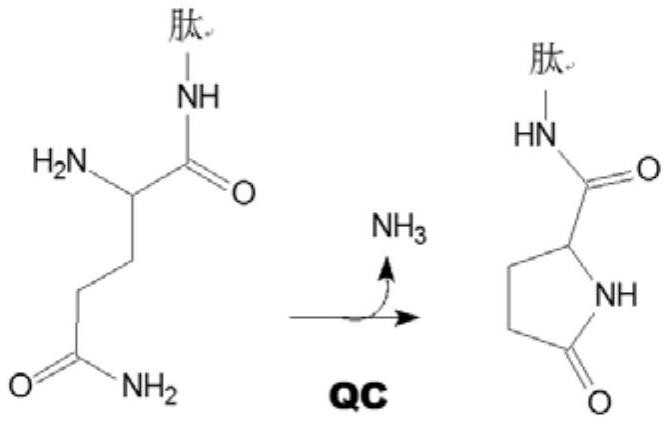
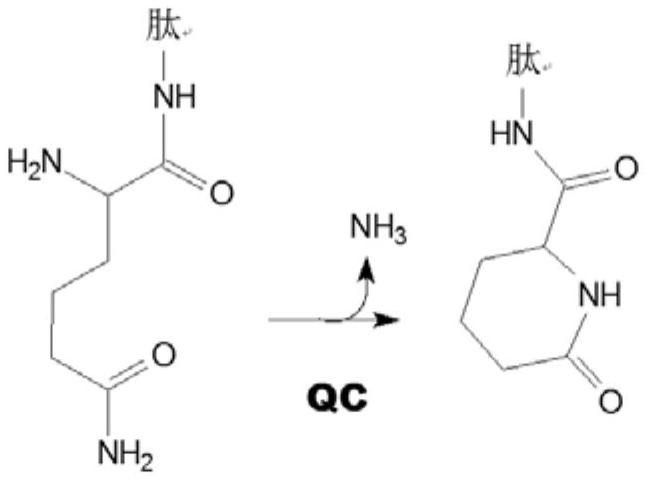
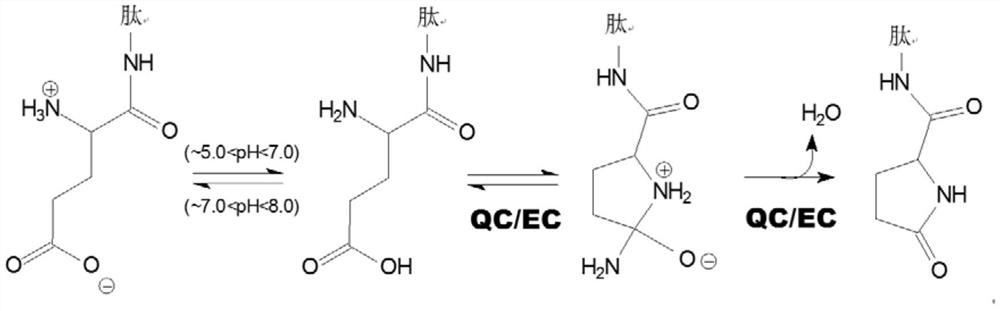
new inhibitor
A compound and alkyl technology, applied in the field of halogenated oxazolidinone derivatives, can solve the problem of not showing sequence homology and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0370] Example
[0371]
[0372]
[0373]
[0374]
[0375]
[0376]
[0377] The present invention also relates to racemates and R-stereoisomers of the compounds of the present invention:
[0378]
[0379]
[0380] General Synthesis Description:
[0381] Method A: Examples 1-3
[0382]
[0383] step 1:
[0384] A mixture of the corresponding fluoro-substituted 4-hydroxybenzonitrile (1 equiv), the corresponding 4-chloro-2-butanol (3 equiv) and potassium carbonate (2 equiv) in acetonitrile was refluxed for 20 h. The reaction was cooled to room temperature and filtered. The filtrate was partitioned between water and ethyl acetate. The organic layer was washed with water, brine; dried over anhydrous sodium sulfate and concentrated in vacuo to obtain crude intermediate MA-S1.
[0385] Step 2:
[0386] 2-Iodoylbenzoic acid (9 equiv) was added to a solution of MA-S1 (crude, 1 equiv) in dichloromethane and dimethyl sulfoxide and stirred at room tempera
Example Embodiment
[0460] Method M: Examples 21–22
[0461]
[0462] step 1:
[0463] (R)-3-Hydroxypyrrolidine (1.5 eq) was added to a stirred solution of the corresponding 4-fluorobenzonitrile (1 eq) and potassium carbonate (1 eq) in dimethylformamide, and the mixture was heated at 80 Stir overnight at °C. The reaction was filtered, washed with ethyl acetate and the filtrate was evaporated in vacuo. The residue was partitioned between water and ethyl acetate. The combined organic layers were washed with water, brine; dried over anhydrous sodium sulfate and concentrated in vacuo to obtain a crude intermediate. Purify by column chromatography on neutral alumina to obtain MN-S1.
[0464] Step 2:
[0465] Category 1 :
[0466] Dess-martin periodinane (2 equiv) was added to a solution of MM-S1 (1 equiv), and the mixture was stirred for 15 h. The reaction was filtered through celite and washed with dichloromethane. The filtrate was washed with water, brine; dried over anhydrous sodium sulfa
Example Embodiment
[0473] Method N: Examples 18–22
[0474]
[0475] step 1:
[0476] At -30°C, n-butyllithium in hexane (2.2M; 2 eq.) was added to a stirred solution of methyltriphenylphosphonium bromide (2 eq.) in THF, and the mixture was heated at 0-5 Stir for 30 min at °C. A solution of the corresponding intermediate ML-S5 or MM-S4 (1 equiv) in THF was added dropwise at -30°C. The temperature was allowed to warm to room temperature, and the mixture was stirred for 2 h. The reaction was quenched with acetic acid and the pH was adjusted to ~5. The solution was extracted with ethyl acetate. The combined organic layers were washed successively with water, brine; dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain a crude intermediate. Purification by column chromatography on silica gel afforded MN-S1.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap