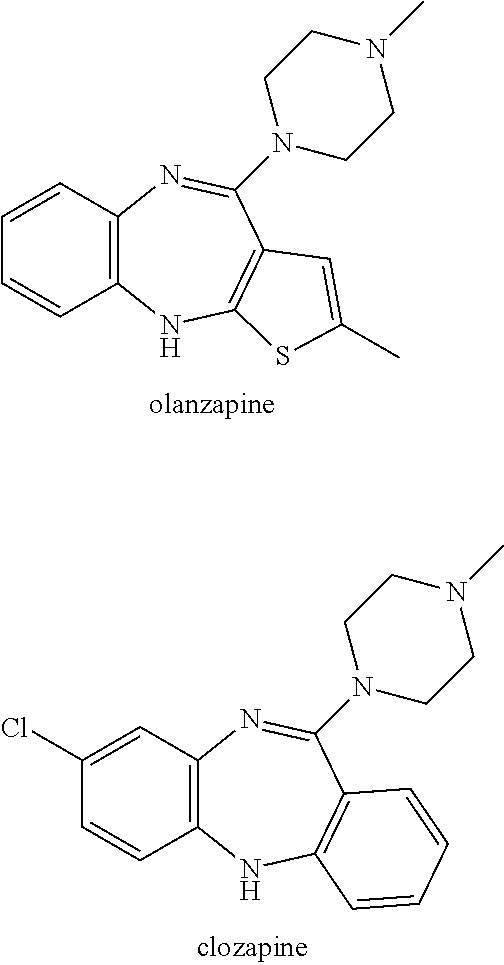
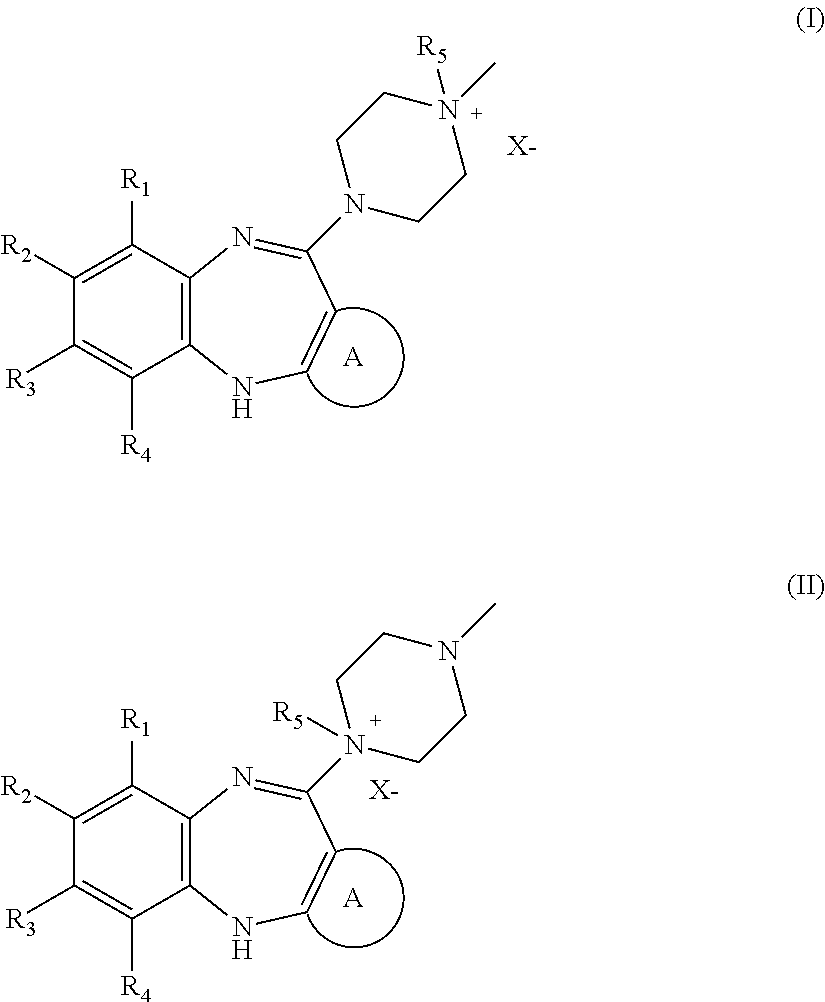
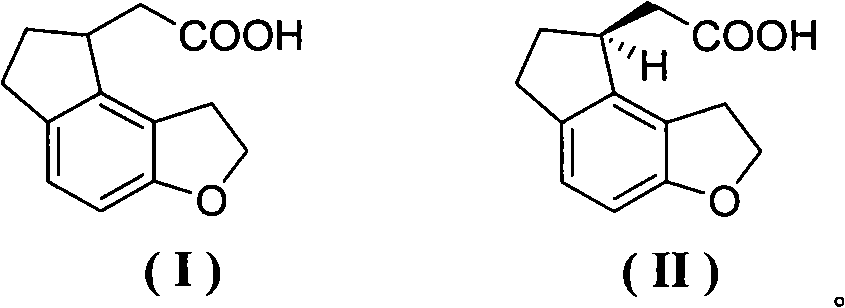
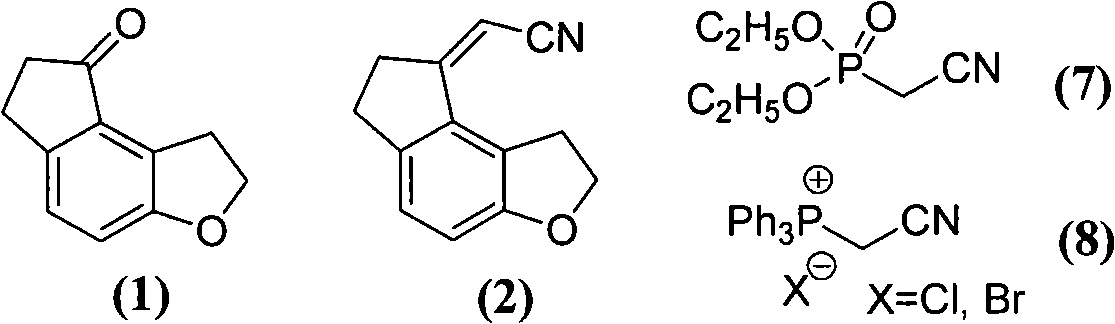
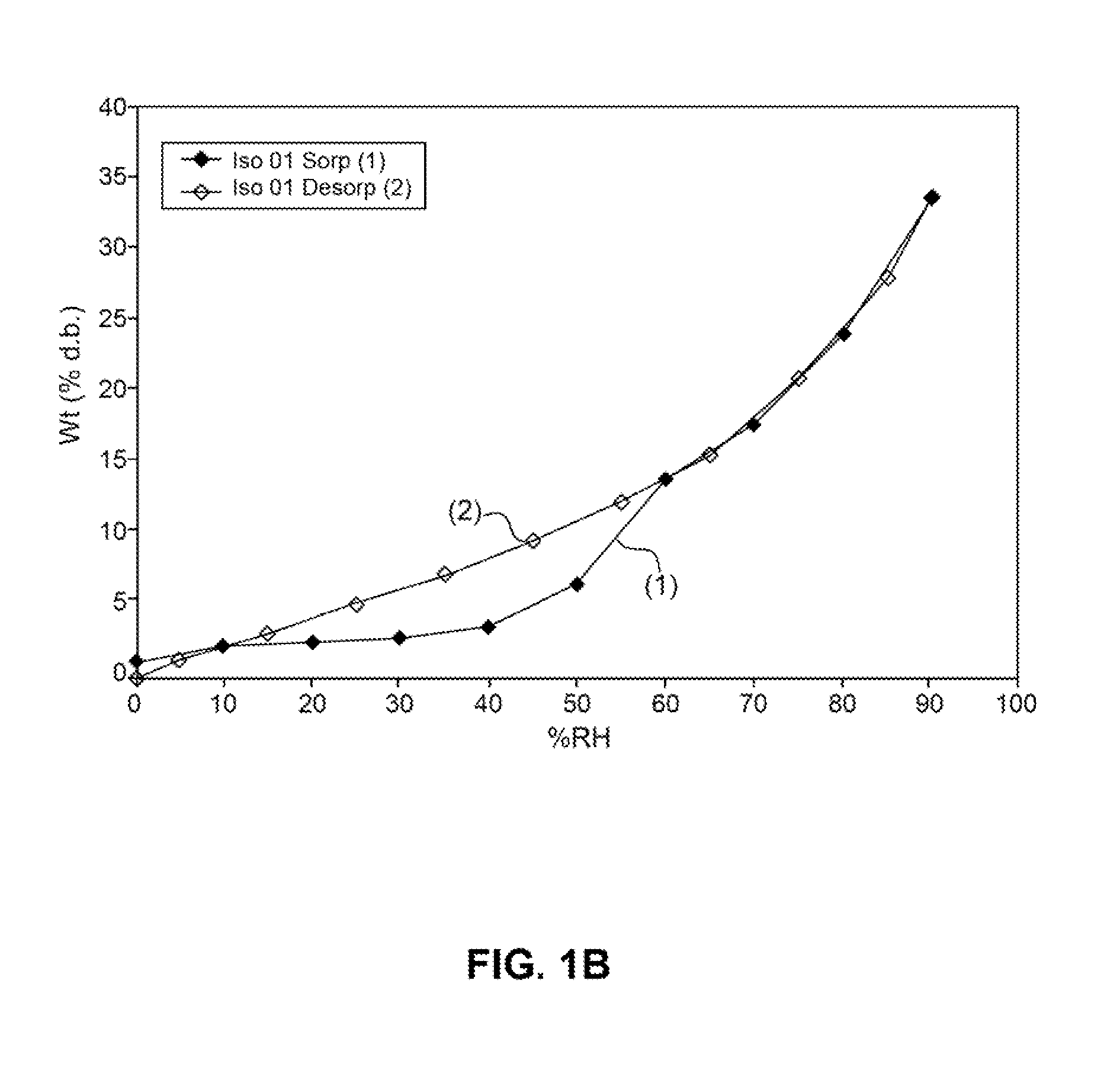
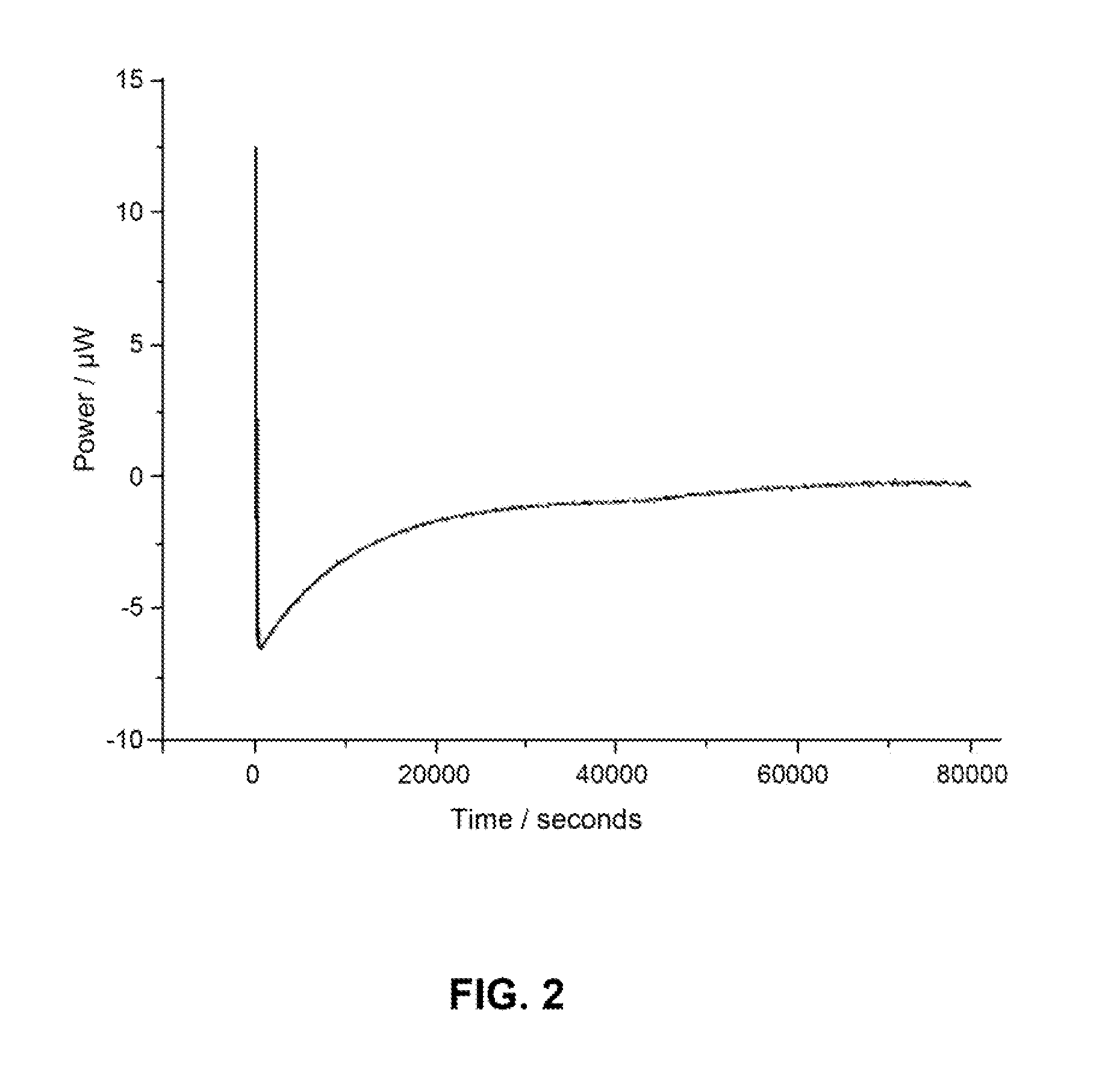
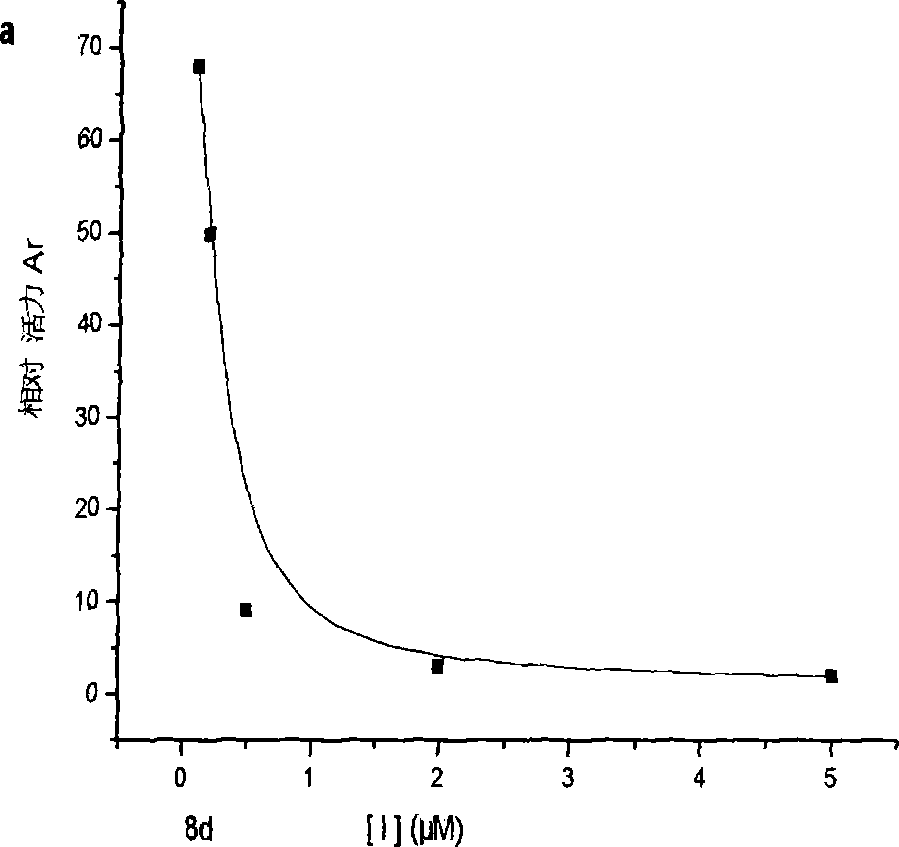
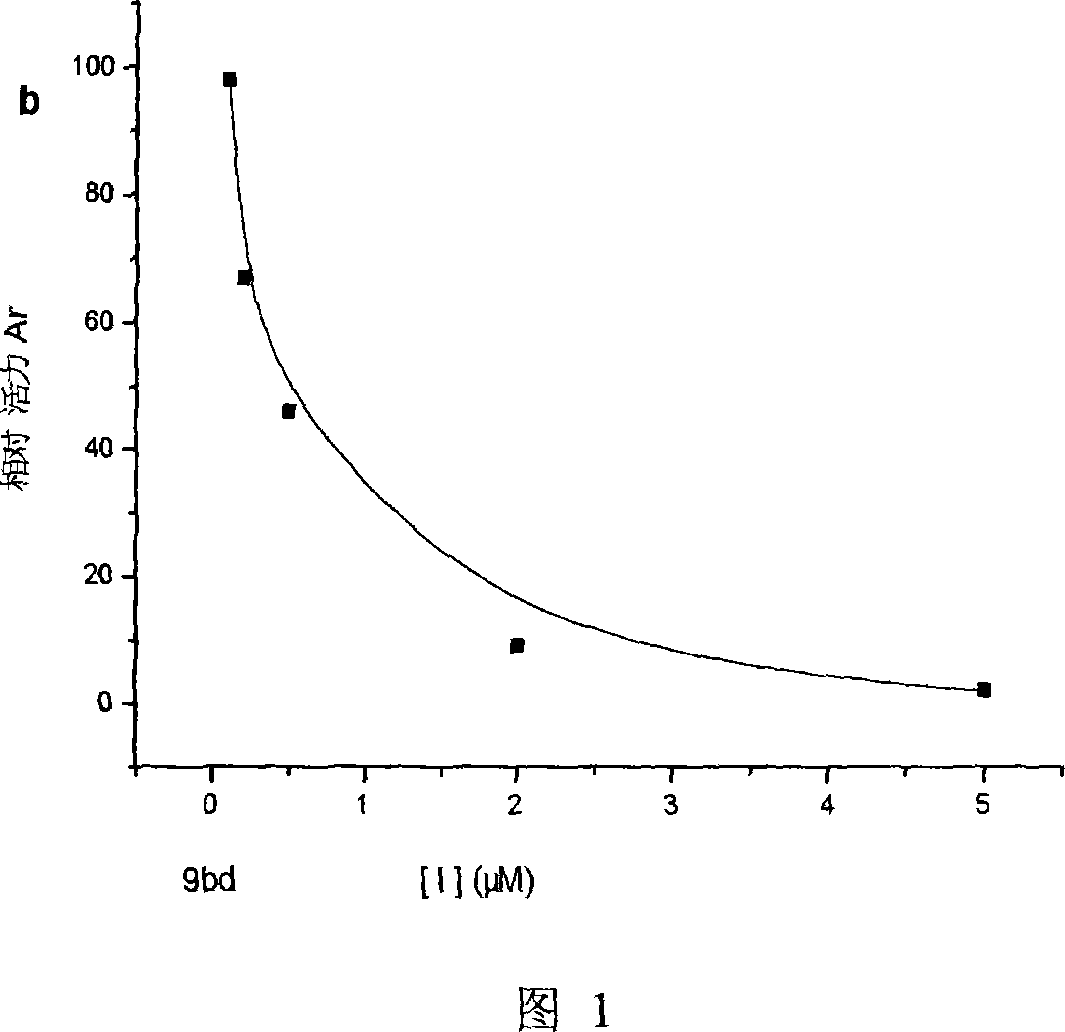
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
173results about "Nervous disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Abuse-resistant opioid dosage form
Owner:PURDUE PHARMA LP
Gastric retention controlled drug delivery system
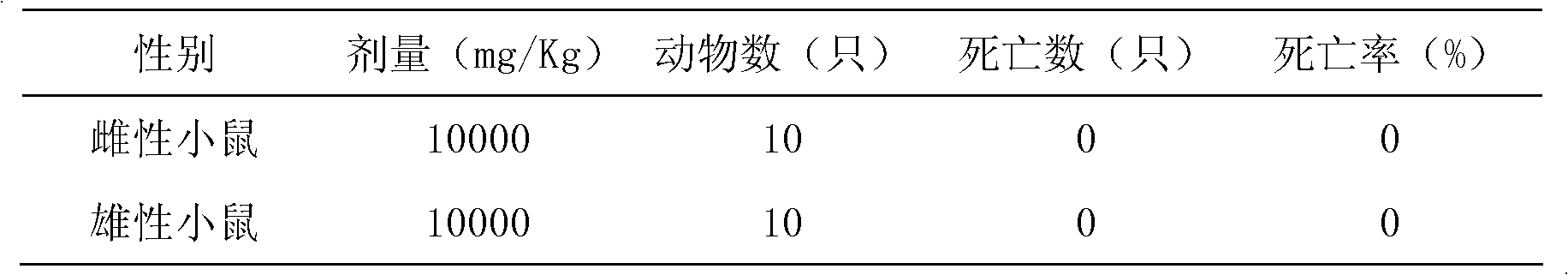
ActiveUS20040180088A1Maintain physical integrityFast swellingOrganic active ingredientsNervous disorderControlled drugsControl release
The present invention provides a gastric retention controlled drug delivery system comprising: (a) a controlled release core comprising a drug, a highly swellable polymer and a gas generating agent, said core being capable of swelling and achieving floatation rapidly while maintaining its physical integrity in gastrointestinal fluids for prolonged periods, and (b) a rapidly releasing coat composition comprising the same drug as in the core and pharmaceutically acceptable excipients, wherein the coating composition surrounds the core such that the system provides a biphasic release of the drug in gastrointestinal fluids.
Owner:SUN PHARMA INDS
Bispecific Anti-vegf/Anti-ang-2 antibodies
Owner:F HOFFMANN LA ROCHE INC
Low Viscosity Liquid Polymeric Delivery System
InactiveUS20090181068A1Heavy metal active ingredientsNervous disorderPolymer sciencePolymer solution
Owner:DUNN RES & CONSULTING
Bacterial strains, compositions including same and probiotic use thereof
Owner:SOROKULOVA IRYNA +1
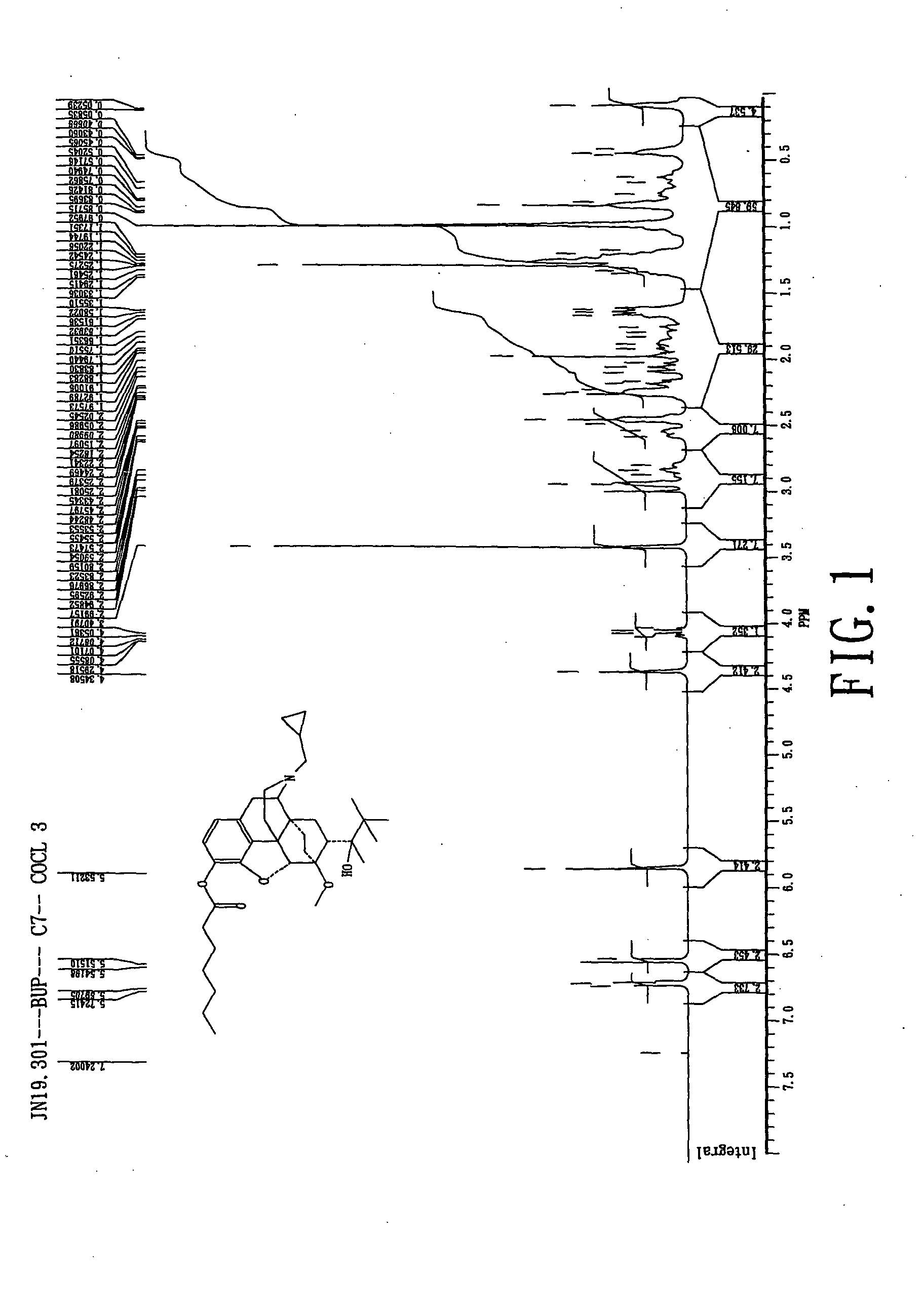
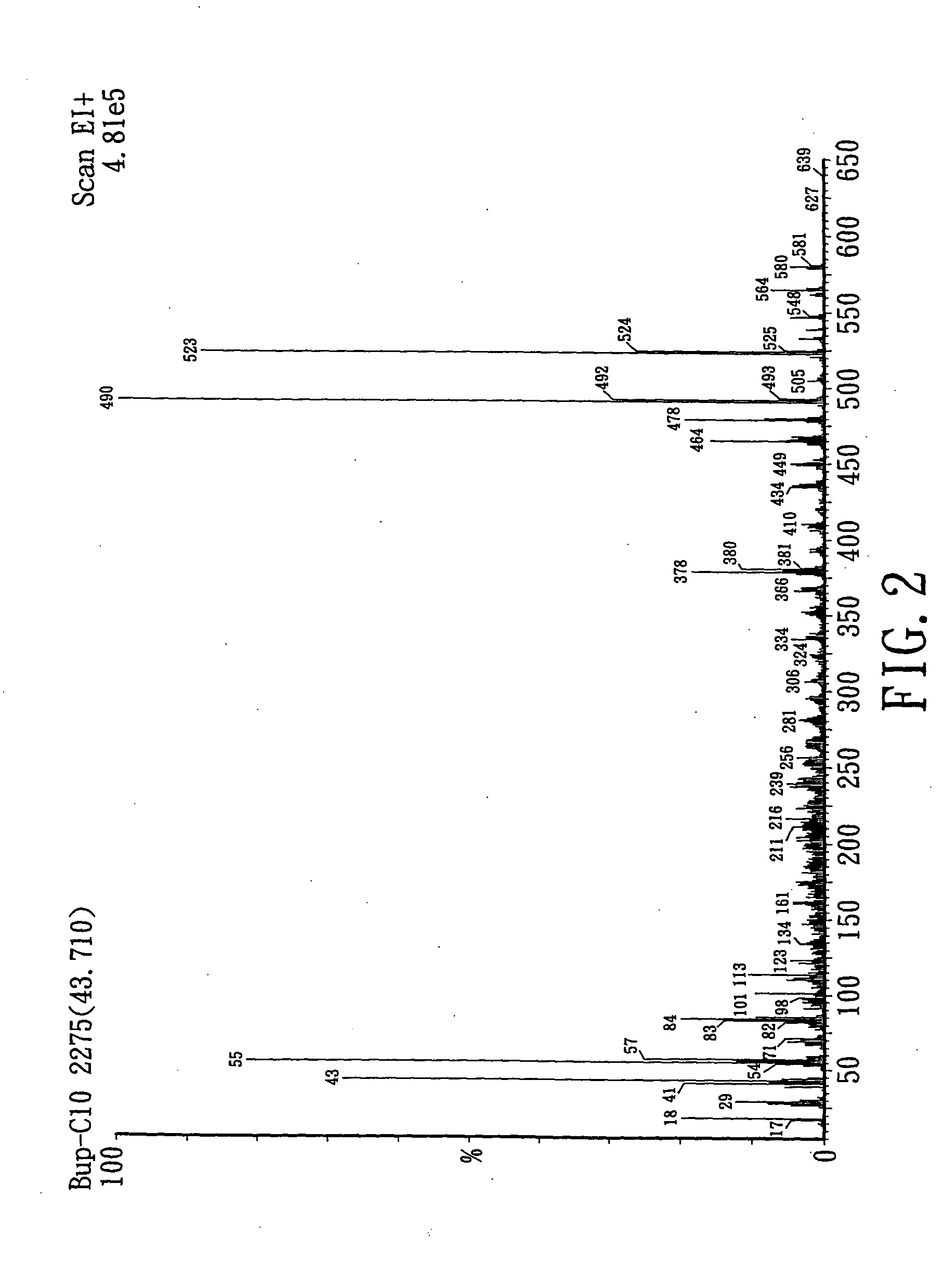
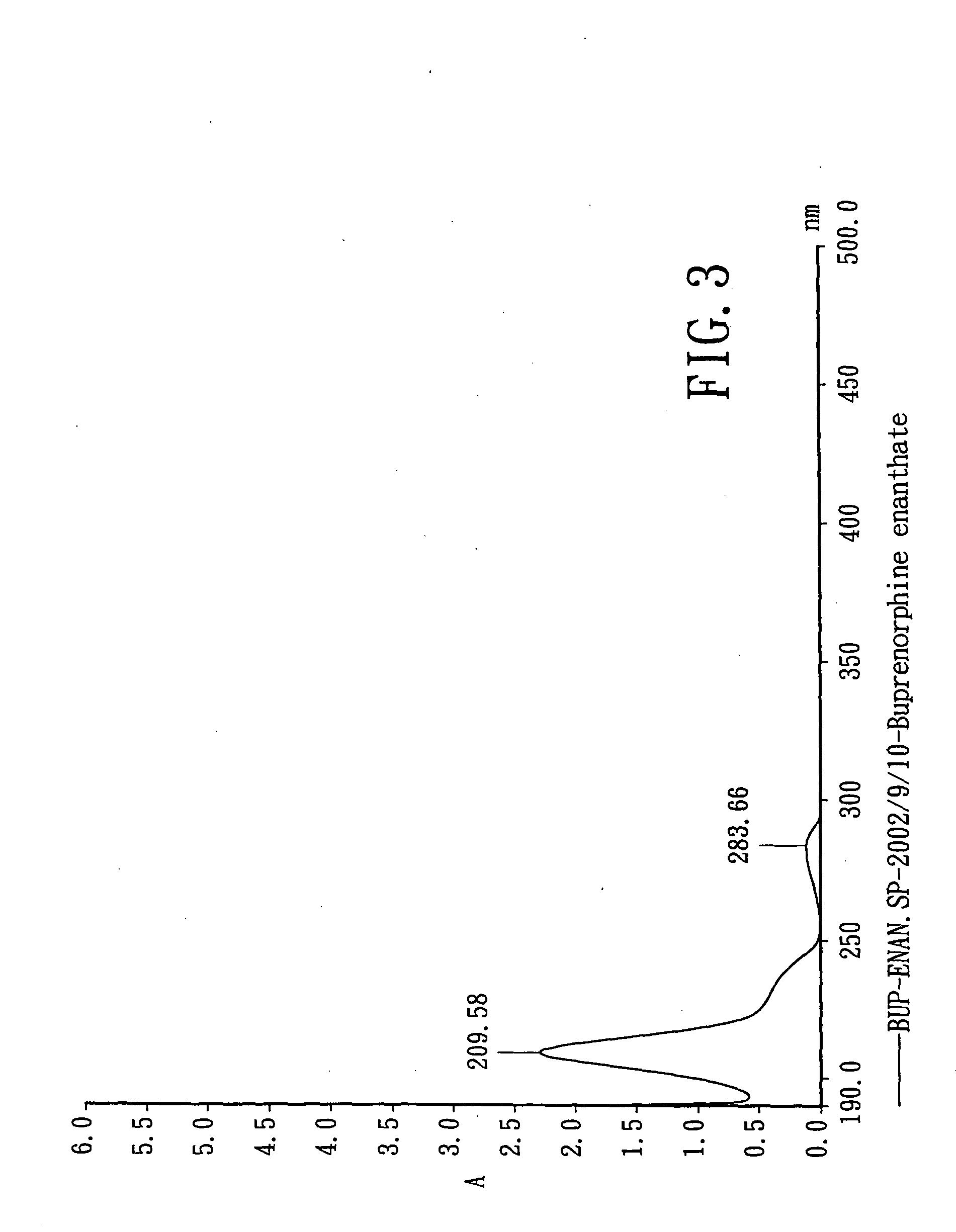
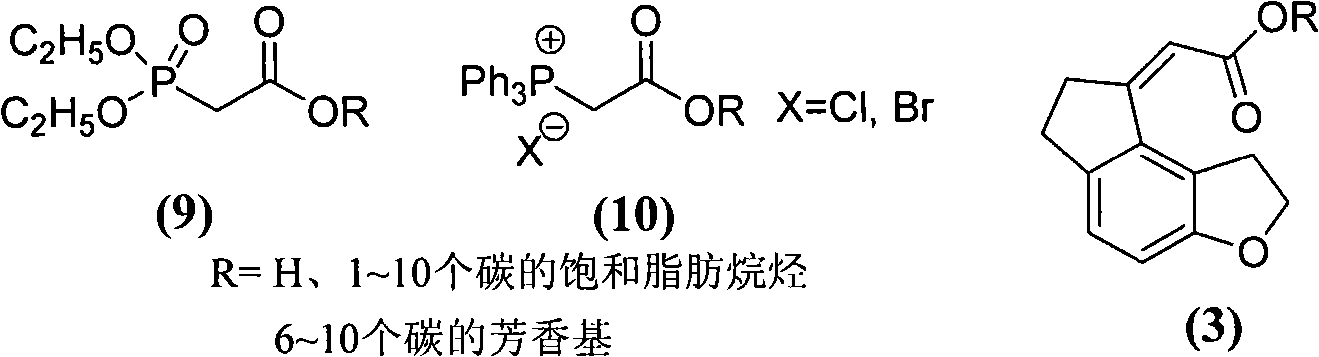
Novel ester derivatives of buprenorphine and their preparation processes, and long acting analgestic pharmaceutical compositions
Owner:CHI MEI MEDICAL CENT
Diaryldiazepine Prodrugs for the Treatment of Neurological and Psychological Disorders
InactiveUS20110166128A1Long duration of actionReduce solubilityBiocideNervous disorderDrug compoundProdrug
Owner:ALKERMES INC
Materials and methods for treating neuropathies and related disorders including those involving a keystone nerve
InactiveUS20160030408A1Increase blood flowAlter perception of painBiocideElectrotherapyDiseaseMedicine
Methods, apparatus, compositions and kits for inhibiting a disorder in a human patient, including non-cerebral neurovascular disorder or muscular headache pain, or loss of motor or sensory function, sympathetic tone or range or fluidity of motion that affect a nerve pathway at more than one locus associated with the disorder to inhibit the disorder. Alternatively or in addition, neuropathy associated with a disorder is treatable by palpating to determine a Keystone nerve essential to the neuropathy, applying pressure to determine a point of maximum discomfort or trigger of increased symptoms to identify a Levin Sign as a locus of initial intervention, and intervening to treat the neuropathy at the location of the Levin Sign by administering a pharmaceutically active agent, internal implanted or external neuro stimulation affecting the nerve pathway to inhibit the neuropathy.
Owner:BHL PATENT HLDG
Uniform delivery of topiramate over prolonged period of time with enhanced dispersion formulation
InactiveUS20050058707A1Improve bioavailabilityPromote absorptionNervous disorderCapsule deliveryTopiramateControlled Release Dosage Form
Compositions and dosage forms for enhanced dispersion of topiramate in a controlled release dosage form delivered as a dry or substantially dry erodible solid at a uniform rate over a prolonged period of time are described.
Owner:ALZA CORP
Compound for preparing ramelteon, preparation method thereof and application thereof
Owner:SICHUAN UNIV
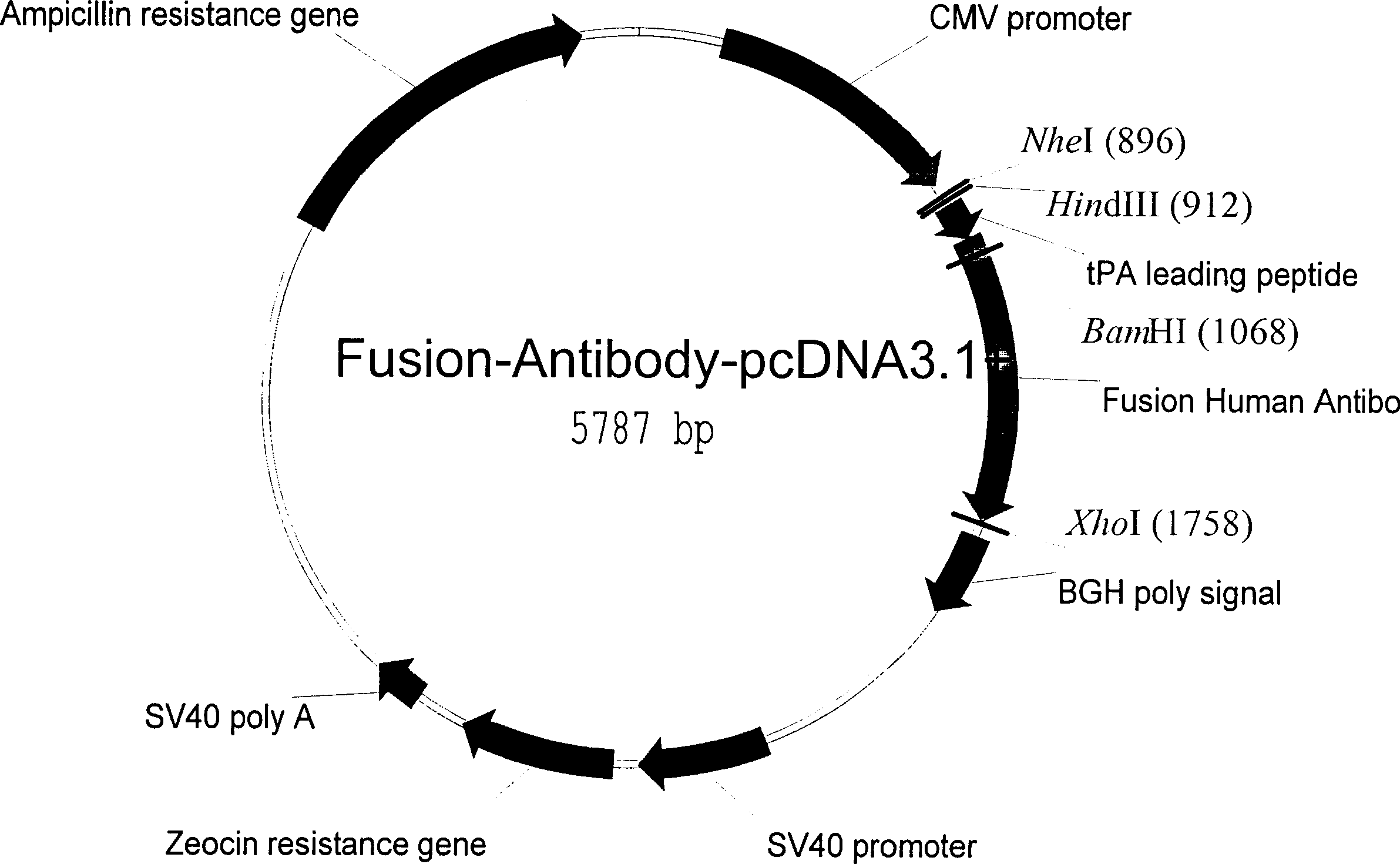
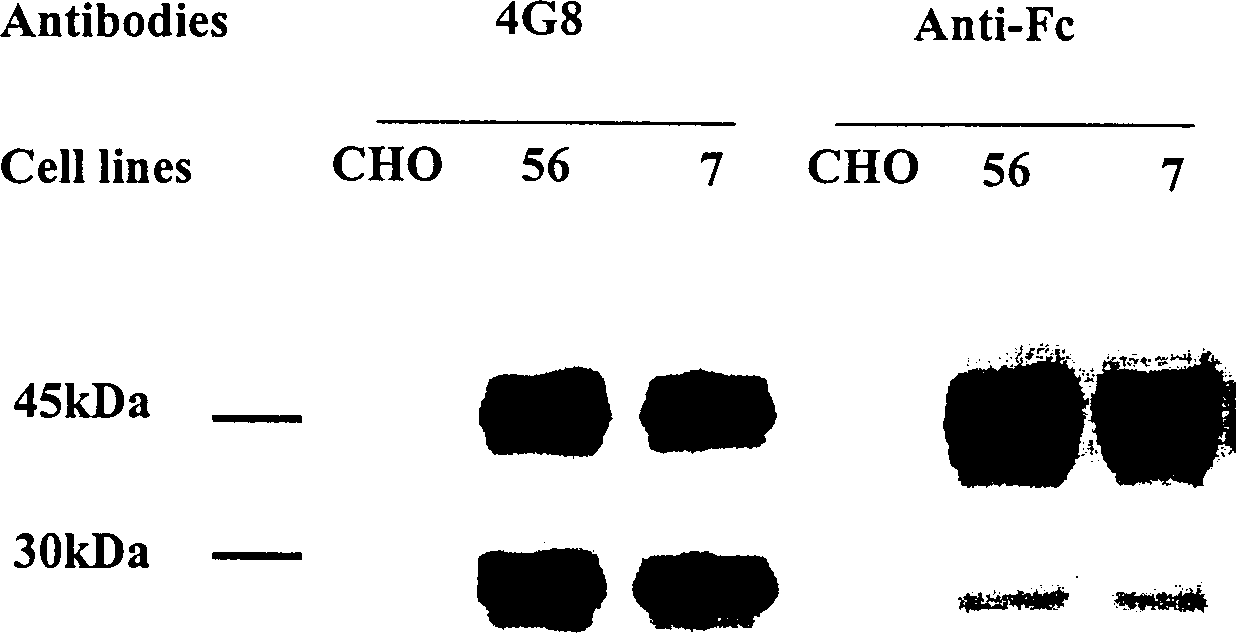
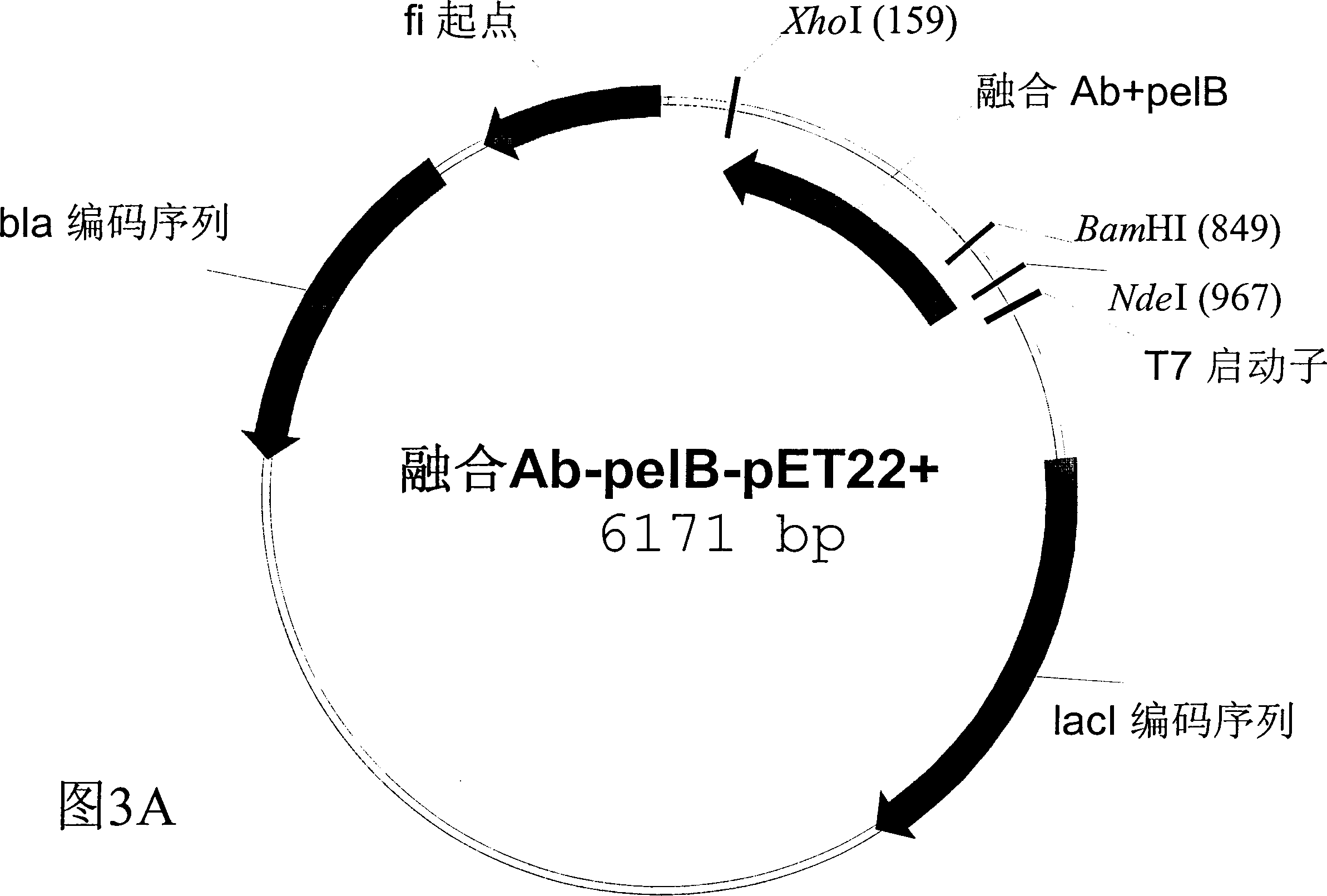
Human fusion antibody for reducing cerebral amyloid fibers associated with senile dementia
Owner:张小如 +1
Immunoconjugates for programming or reprogramming of cells
InactiveUS20180117171A1Enhance Th immunityIncrease Th responseNervous disorderAntipyreticAutoimmune responsesReprogramming
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Predictive biomarkers of clinical response to glatiramer acetate therapy in multiple sclerosis
A method for treating a subject afflicted with an autoimmune disease with a pharmaceutical composition comprising glatiramer acetate and a pharmaceutically acceptable carrier, comprising the steps of administering a therapeutic amount of the pharmaceutical composition to the subject, determining whether the subject is a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder by measuring the value of a biomarker selected from the group consisting of IL-10 concentration, IL-17 concentration, IL-18 concentration, TNF-α concentration, BDNF concentration, caspase-1 concentration, IL-10 / IL-18 ratio and IL-10 / IL-17 ratio in the blood of the subject, and comparing the measured value to a reference value for the biomarker to identify the subject as a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder, and continuing the administration if the subject is identified as a glatiramer acetate responder, or modifying treatment of the subject if the subject is identified as a glatiramer acetate hypo- / non-responder.
Owner:TEVA PHARMA IND LTD
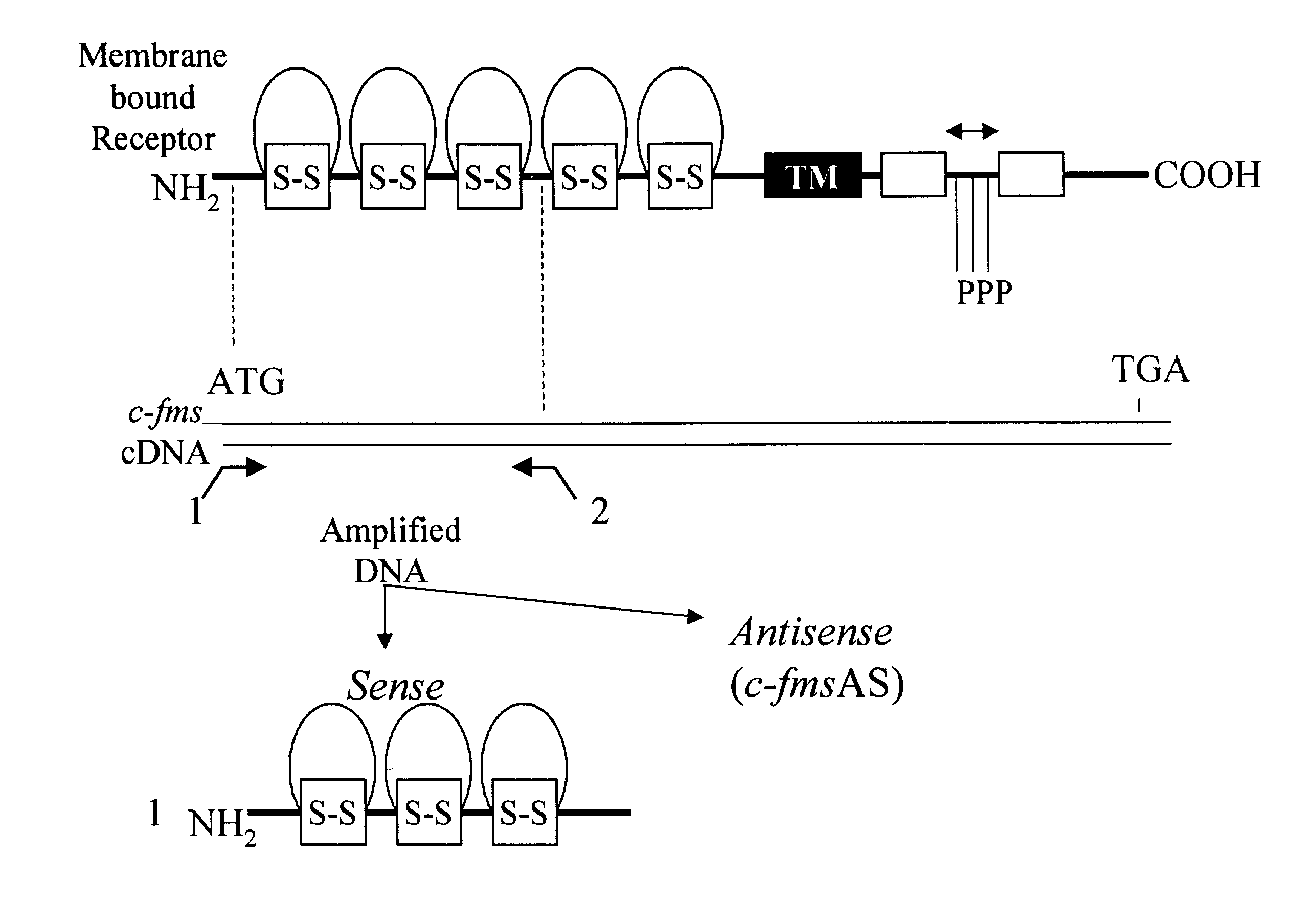
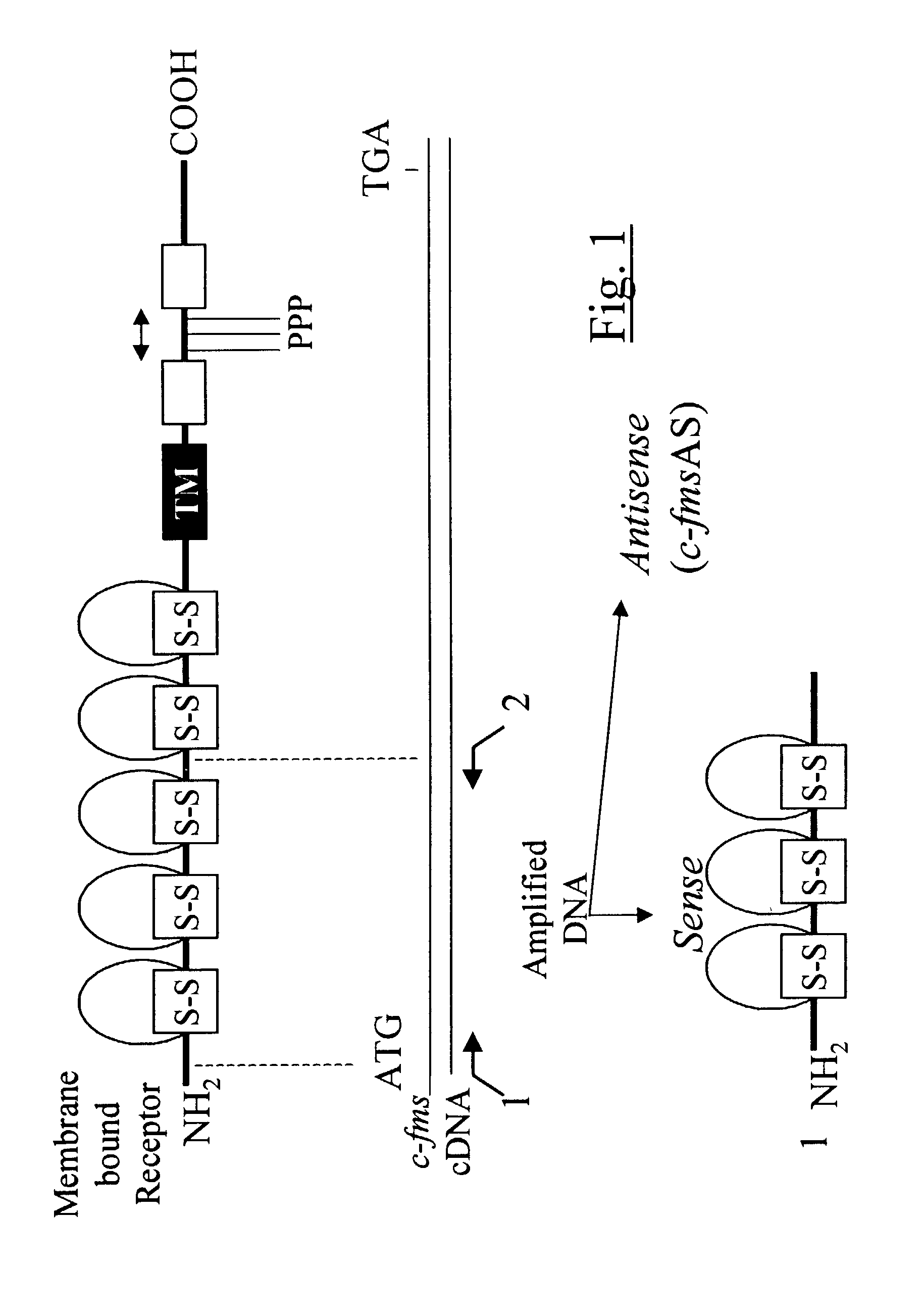
Methods for inhibiting macrophage colony stimulating factor and c-FMS-dependent cell signaling
Owner:RAJAVASHISTH TRIPATHI
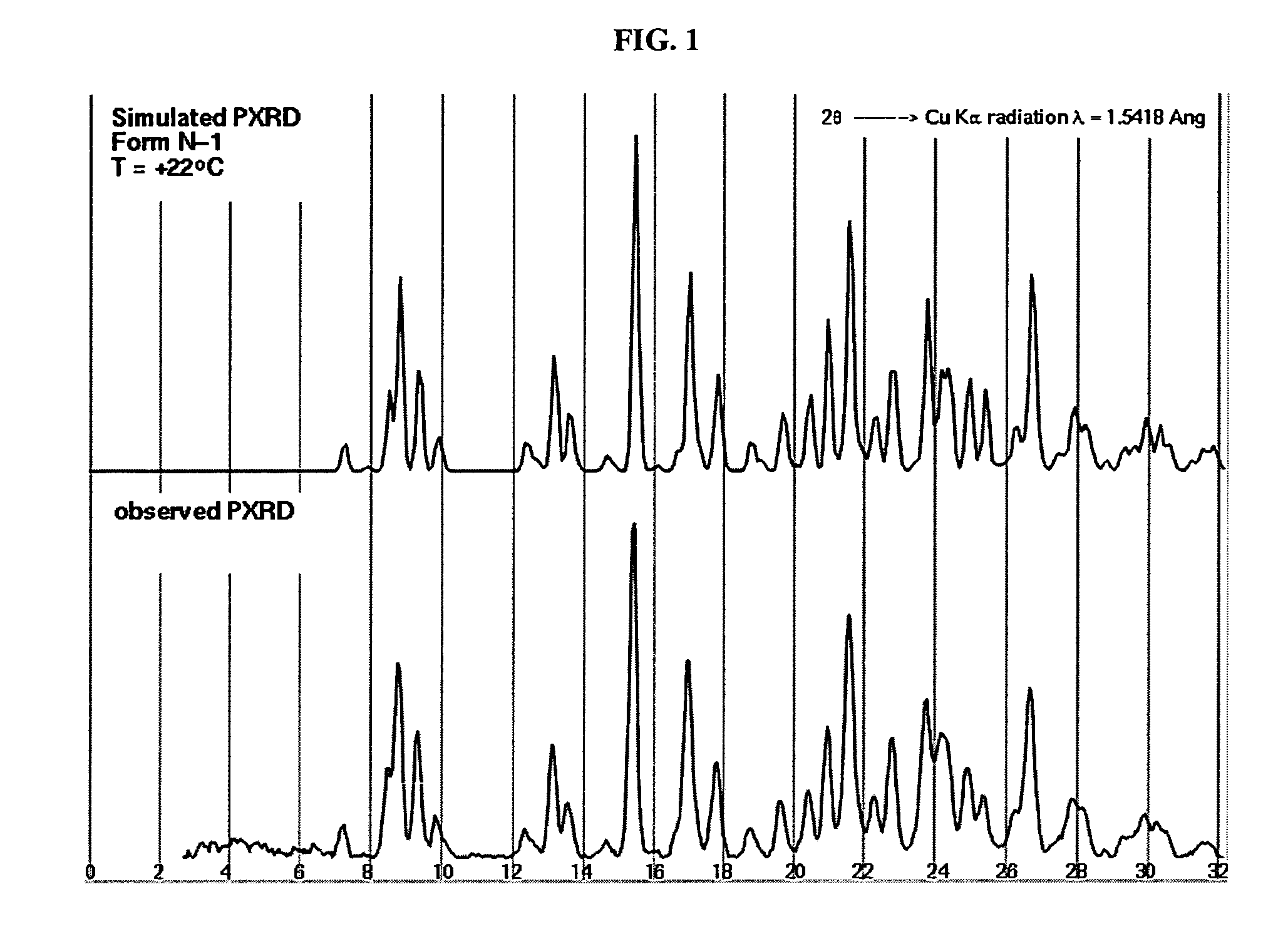
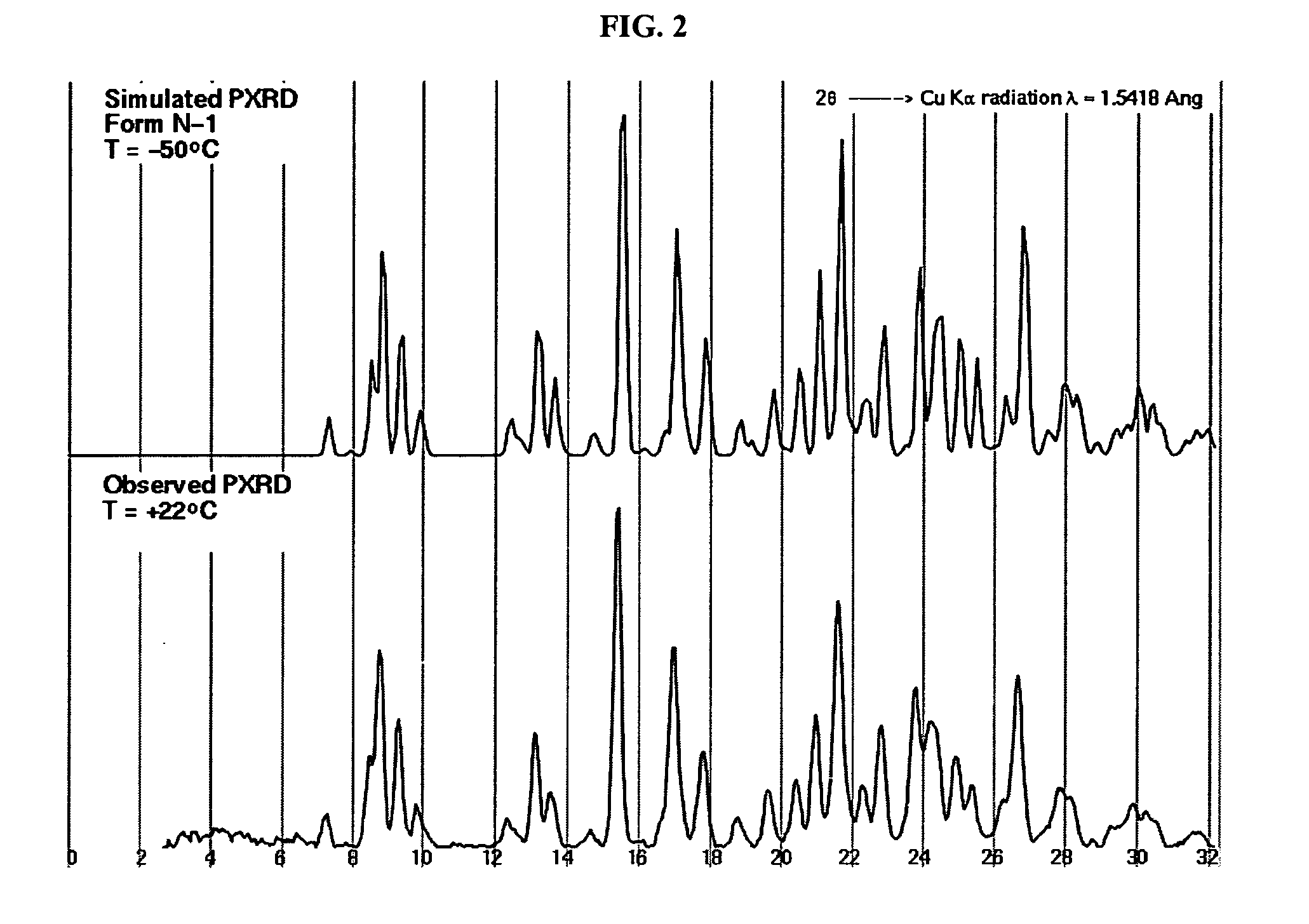
Crystalline forms and process for preparing spiro-hydantoin compounds
Owner:BRISTOL MYERS SQUIBB CO
Novel compounds as cannabinoid receptor ligands
Disclosed herein are cannabinoid receptor ligands of formula (I)wherein Y, X1, X2, X3, R1, and R2 are as defined in the specification. Compositions comprising such compounds, and methods for treating conditions and disorders using such compounds and compositions are also disclosed.
Owner:ABBVIE INC
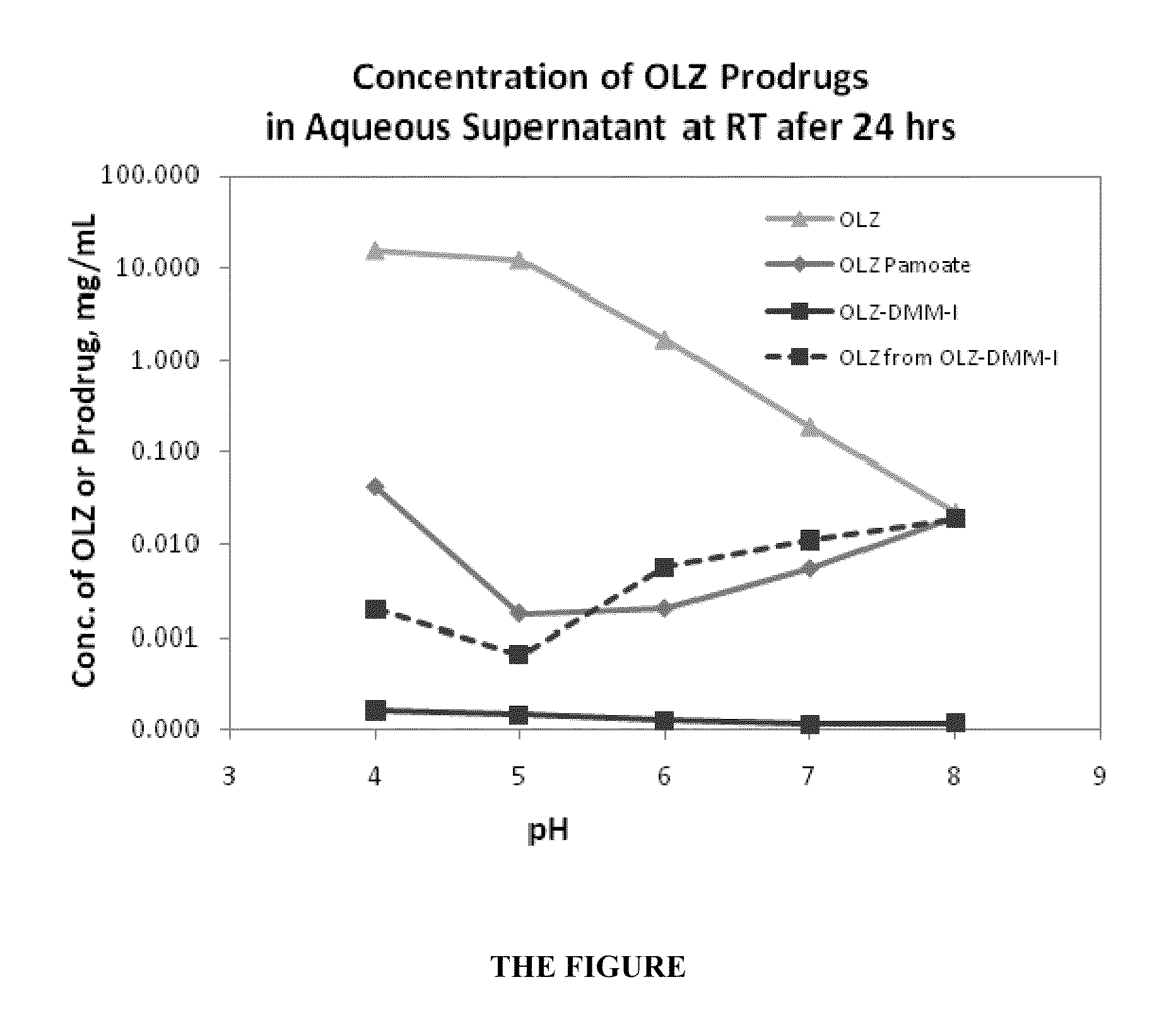
Packaged modified release gamma-hydroxybutyrate formulations having improved stability
InactiveUS20190183806A1Stable dissolution profileMaintain chemical stabilityNervous disorderInorganic non-active ingredientsGamma hydroxybutyrateImproved solubility
Packaged formulations of gamma-hydroxybutyrate having improved dissolution and chemical stability, packaging for supporting said stability, and therapeutic uses thereof.
Owner:FLAMEL IRELAND
Chinese medicinal composition of medicine pillow for assisting sleep
InactiveCN101366906AReasonable preparationCompatibility is reasonablePillowsNervous disorderCarthamusGynostemma
The present invention relates to a traditional Chinese medicine composition of a sleep helping and nerves calming medicine-filled pillow, which belongs to the field of bed health care products. The composition comprises the following materials in weight portion: 20 to 30 portions of ginseng, 20 to 30 portions of angelica, 20 to 30 portions of caulis polygoni multiflori, 15 to 25 portions of grassleaved sweetflag rhizome, 20 to 30 portions of uncaria, 25 to 35 portions of radix salviae miltiorrhizae, 20 to 40 portions of albizzia julibrissin, 30 to 40 portions of spina date seed, 30 to 40 portions of semen biotae shelled, 20 to 30 portions of licorice, 25 to 35 portions of senega, 20 to 30 portions of safflower, 30 to 40 portions of mulberry leaves, 30 to 40 portions of Gynostemma pentaphylla, 20 to 40 portions of lily and 20 to 30 portions of poria cum radix pini. The traditional Chinese medicine composition of the sleep helping and nerves calming medicine-filled pillow has no toxic side effects on a human body, completely adopts pure natural products as materials, enhances the vigor of the human body in sleeping state, nourishes heart and blood, calms nerves, relaxes veins, and improves immunity. Particularly, the composition is suitable for the symptoms such as sleeplessness, dreaminess, short sleep, vexation, forgettery, hardness to fall asleep, and the like.
Owner:李富祥
Cariprazine tartrate, preparation method therefor and medical use thereof
ActiveCN105218484ANot easy to absorb moistureImprove solubilityNervous disorderOrganic chemistry methodsCariprazinePharmaceutical drug
Owner:ANHUI HEALSTAR PHARM CO LTD
Clinic compliant method for banking human placental mesenchymal cells
Owner:AFFILIATED HOSPITAL OF NINGXIA MEDICAL UNIV
Formulations and Dosage Forms of Oxidized Phospholipids
Owner:VASCULAR BIOGENICS
Bean-curd pectin analogues, and its use in preparation of medicine for preventing senile dementia
ActiveCN101029064ASmall toxicityGood curative effectOrganic active ingredientsNervous disorderAcetylcholinesteraseCurative effect
Owner:KUNMING BAKER NORTON PHARMA
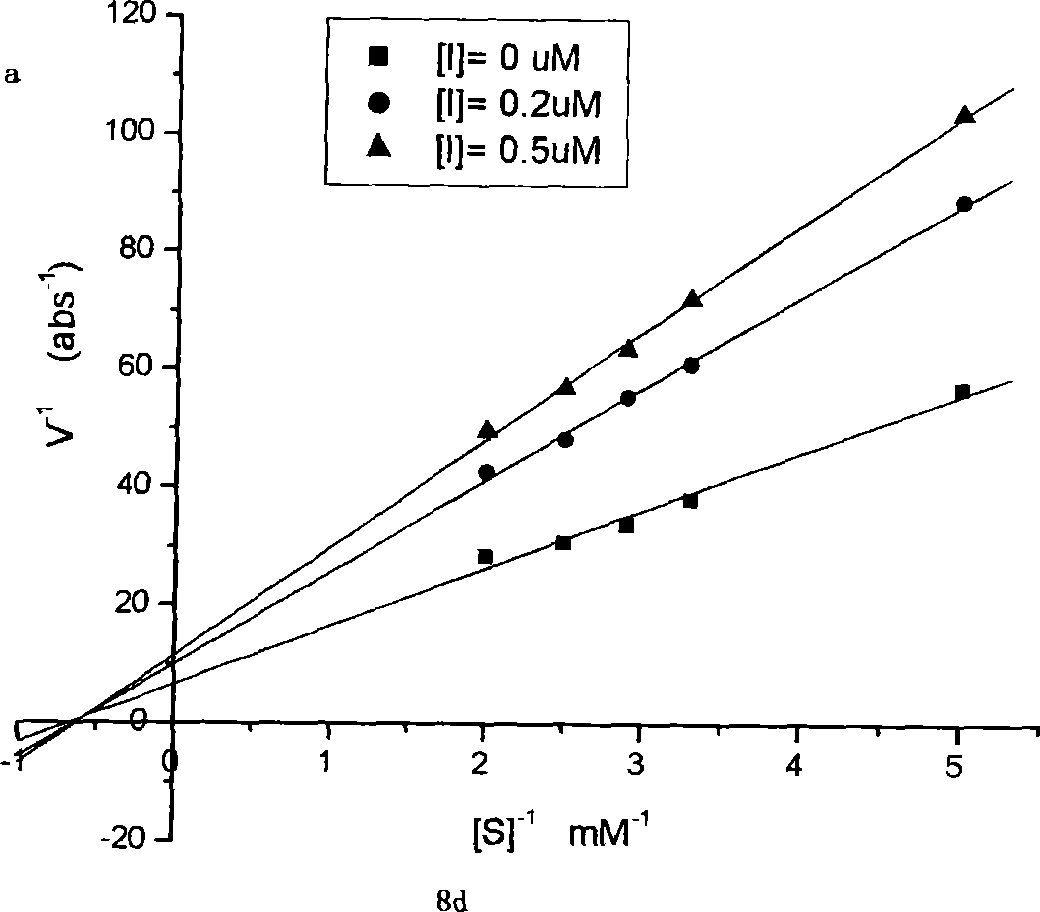
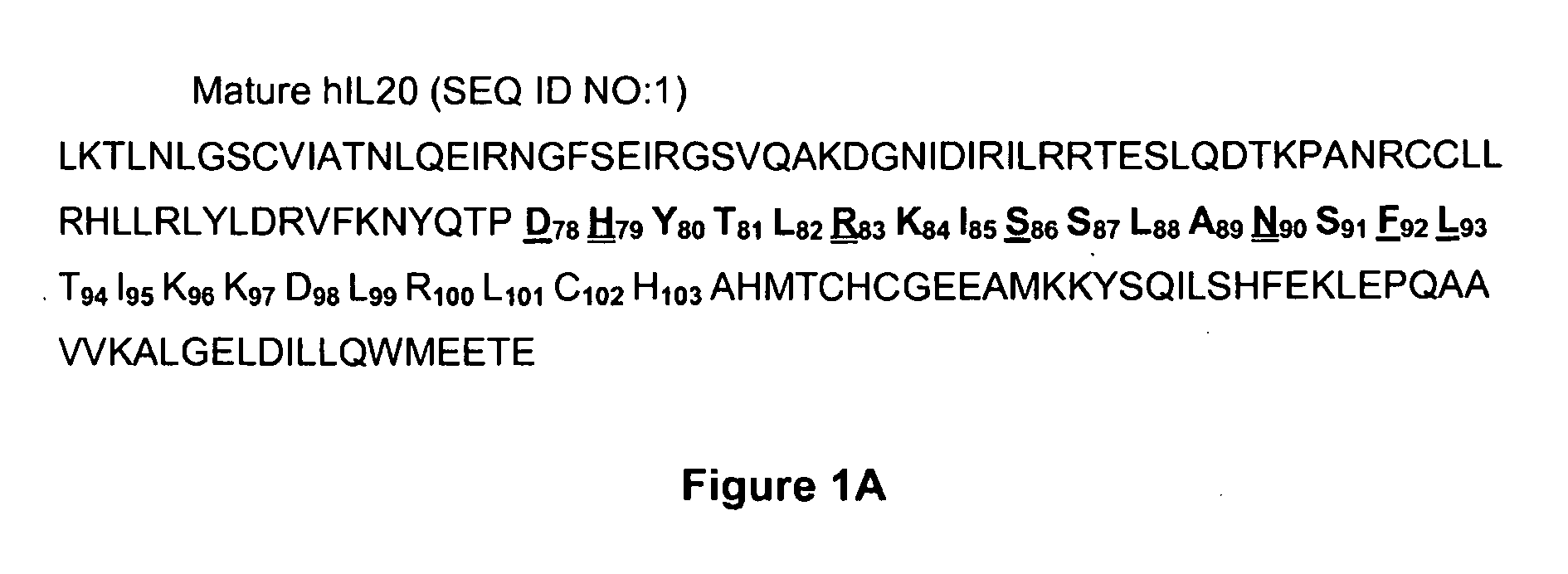
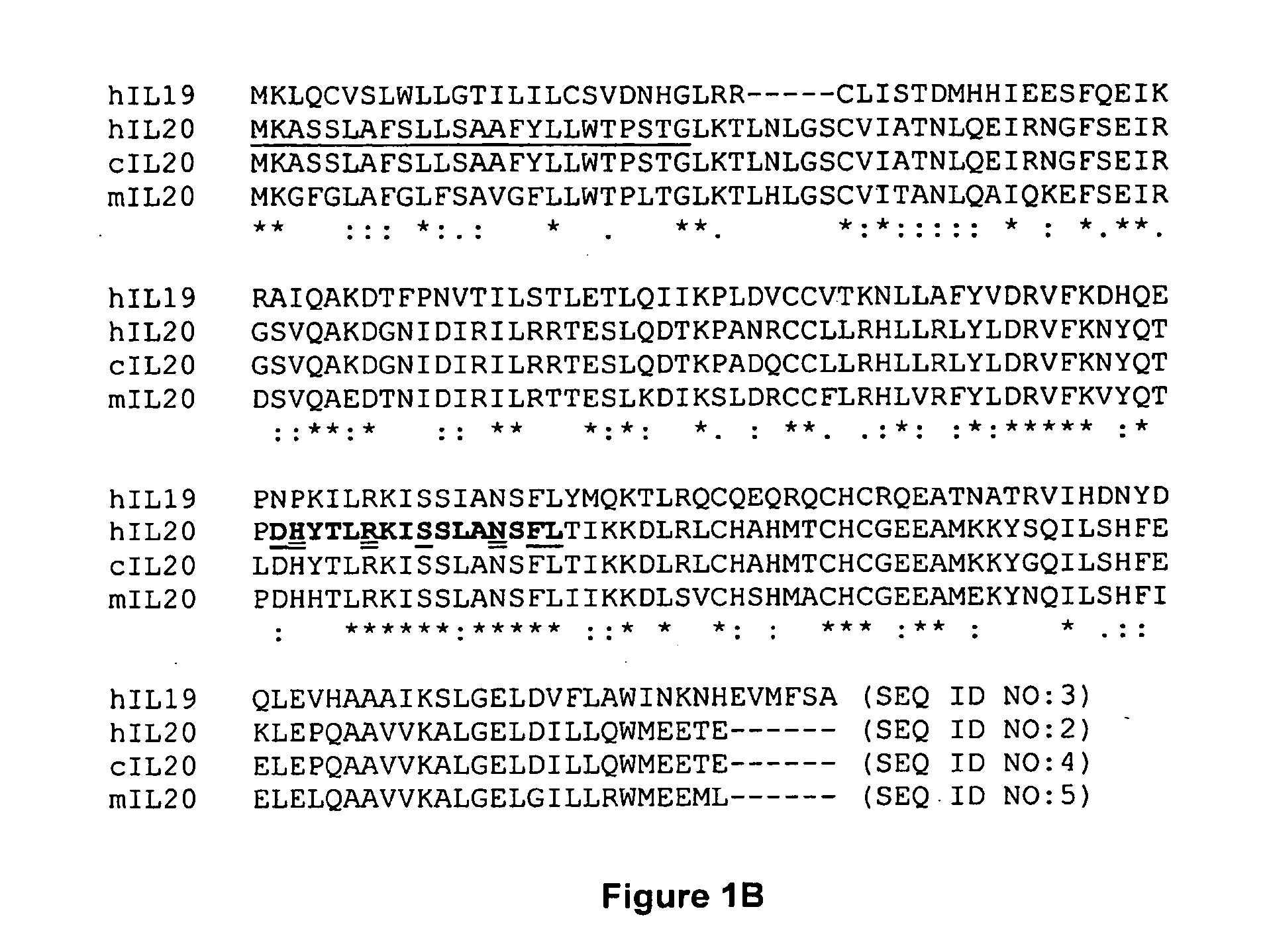
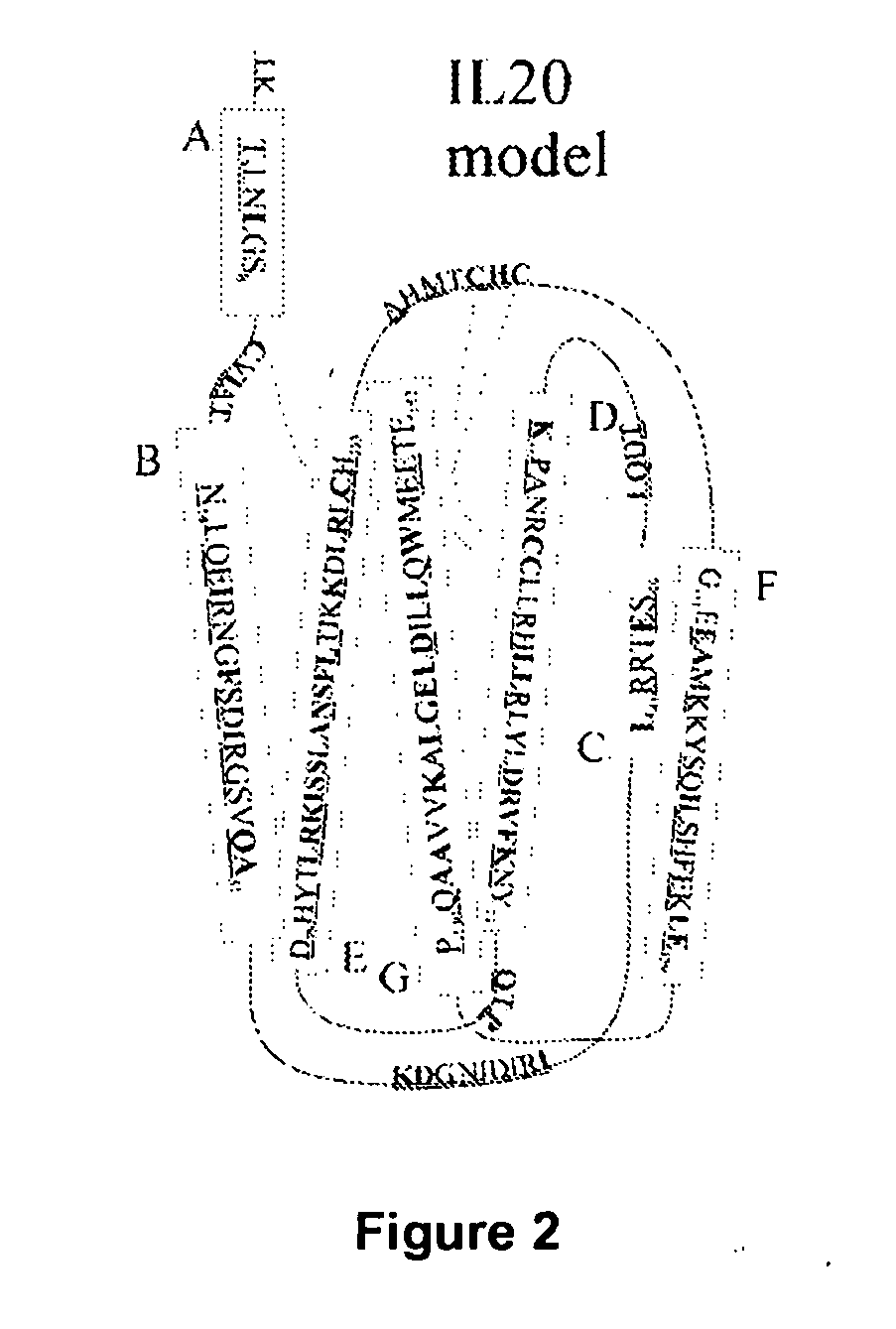
Anti-Human Interleukin-20 Antibodies
Owner:NOVO NORDISK AS
Mucosal Bioadhesive SLow Release Carrier for Delivering Active Principles
A mucosal bioadhesive slow release carrier comprising an active principle and devoid of starch, lactose, which can release the active principal for a duration of longer than 20 hours. This bioadhesive carrier contains a diluent, an alkali metal alkylsulfate, a binding agent, at least one bioadhesive polymer and at least one sustained release polymer, as well as a method for its preparation.
Owner:ONXEO SA
Traditional Chinese medicine health care product, its preparation method and its application
ActiveCN102552335AImprove sleepingImprove anemiaNervous disorderAnthropod material medical ingredientsSide effectCordyceps cicadae
Owner:ZHEJIANG BIOASIA PHARMA CO LTD
Treatment of neurodegenerative diseases by targeting mirna
ActiveUS20130184331A1Inhibit functioningImprove regenerative abilityOrganic active ingredientsNervous disorderMedicinePharmaceutical drug
The present invention relates to a pharmaceutical composition for preventing or treating neurodegenerative diseases by targeting a specific miRNA. In addition, the present invention relates to a kit for diagnosing neurodegenerative diseases. A miR-206 target found in the present invention, which is highly expressed in both animal models of Alzheimer's disease and human brain samples, is a substantial treatment target selected without artifact errors. An antisense oligonucleotide of the present invention as an inhibitor for miR-206 suggests a successful result in treatment of neurodegenerative diseases by targeting miRNA. The antisense oligonucleotide of the present invention inhibits the function of miR-206 to greatly increase the levels of BDNF and IGF-1 and to increase the regeneration of synapses, thereby treating neurodegenerative diseases, particularly Alzheimer's disease.
Owner:SEOUL NAT UNIV R&DB FOUND
Therapeutic compositions and methods of treatment with capsianoside-type compounds
The present invention discloses the use of certain compounds as therapeutic agents, and in particular as analgesics and anti-inflammatory agents. Such compounds include, for example, certain diterpene monoglycosides and diterpene diglycosides. The compounds of the present invention may be synthesized or isolated from the fruit of the genus Capsicum, and in particular may be isolated from sweet bell peppers (C. annuum). Pharmaceutically-acceptable salts, enantiomers, diasteriomers, racemic mixtures, enantiomerically-enriched mixtures, solvates, and prodrugs of such compounds are also disclosed. Pharmaceutical compositions and methods of using such compounds, including pharmaceutical compositions and methods of using such compounds in combination with one or more active ingredients, are also disclosed.
Owner:BMB PATENT HLDG CORP
Oxygen-fluorine liposome microbubble and preparation method thereof
InactiveCN103212094AImprove performanceUniform particle sizeNervous disorderEnergy modified materialsPolyethylene glycolPhospholipid
Owner:CHILDRENS HOSPITAL OF CHONGQING MEDICAL UNIV
Controlled release formulation for treating sleep disorders
The invention relates to a controlled-release formulation for preventing and / or treating sleep disorders comprising Zaleplon or a pharmaceutically acceptable salt thereof in immediate release form and Zolpidem or a pharmaceutically acceptable salt thereof in sustained release form, wherein Zaleplon or a pharmaceutically acceptable salt thereof and Zolpidem or a pharmaceutically acceptable salt thereof are released in two phases where the first phase is a immediate release phase of Zaleplon or a pharmaceutically acceptable salt thereof and the second phase is a sustained release phase of Zolpidem or a pharmaceutically acceptable salt thereof.
Owner:TAIWAN BIOTECH
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap