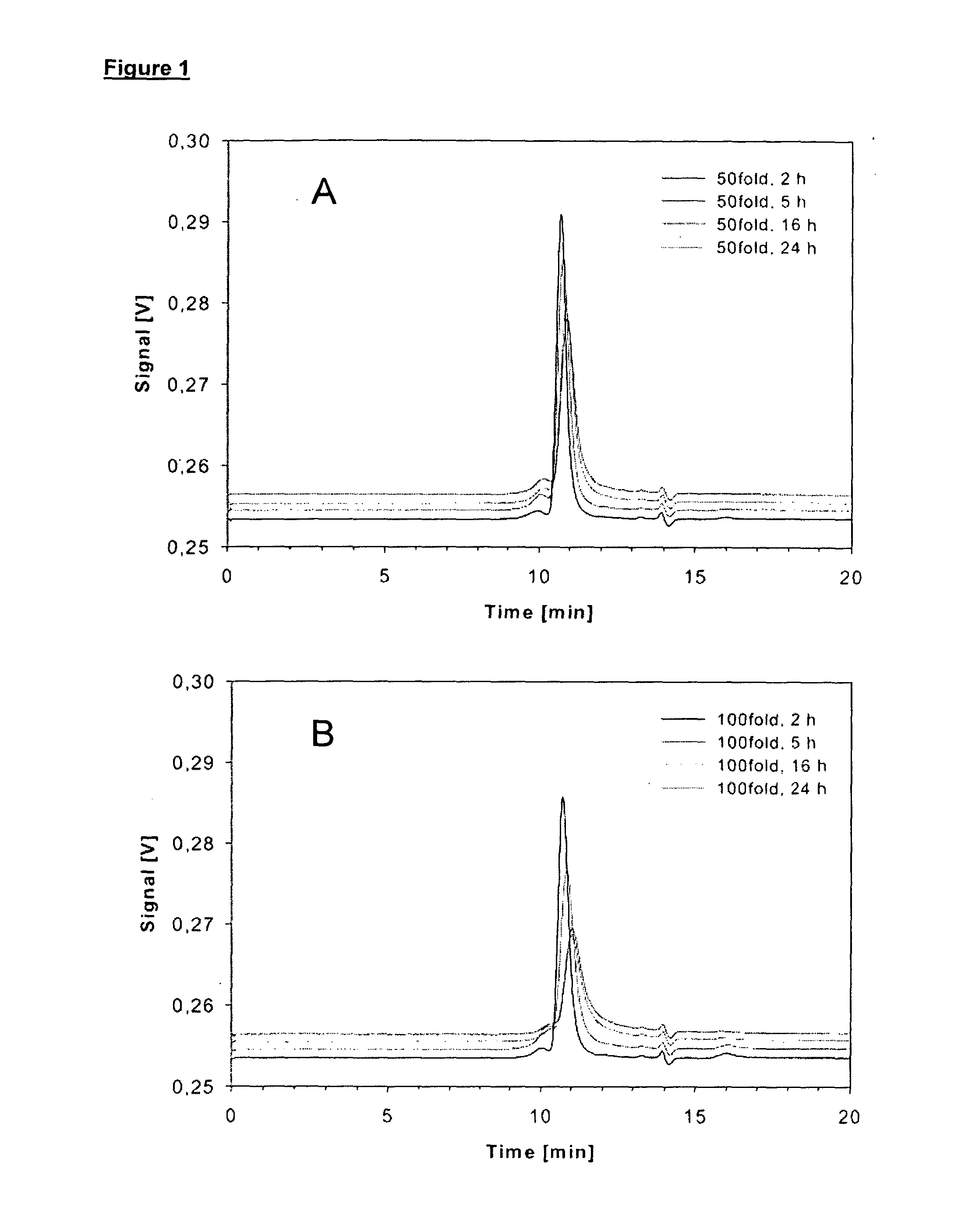
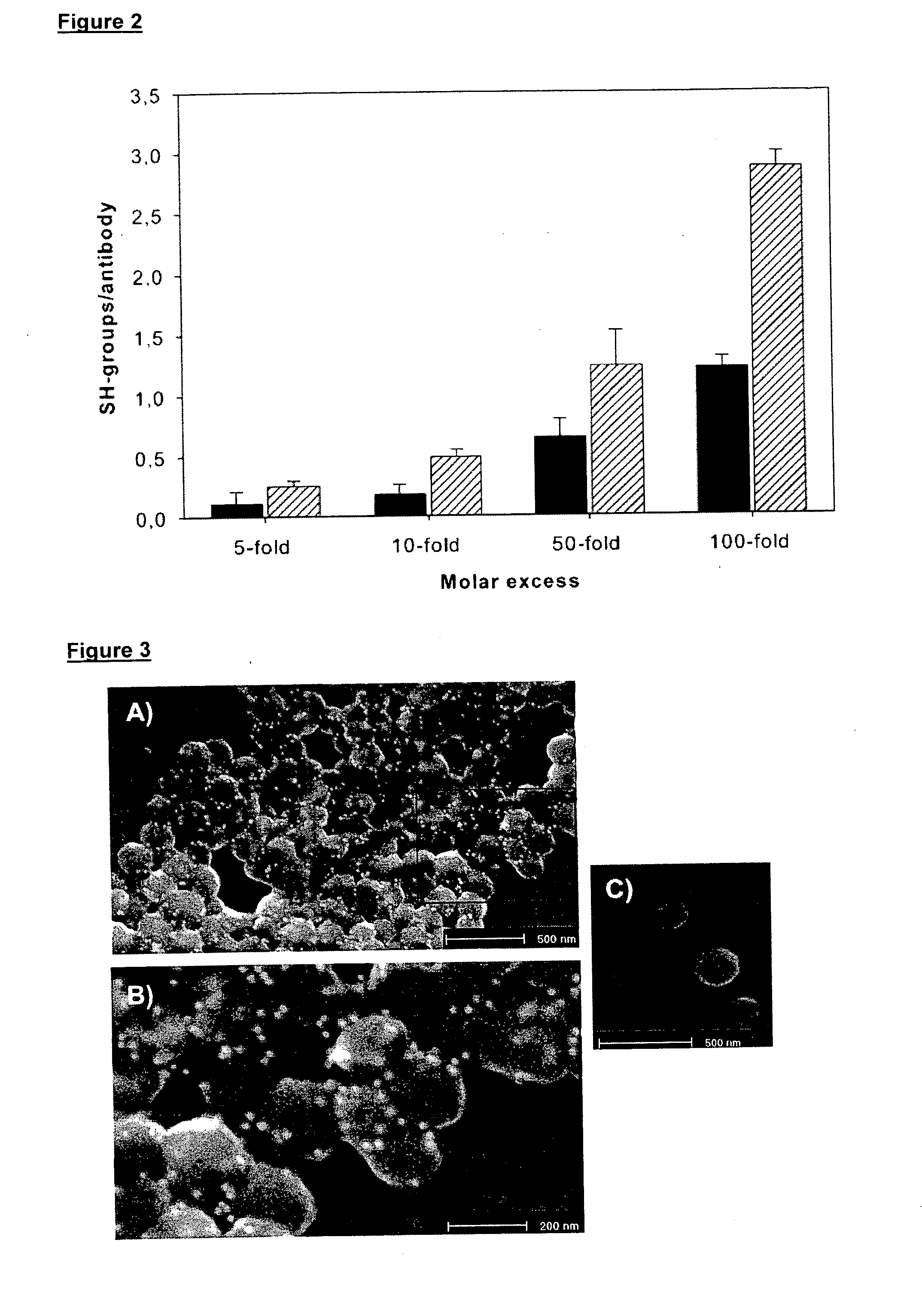
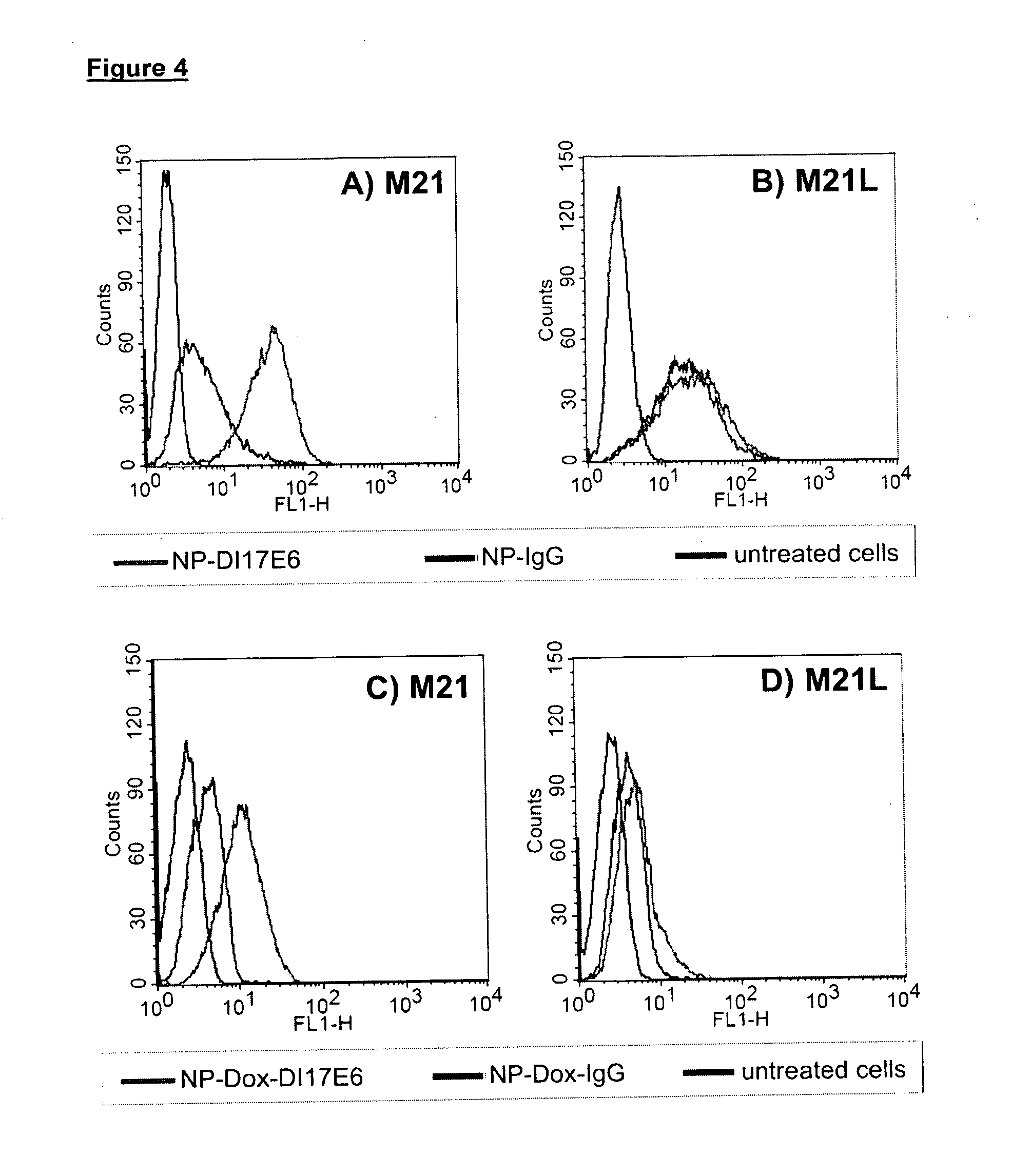
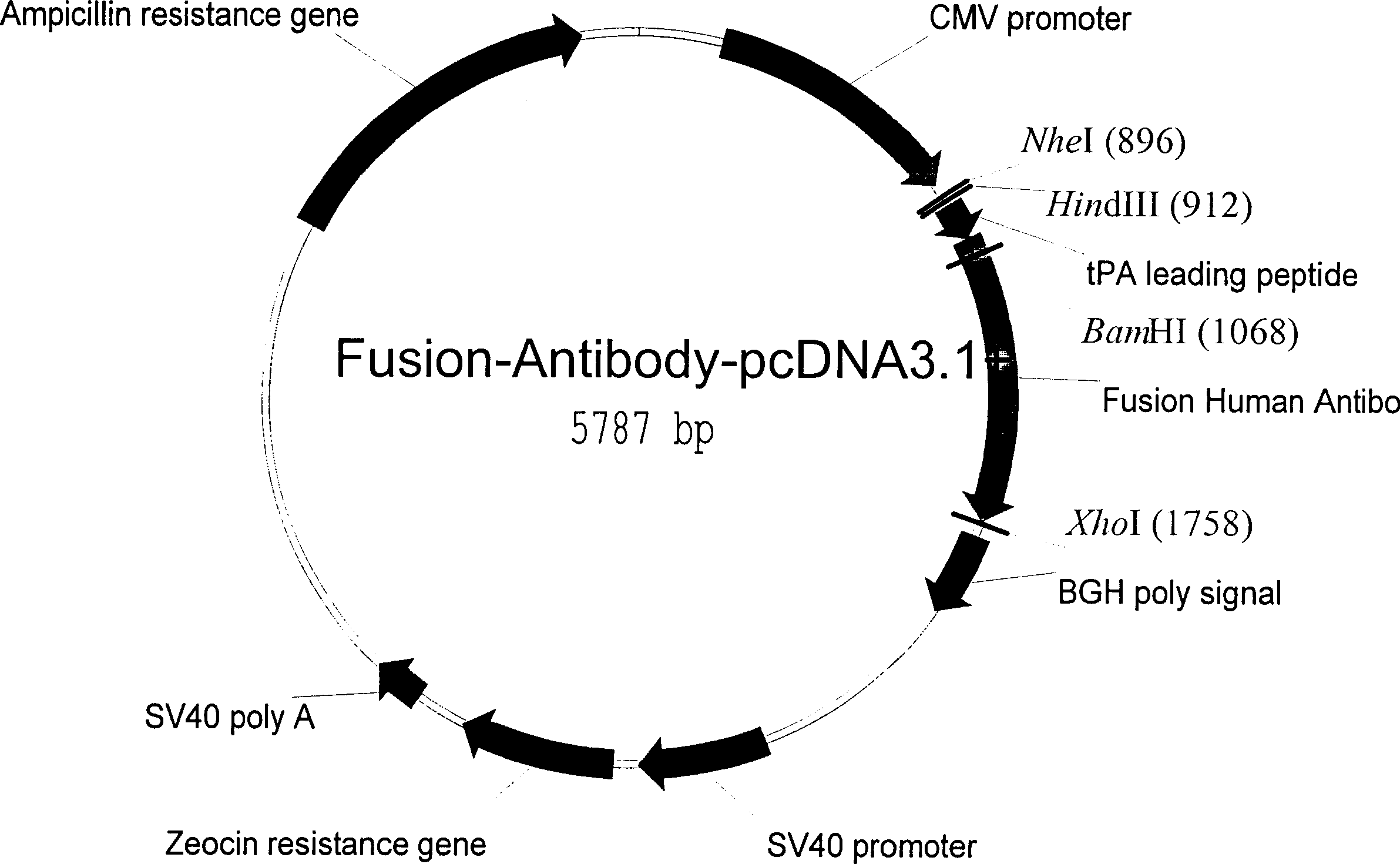
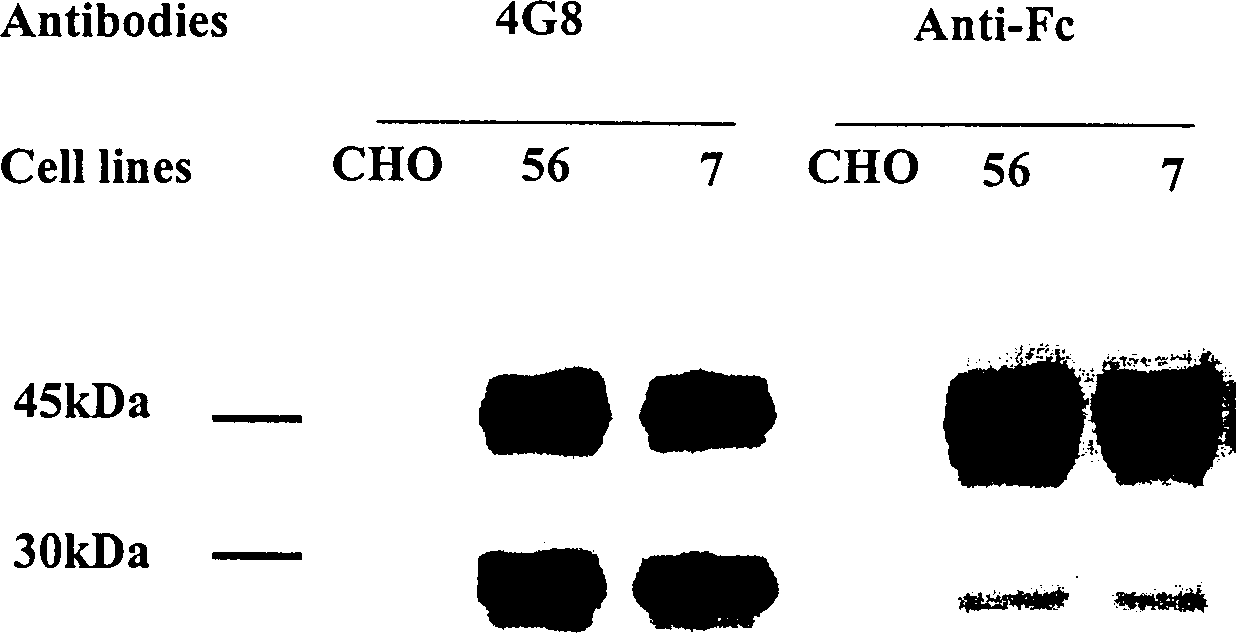
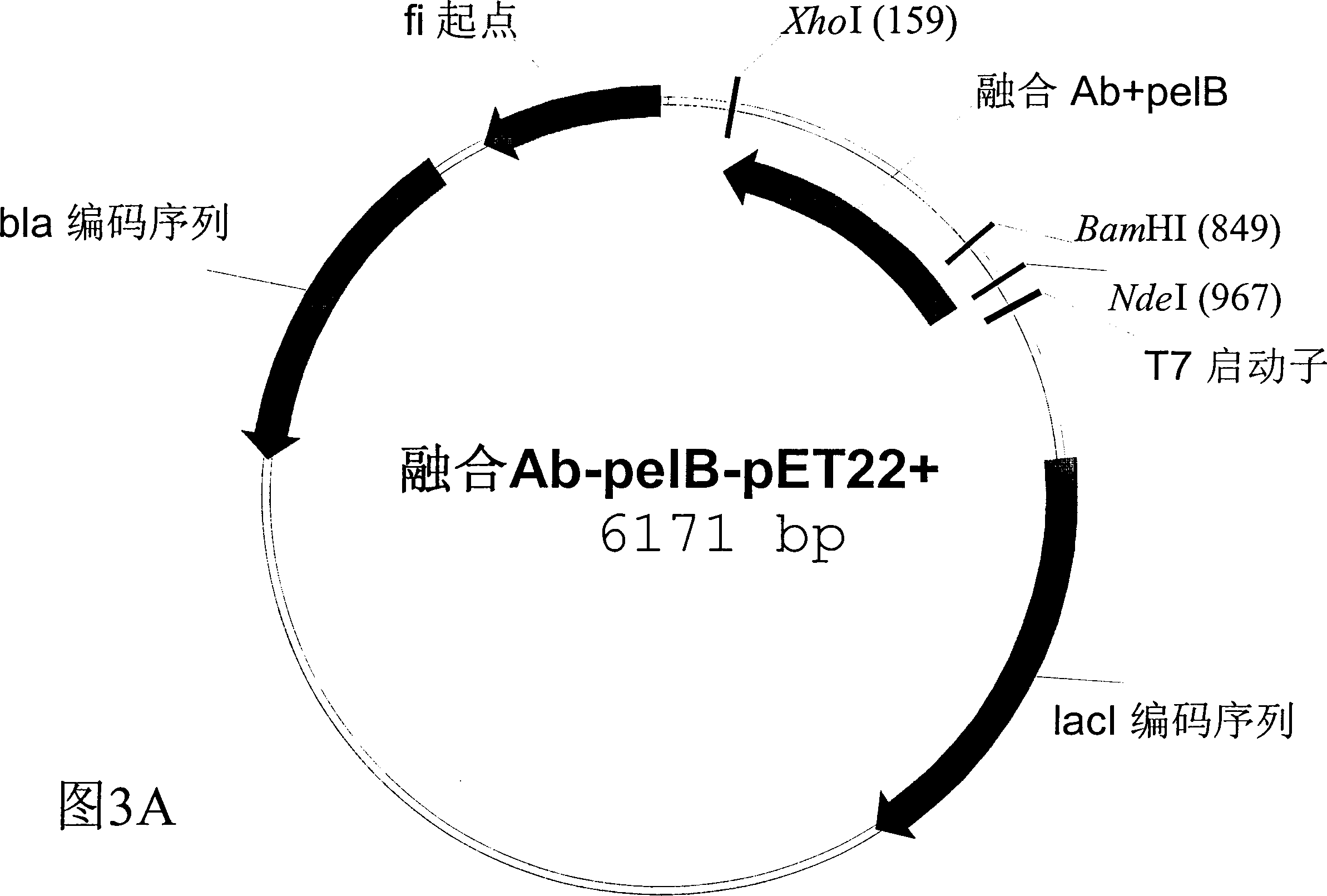
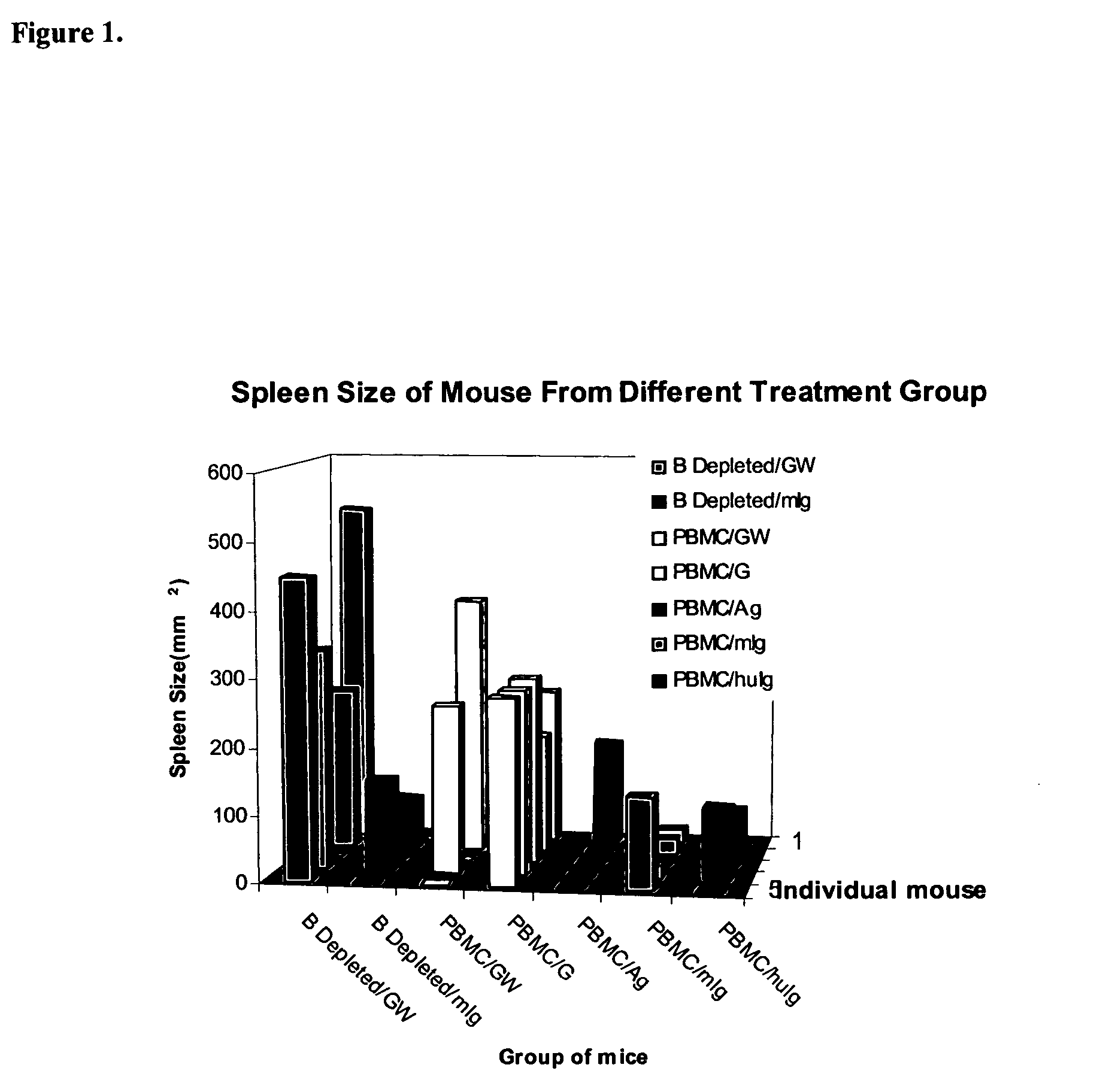
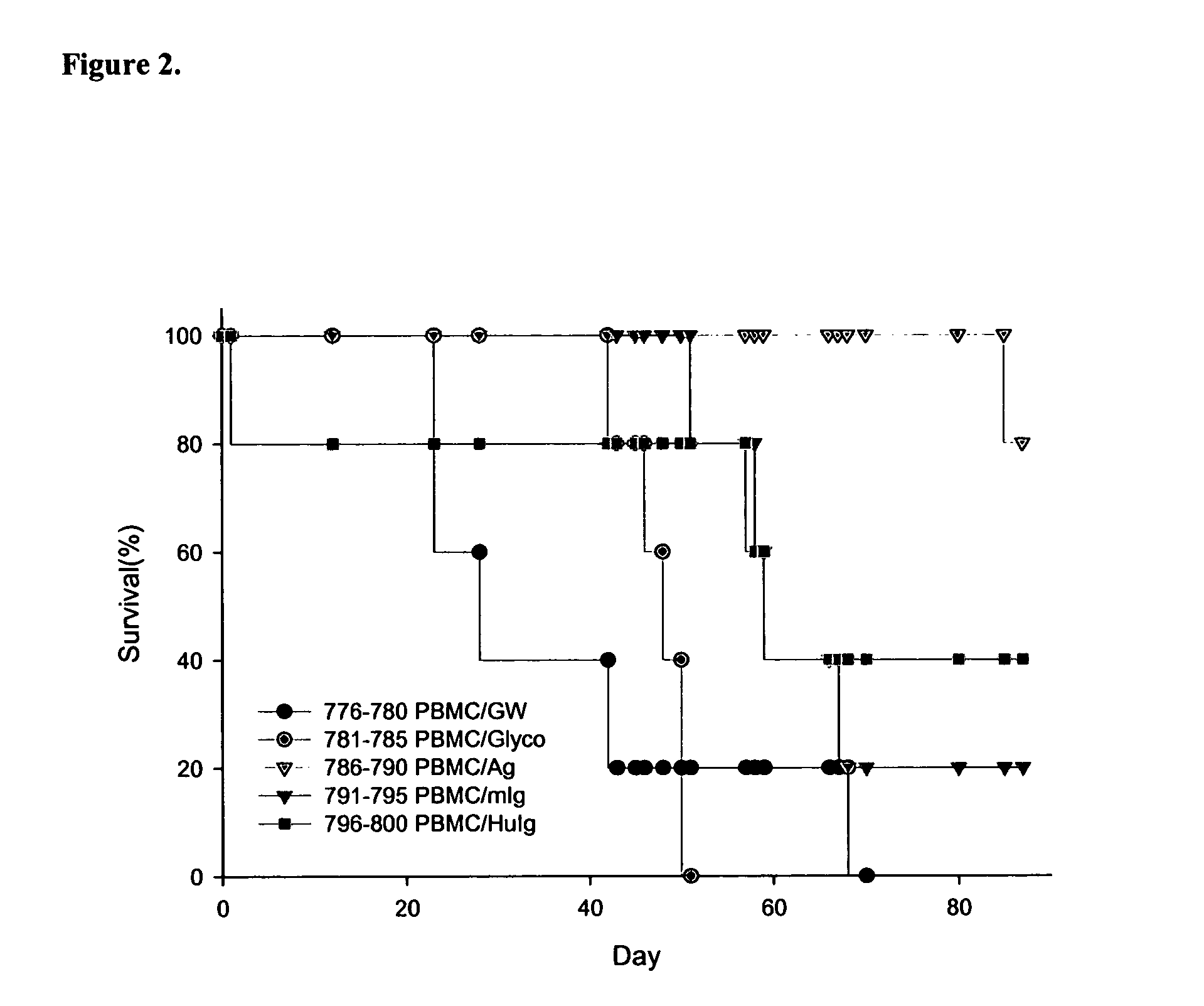
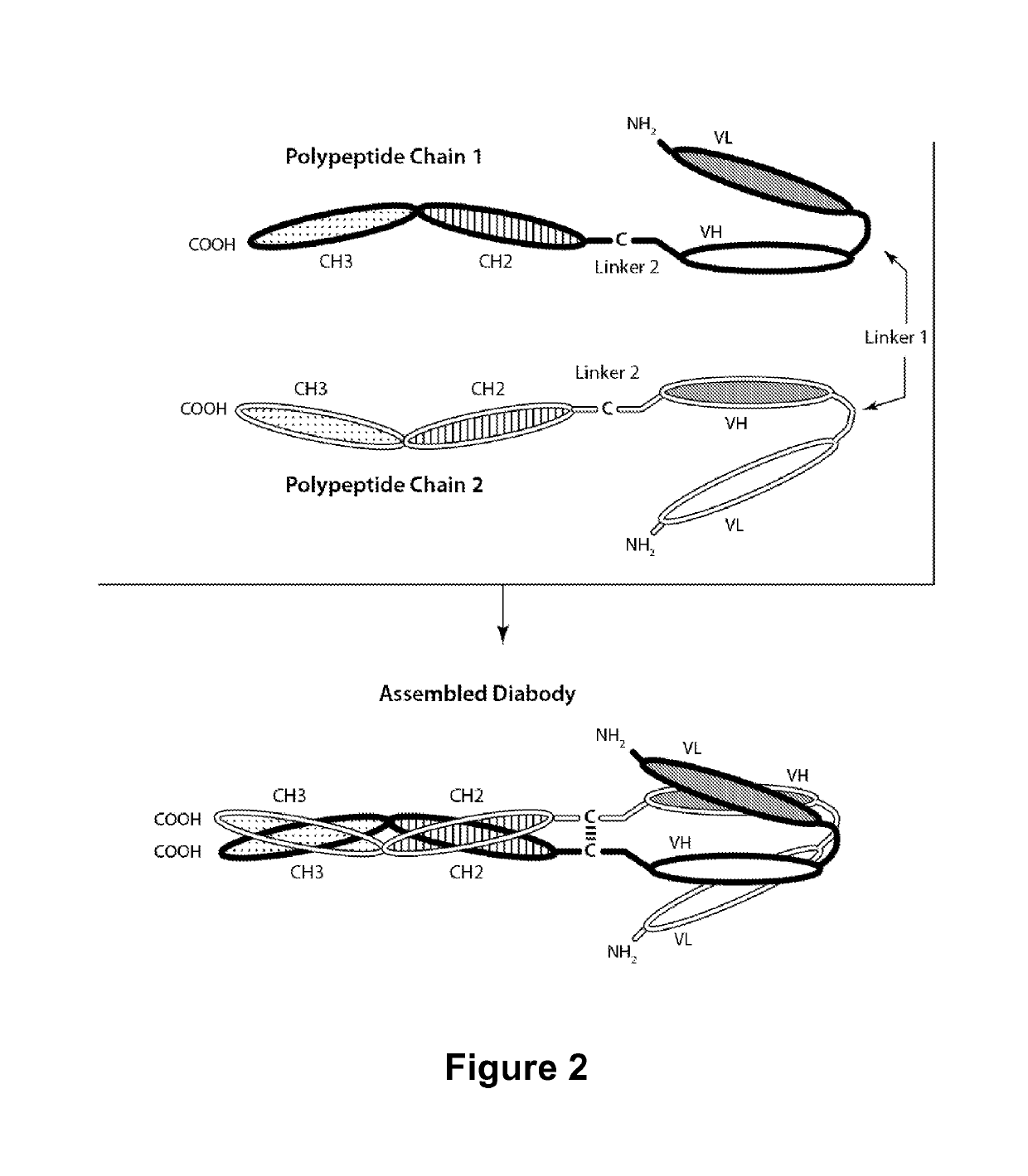
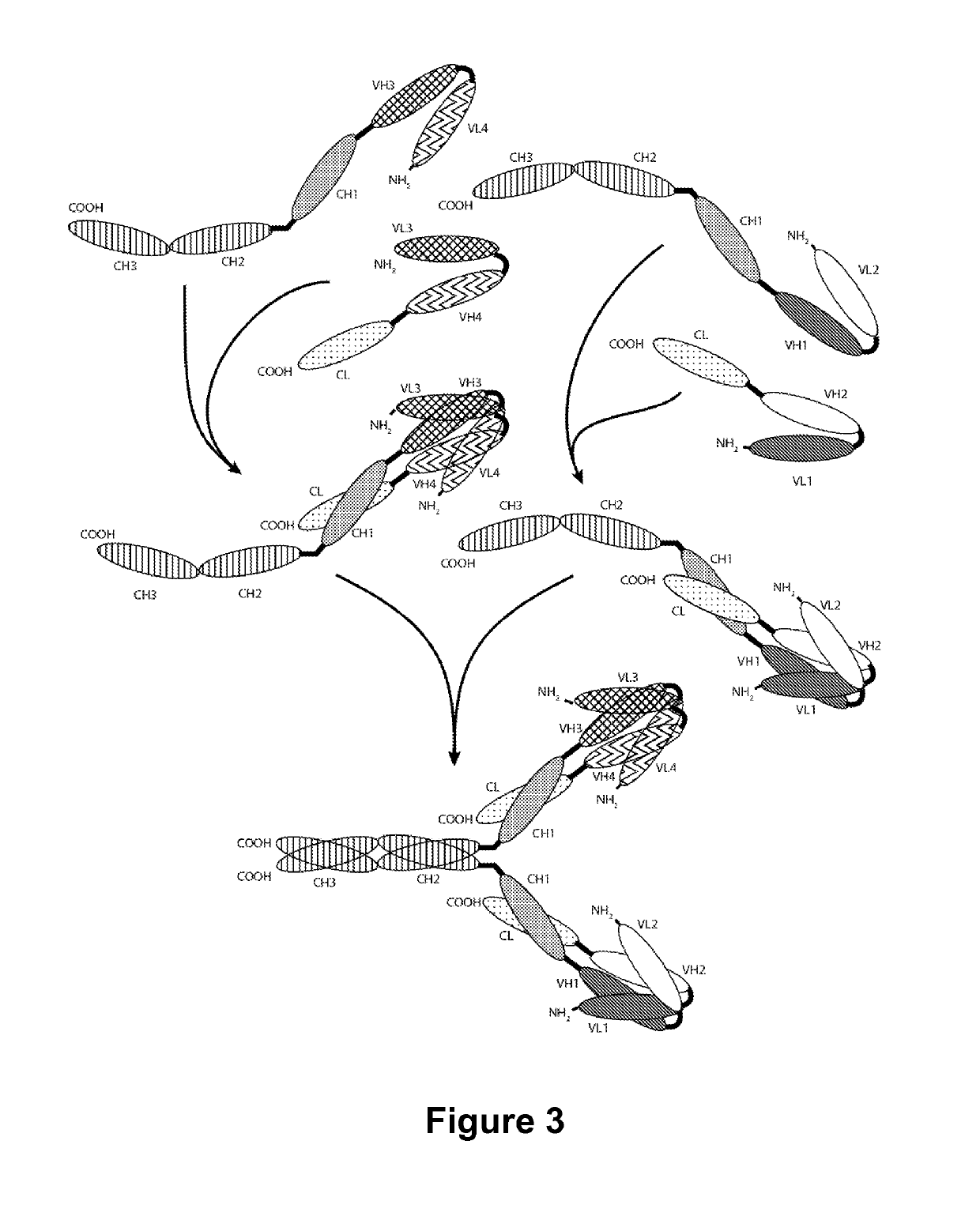
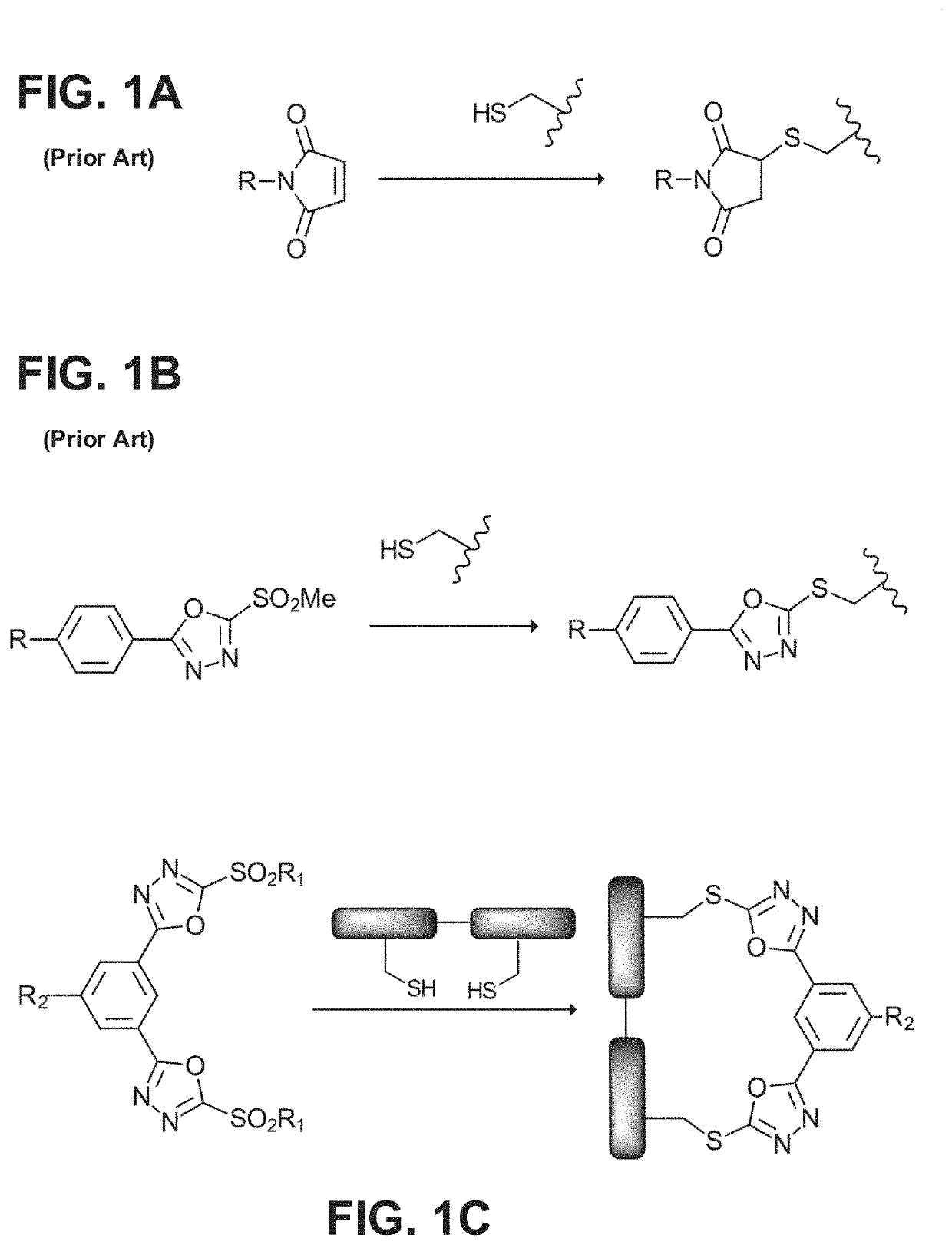
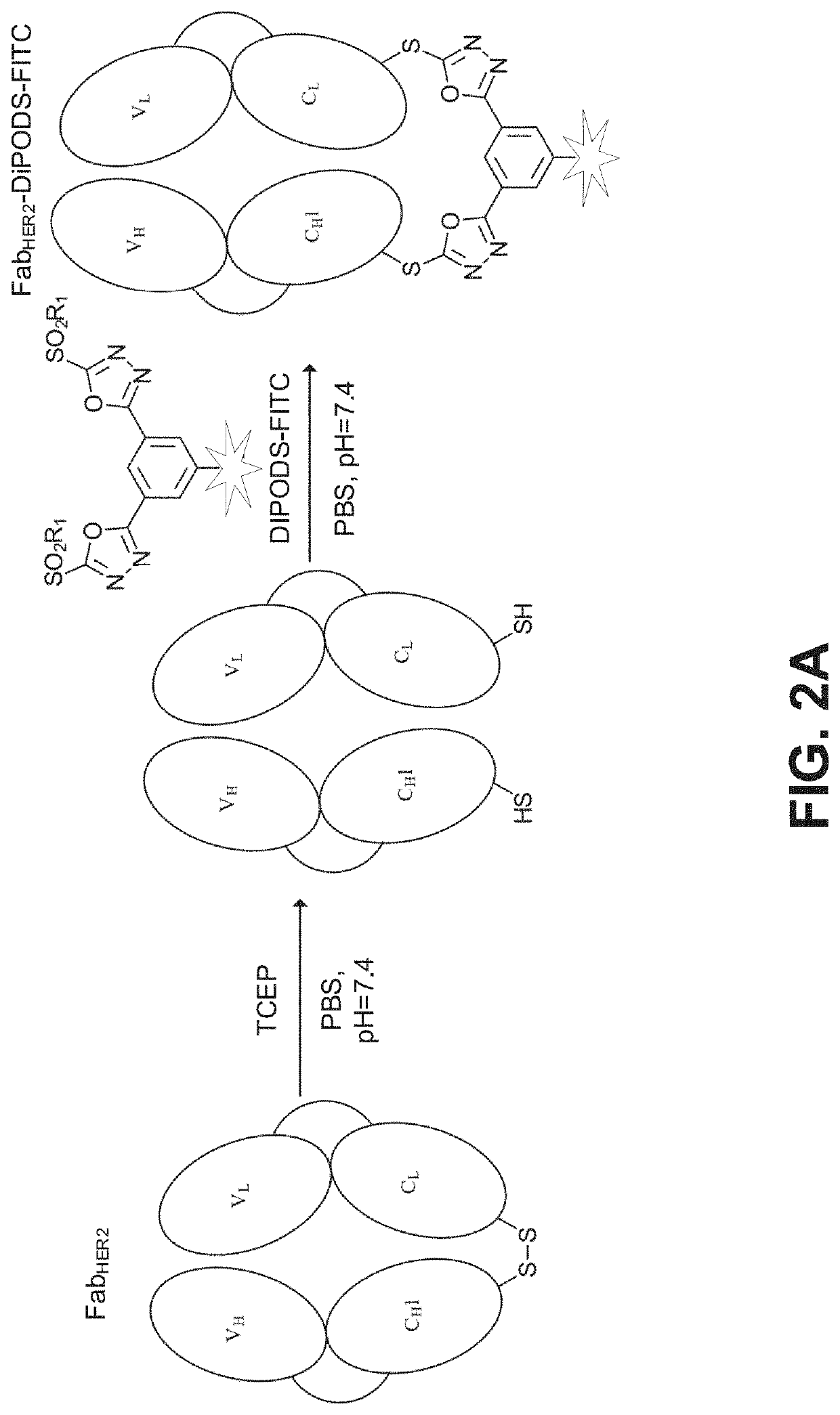
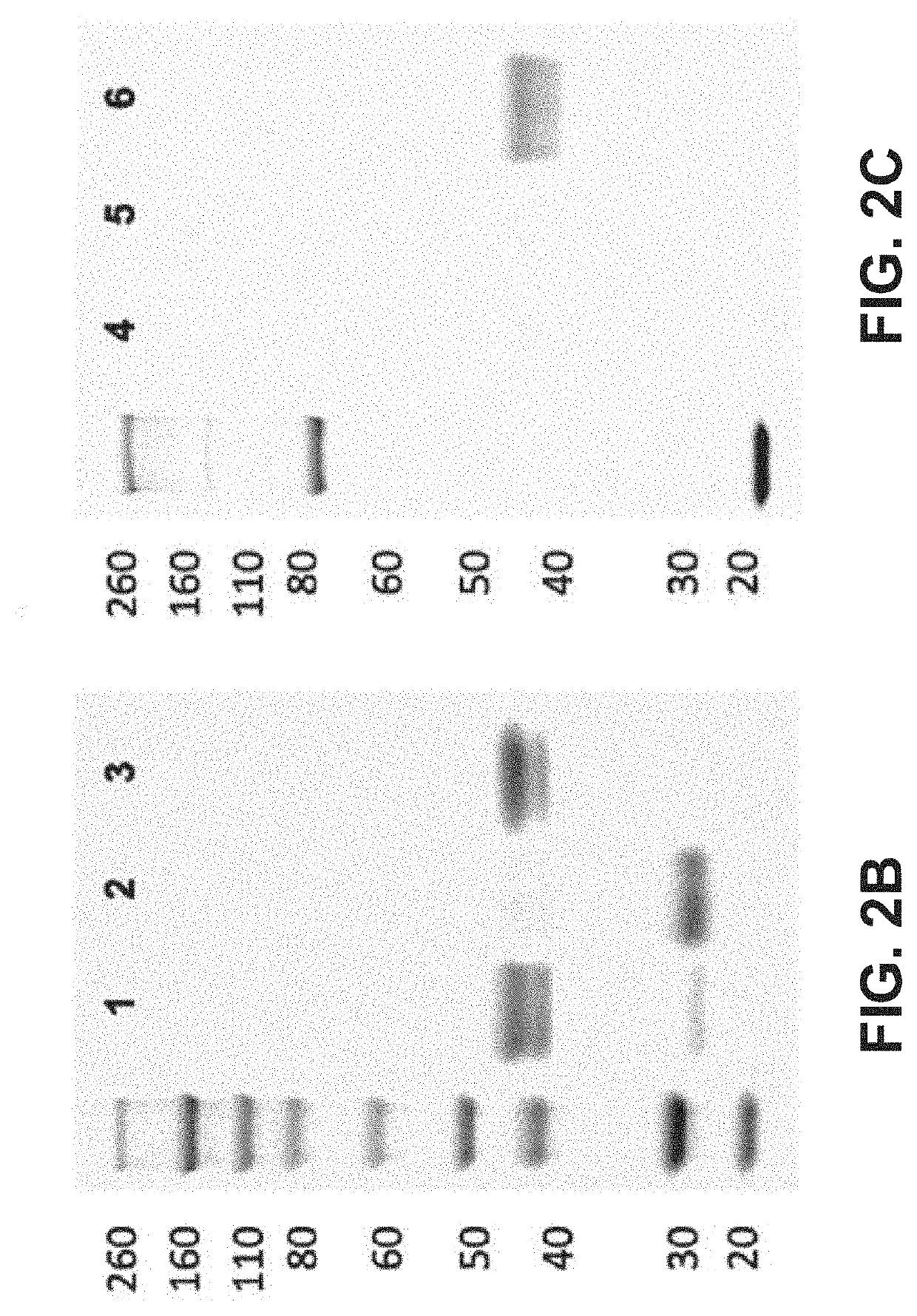
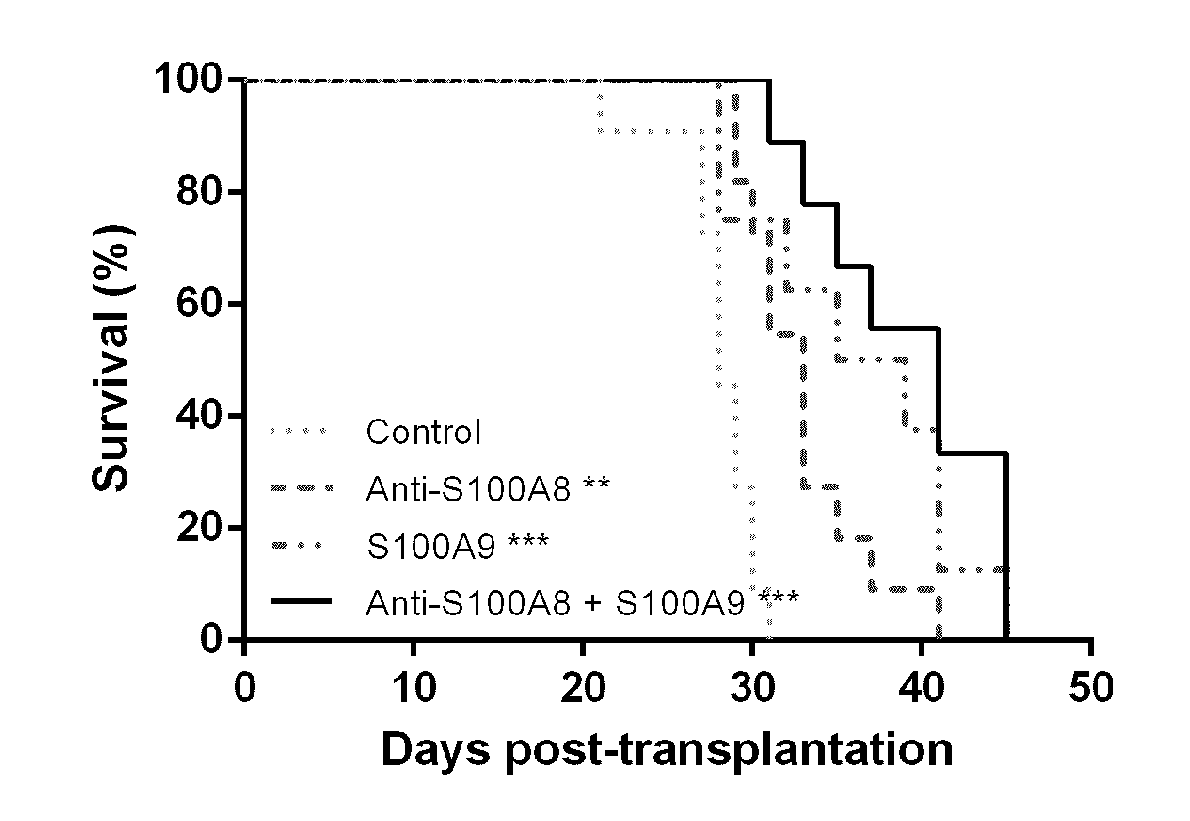
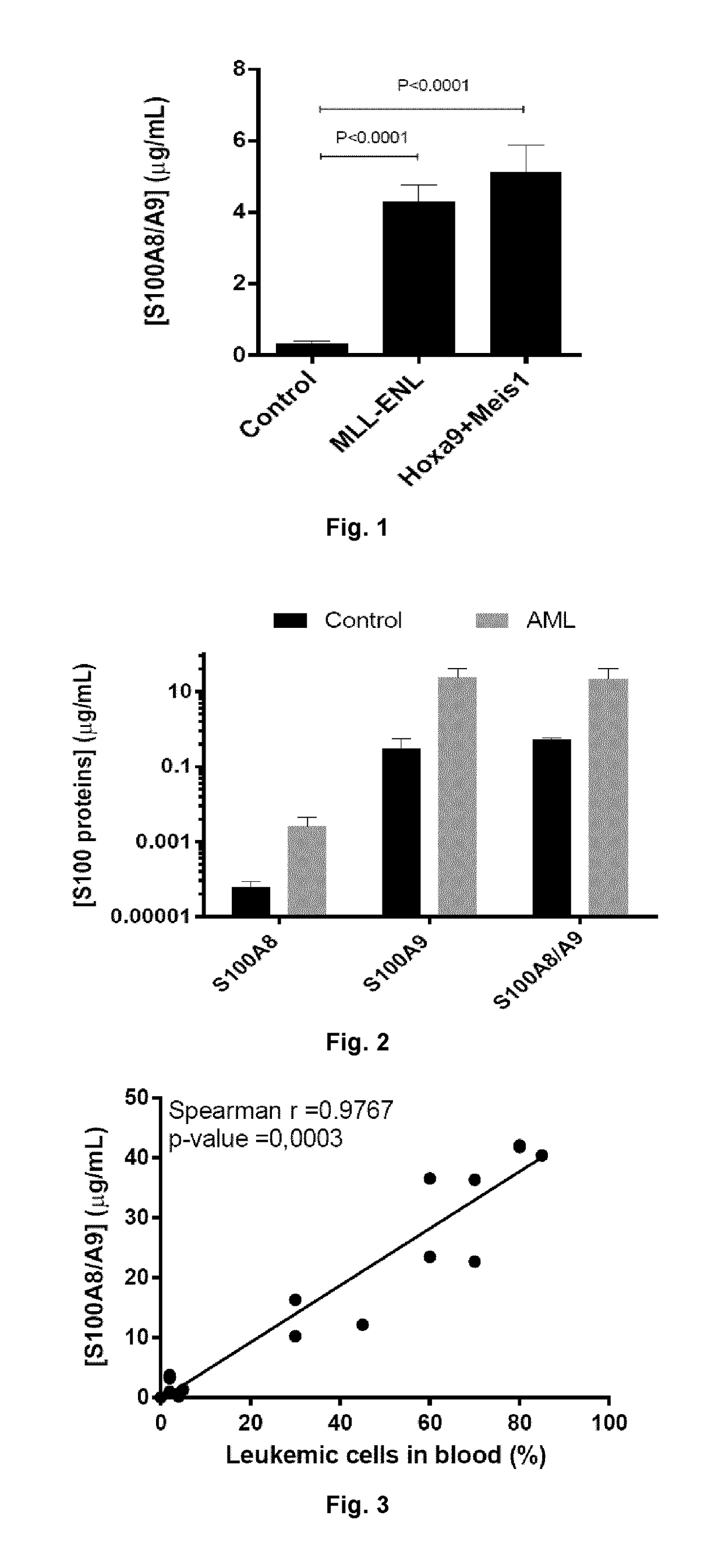
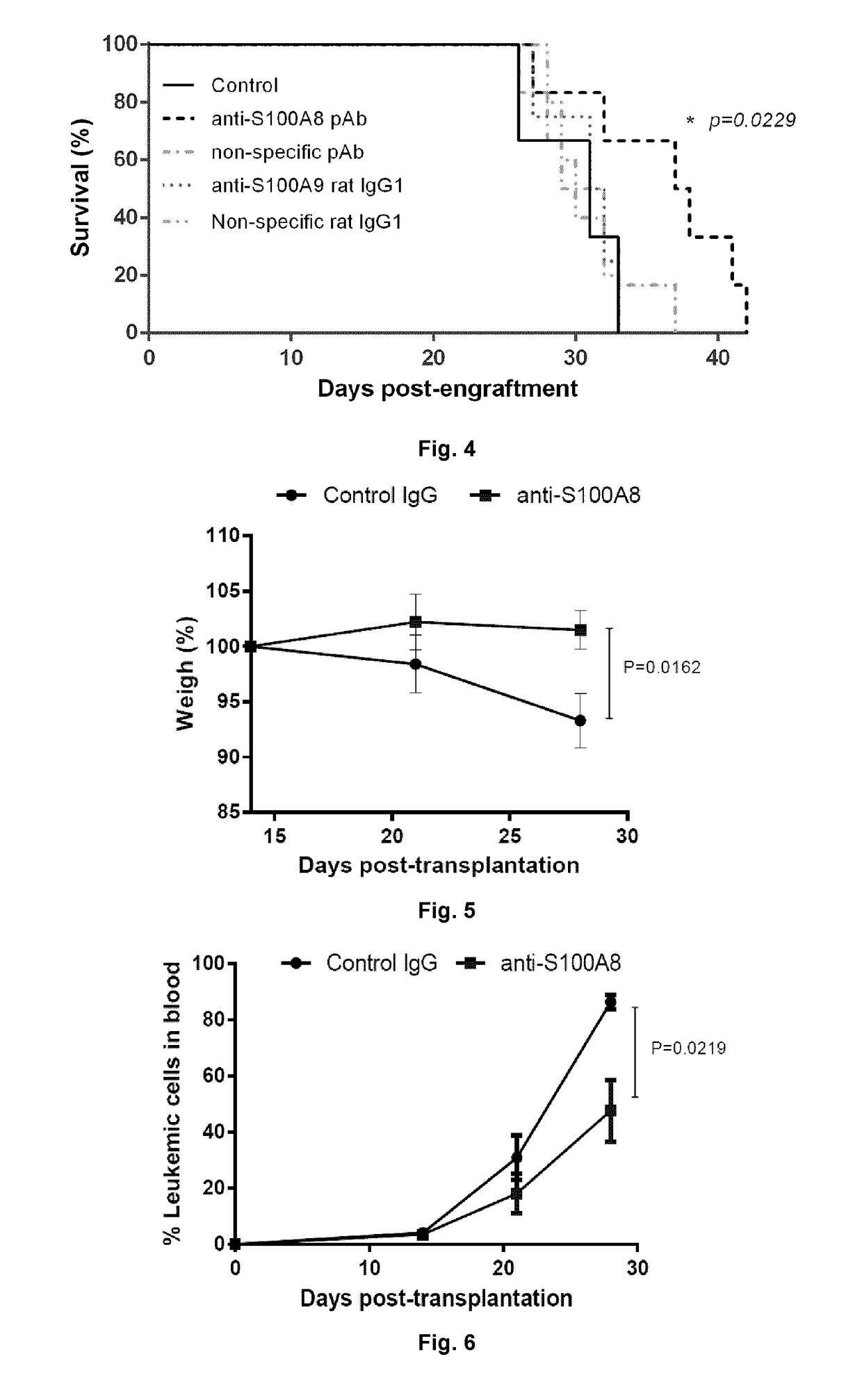
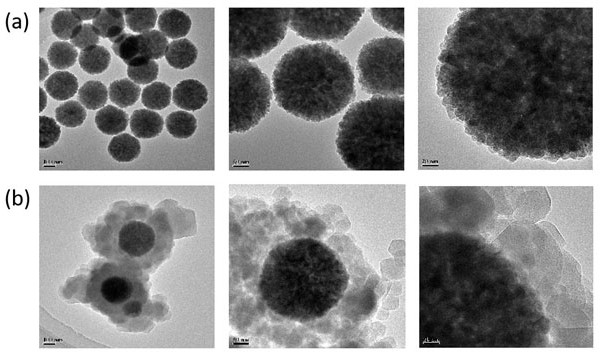
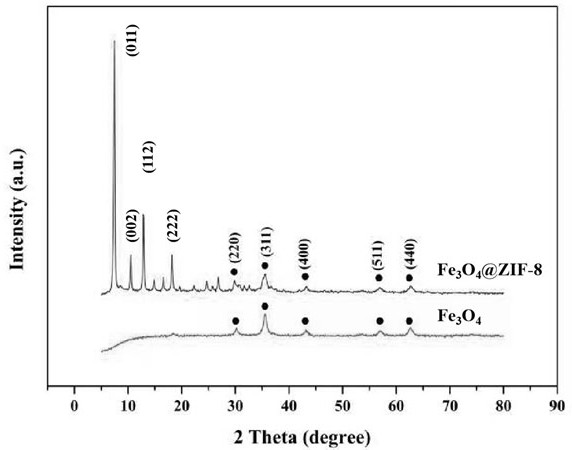
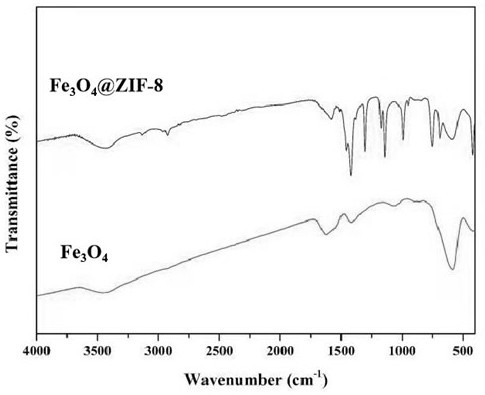
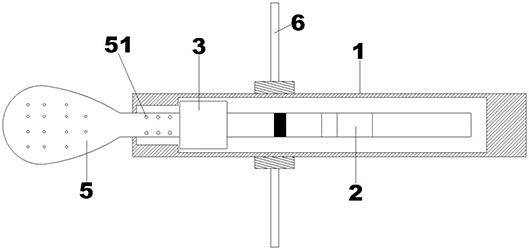
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein produced mainly by plasma cells that is used by the immune system to neutralize pathogens such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen, via the fragment antigen-binding (Fab) variable region. Each tip of the "Y" of an antibody contains a paratope (analogous to a lock) that is specific for one particular epitope (similarly, analogous to a key) on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can tag a microbe or an infected cell for attack by other parts of the immune system, or can neutralize its target directly (for example, by inhibiting a part of a microbe that is essential for its invasion and survival). Depending on the antigen, the binding may impede the biological process causing the disease or may activate macrophages to destroy the foreign substance. The ability of an antibody to communicate with the other components of the immune system is mediated via its Fc region (located at the base of the "Y"), which contains a conserved glycosylation site involved in these interactions. The production of antibodies is the main function of the humoral immune system.
Non-mammalian GnRH analogs and uses thereof in the immune system
InactiveUS20050043245A1Effective supervisionHigh affinityPeptide/protein ingredientsLuteinising hormone-releasing hormoneDiseaseD-Arginine
Specially designed non-mammalian GnRH, its analogs, or biometics resistant to degradation by peptidase, are disclosed. The GnRH analogs are further defined as analogs of GnRH II or salmon GnRH. These non-mammalian analogs incorporate D-arginine, D-leucine, D-tBu-Serine, D-Trp or other active D amino acids at position 6 and ethylamide, aza-Gly-amide or other Gly amide at position 10. The D-Arg (6)—GnRH II-ethylamide, D-Arg (6)—GnRH II-aza-Gly (10)-amide, the D-Arg (6)—salmon GnRH ethylamide, and D-Arg (6)—salmon GnRH-aza-Gly (10)-amide analogs are also provided, and demonstrate preferential binding to immune system non-mammalian GnRH receptors. These non-mammalian GnRH or its analogs, or long-acting preparation, biometics or their antibodies may be used in pharmaceutical preparation, and specifically in treatment of various immune system disorders. The non-mammalian GnRH or its analogs are also provided in pharmaceutical preparations that may be used clinically for treating immune system disorders when used in very low doses and administered in pulsatile fashion. The aza-Gly (10) amide non-mammalian analogs are yet other embodiments of the non-mammalian GnRH or its analogs provided as a part of the invention. The use of agents that regulate the production or antibodies or In addition, the detection of non-mammalian GnRH or GnRH II or the non-mammalian GnRH receptors may be used as a diagnostic tool.
Owner:SILER KHODR THERESA
Anti integrin antibodies linked to nanoparticles loaded with chemotherapeutic agents
InactiveUS20120263739A1Good curative effectGood effectPowder deliveryNanomedicineDoxorubicinAntibody
Owner:MERCK PATENT GMBH
Uses for and article of manufacture including her2 dimerization inhibitor pertuzumab
InactiveUS20130095172A1Prolong progression-free survivalReduce riskOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMetastatic gastric cancerHER2 Positive Breast Cancer
The present application describes uses for and articles of manufacture including Pertuzumab, a first-in-class HER2 dimerization inhibitor. In particular, the application describes methods for extending progression free survival in a HER2-positive breast cancer patient population; combining two HER2 antibodies to treat HER2-positive cancer without increasing cardiac toxicity; treating early-stage HER2-positive breast cancer; treating HER2-positive cancer by co-administering a mixture of Pertuzumab and Trastuzumab from the same intravenous bag; treating HER2-positive metastatic gastric cancer; treating HER2-positive breast cancer with Pertuzumab, Trastuzumab and Vinorelbine; treating HER2-positive breast cancer with Pertuzumab, Trastuzumab and aromatase inhibitor; and treating low HER3 ovarian, primary peritoneal, or fallopian tube cancer. It also describes an article of manufacture comprising a vial with Pertuzumab therein and a package insert providing safety and / or efficacy data thereon; a method of making the article of manufacture; and a method of ensuring safe and effective use of Pertuzumab related thereto. In addition the application describes an intravenous (IV) bag containing a stable mixture of Pertuzumab and Trastuzumab suitable for administration to a cancer patient.
Owner:GENENTECH INC
Human fusion antibody for reducing cerebral amyloid fibers associated with senile dementia
Owner:张小如 +1
Anti-CD137 antibody as an agent in the treatment of cancer and glycosylation variants thereof
InactiveUS20060182744A1Reduce and prevent inactivationEasy to manufactureImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAnticarcinogenCD137
Owner:GTC BIOTHERAPEUTICS INC
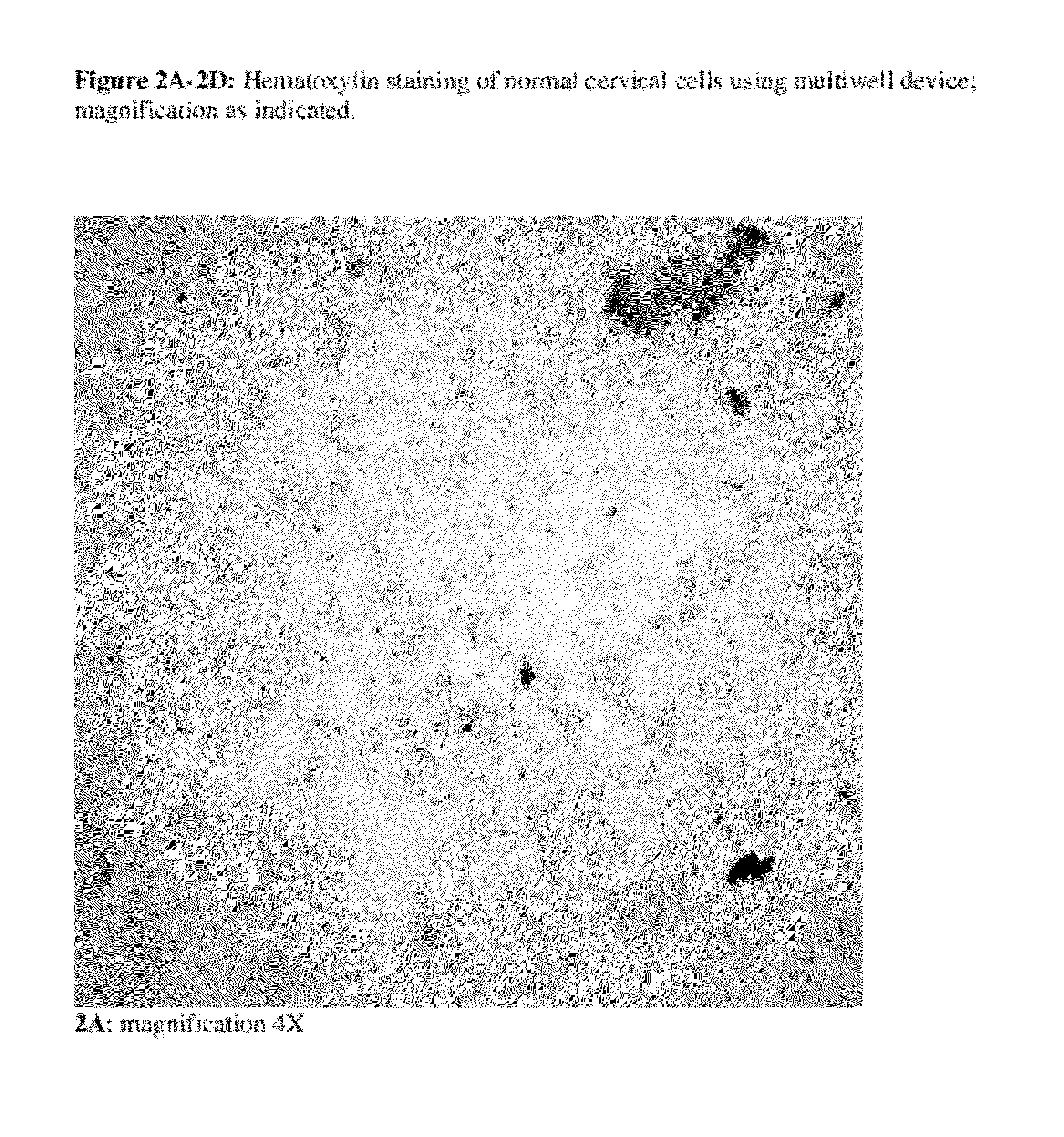
Device and Methods for the Detection of Cervical Disease
InactiveUS20120156698A1Improve accuracyBioreactor/fermenter combinationsBiological substance pretreatmentsAntibodyDysplasia
Owner:MILAGEN
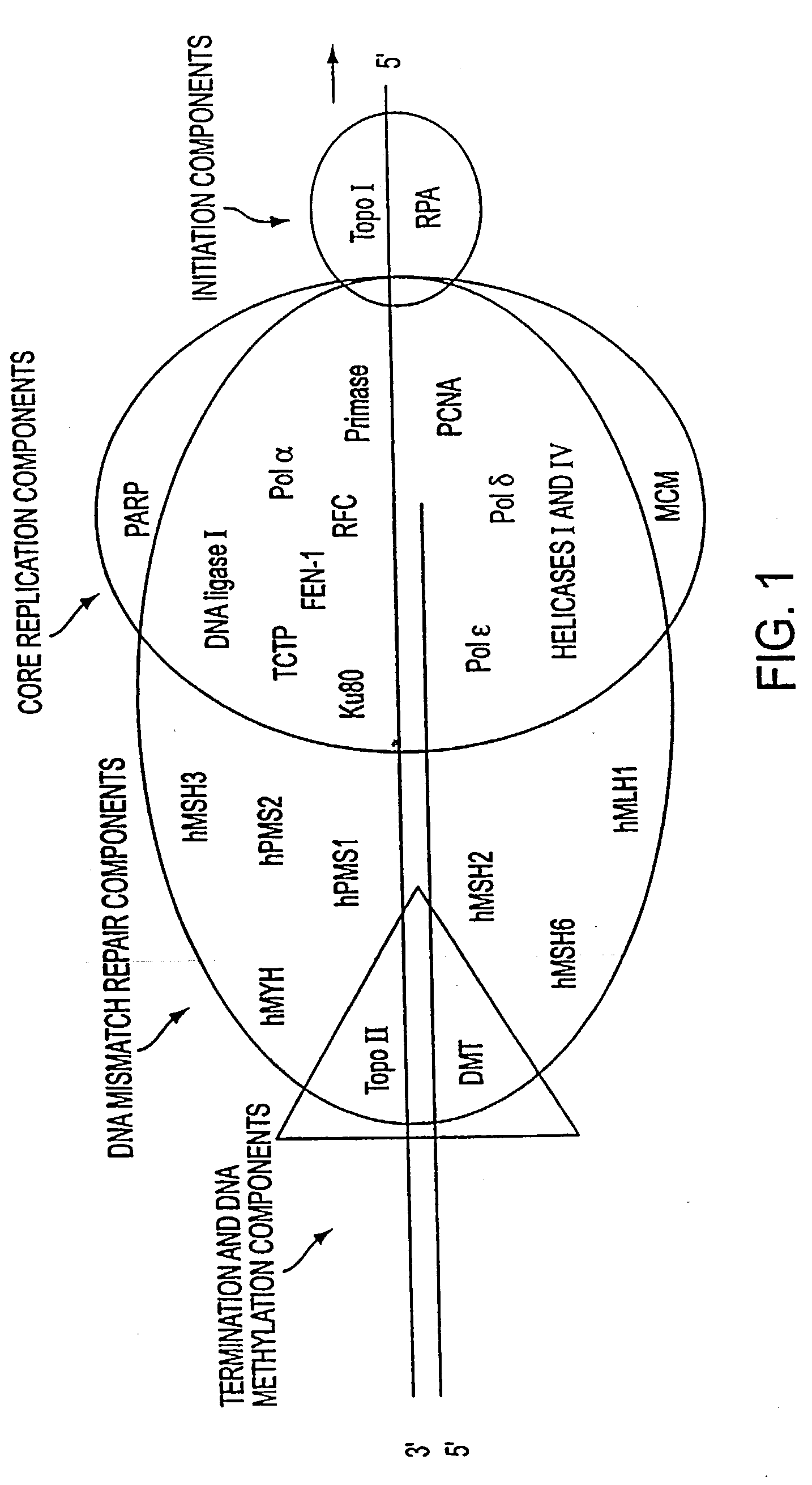
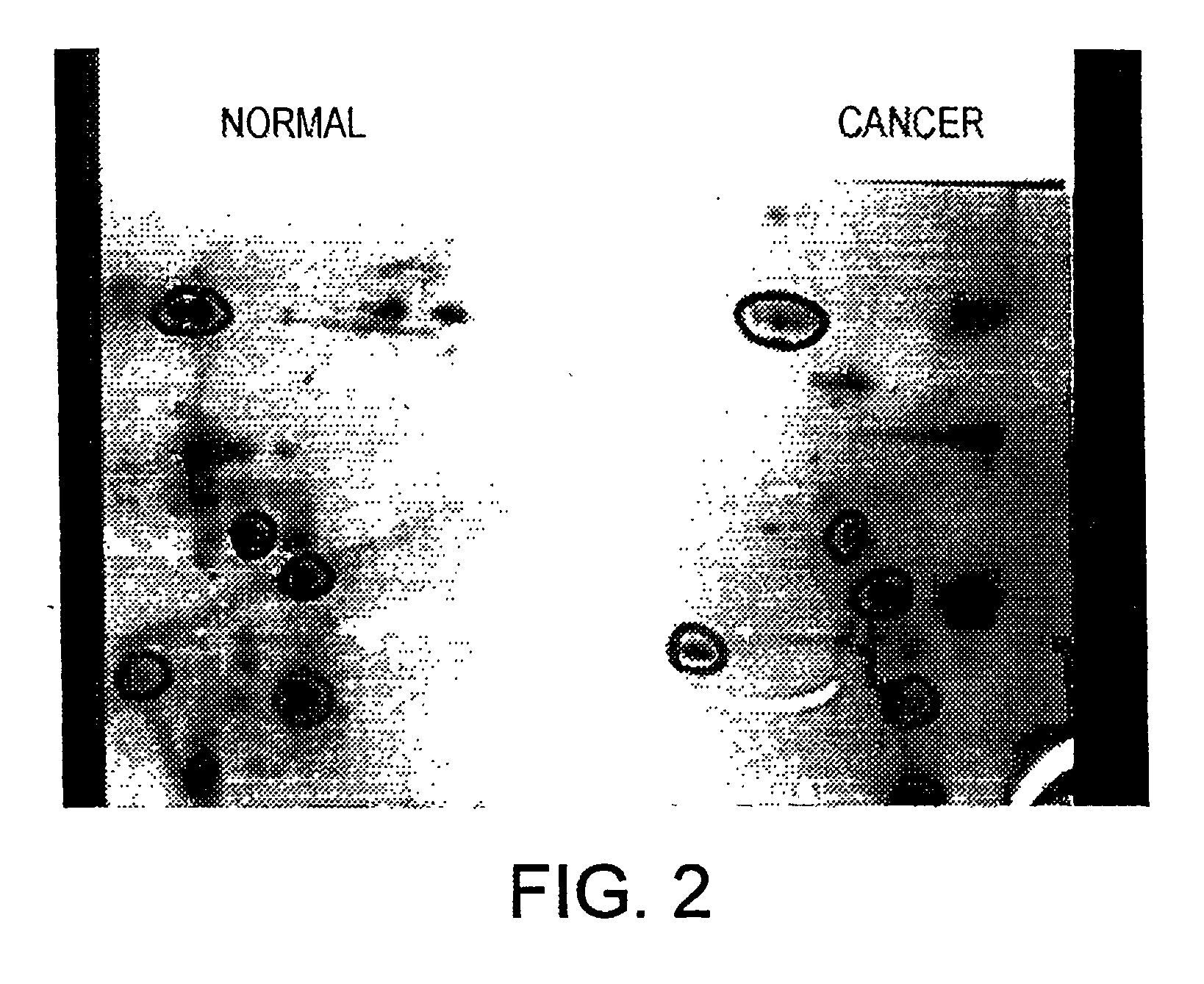
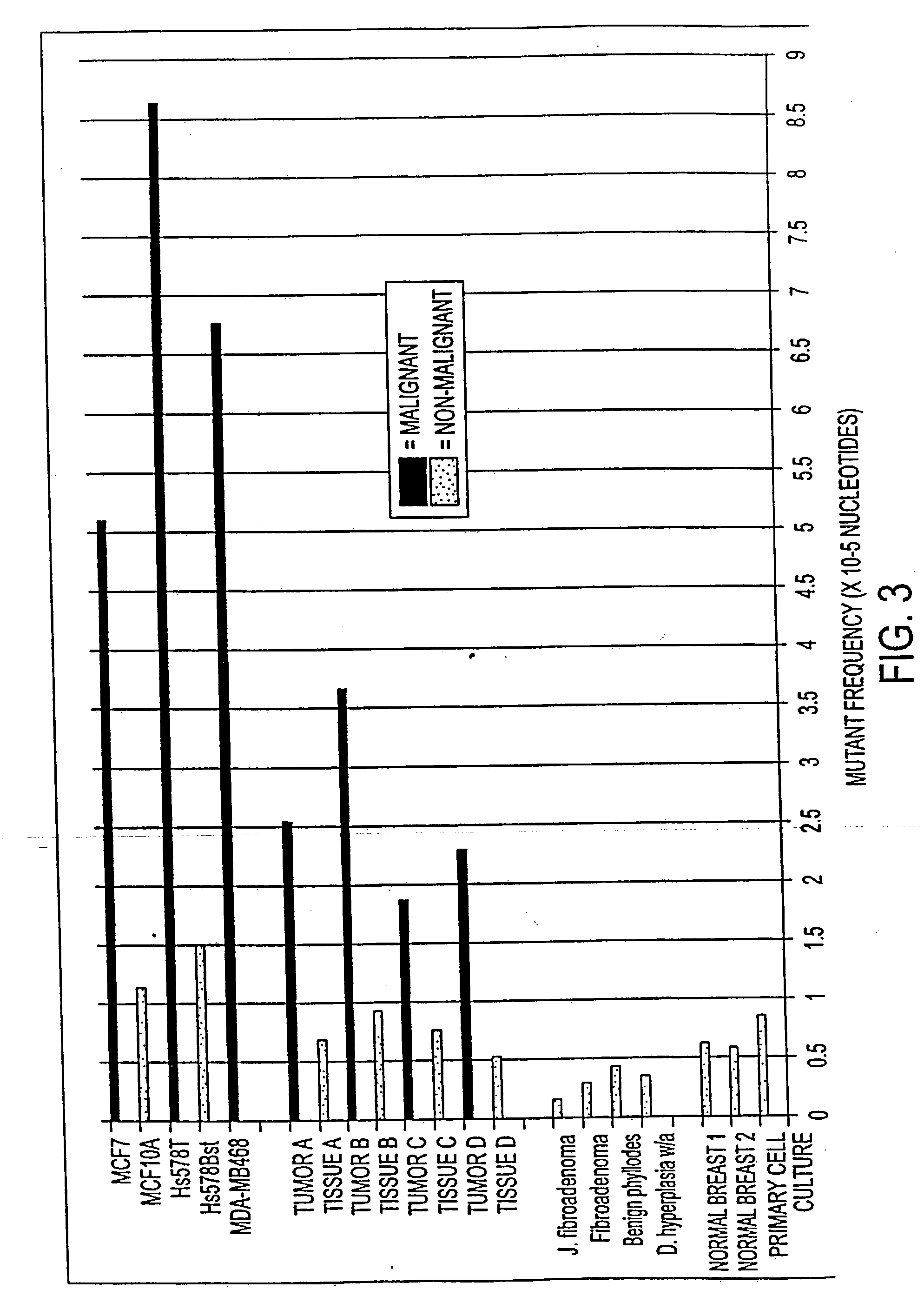
Altered DNA synthesome components as biomarkers for malignancy
InactiveUS20060073477A1Guaranteed functionChange activityPeptide/protein ingredientsMicrobiological testing/measurementMalignant phenotypeNeoplasm
Owner:SCHNAPER LAUREN
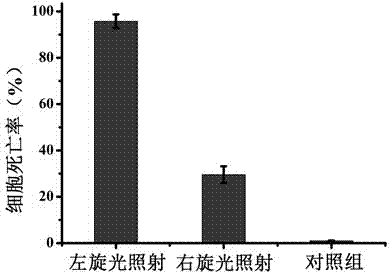
Preparation method and application of plasma chiral nano-gold dimer based on polarization effect
The invention discloses a preparation method and application of a plasma chiral nano-gold dimer based on a polarization effect, and belongs to the technical field of material chemistry. The plasma chiral nano-gold dimer based on the polarization effect is prepared by the surface finish of a chiral nano-gold dimer, the coupling of the chiral nano-gold dimer to photosensitive molecules and the coupling of the chiral nano-gold dimer to a targeted antibody; then a killing effect of the chiral nano-gold dimer on cells under different beams of polarized light and a therapeutic effect of the chiral nano-gold dimer on living body tumors under the different beams of polarized light are detected. The invention provides the preparation method of the plasma chiral nano-gold dimer based on the polarization effect, a powerful platform can be provided for selective photodynamic therapy by utilizing the irradiation of different beams of exciting light, and the method can play an important role in the clinical medicine and the biotechnology.
Owner:JIANGNAN UNIV
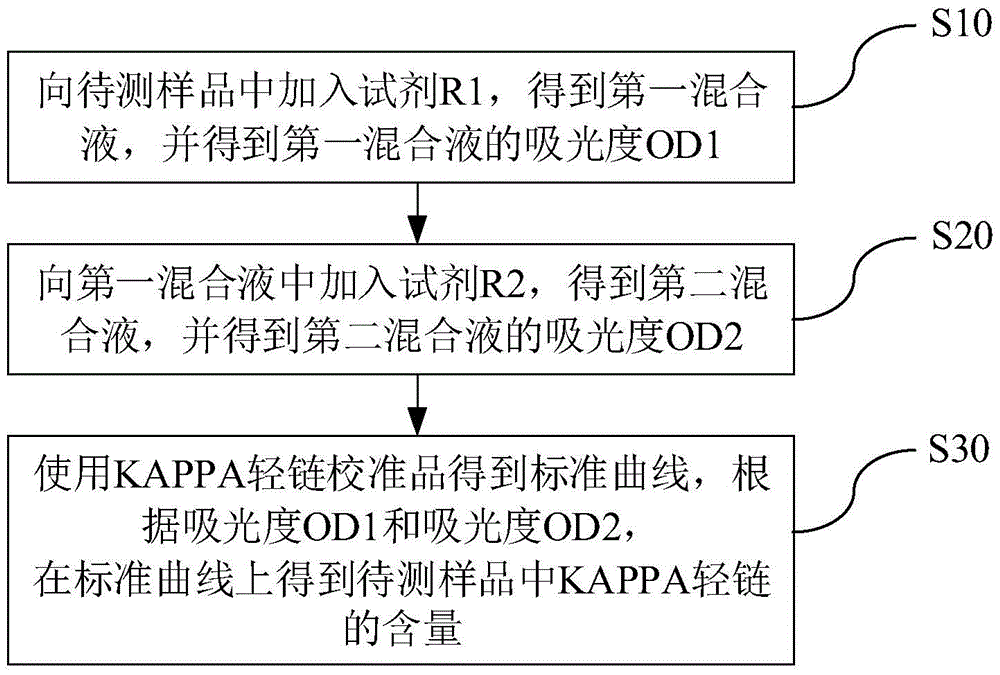
Kit and method for detecting content of KAPPA light chain and application of kit
ActiveCN105738300ADetection is simple and fastEasy to useDisease diagnosisColor/spectral properties measurementsAntigenC1-inhibitor
Owner:潍坊三维生物工程集团有限公司
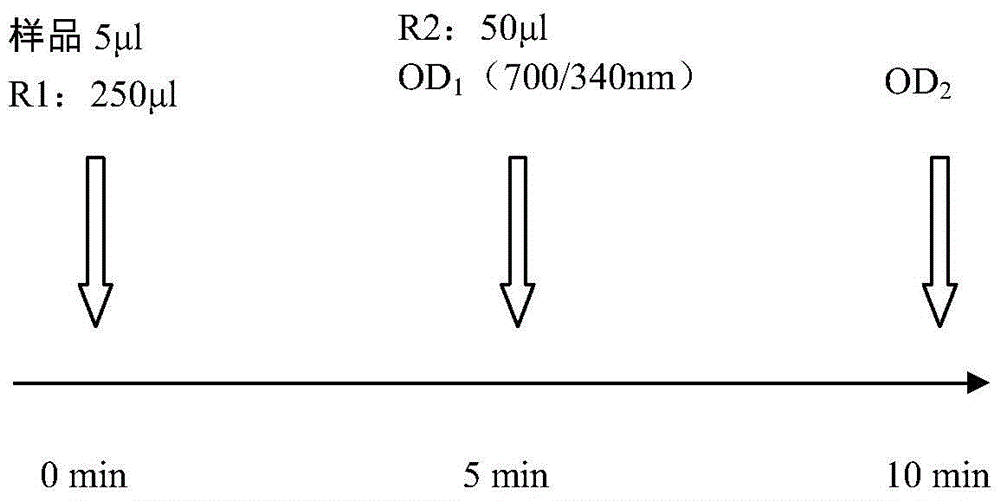
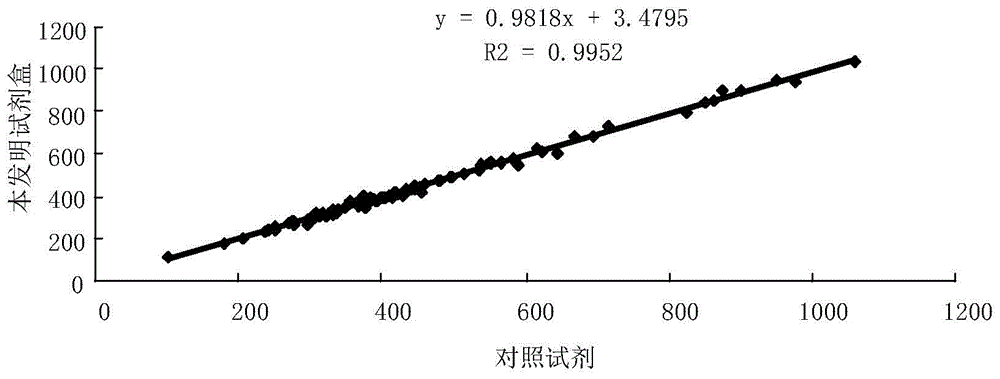
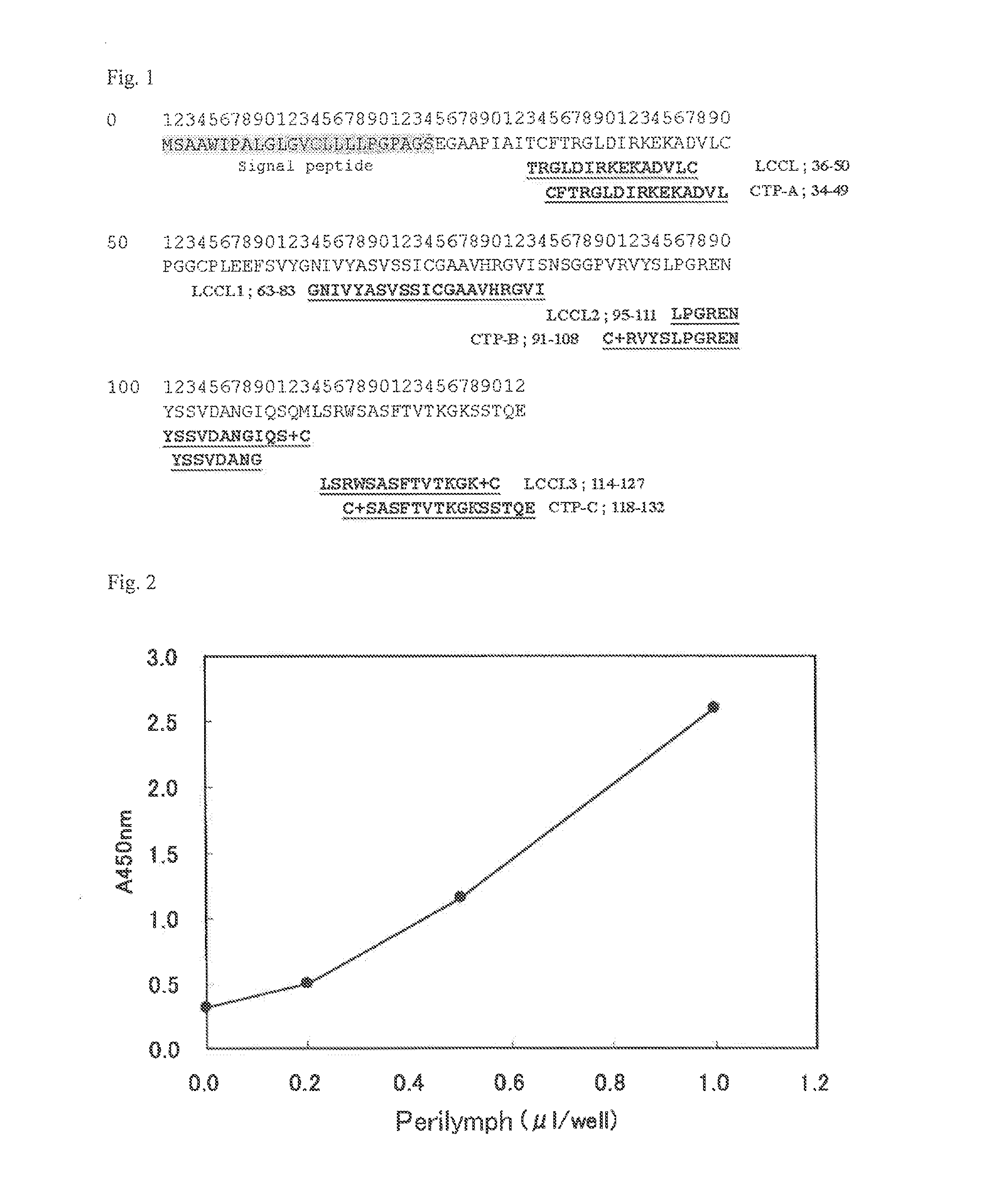
Antibody reacting with native cochlin-tomoprotein (CTP) and method for measuring ctp using same
ActiveUS20140030742A1Accurate and Rapid DiagnosisSimple procedureImmunoglobulins against animals/humansDisease diagnosisAntigenAmino acid
Owner:SAITAMA MEDICAL UNIVERSITY
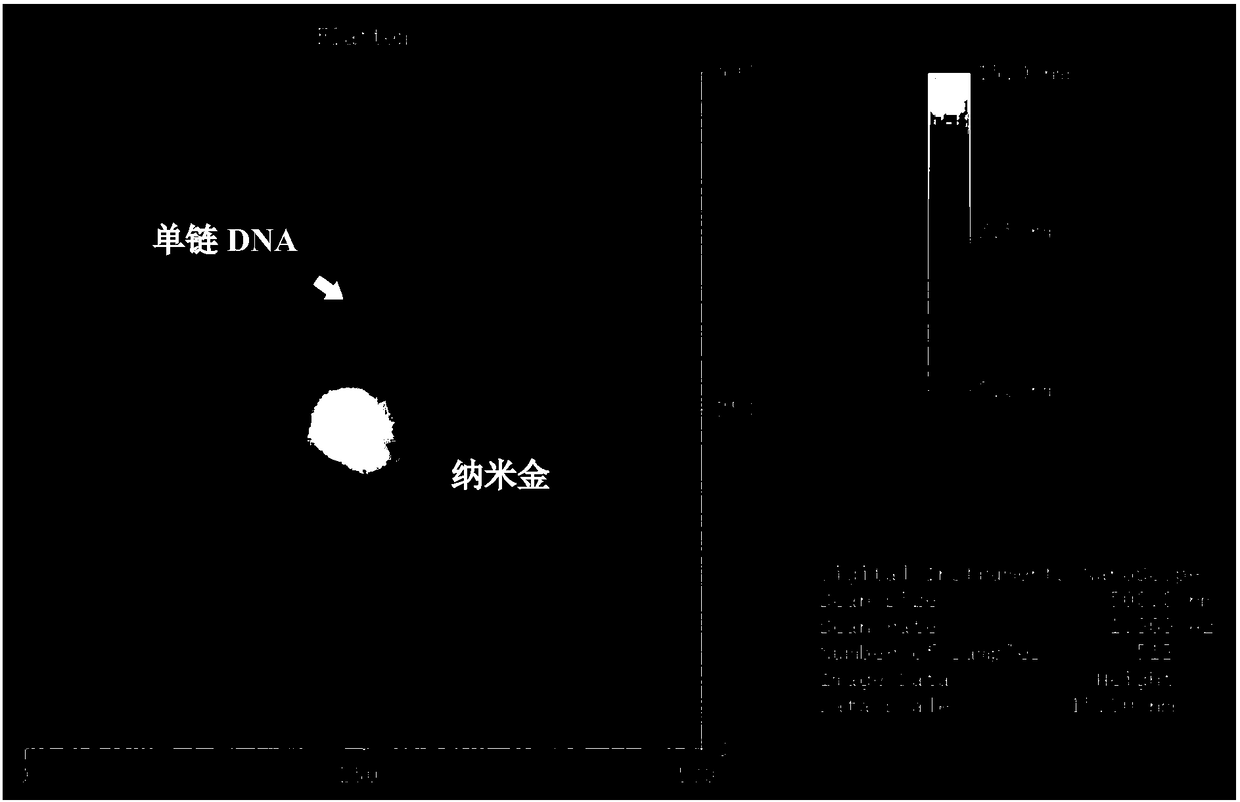
Method for preparing core-shell SERS structure based on amplification of nucleic acid strand by terminal deoxynucleotidyl transferase
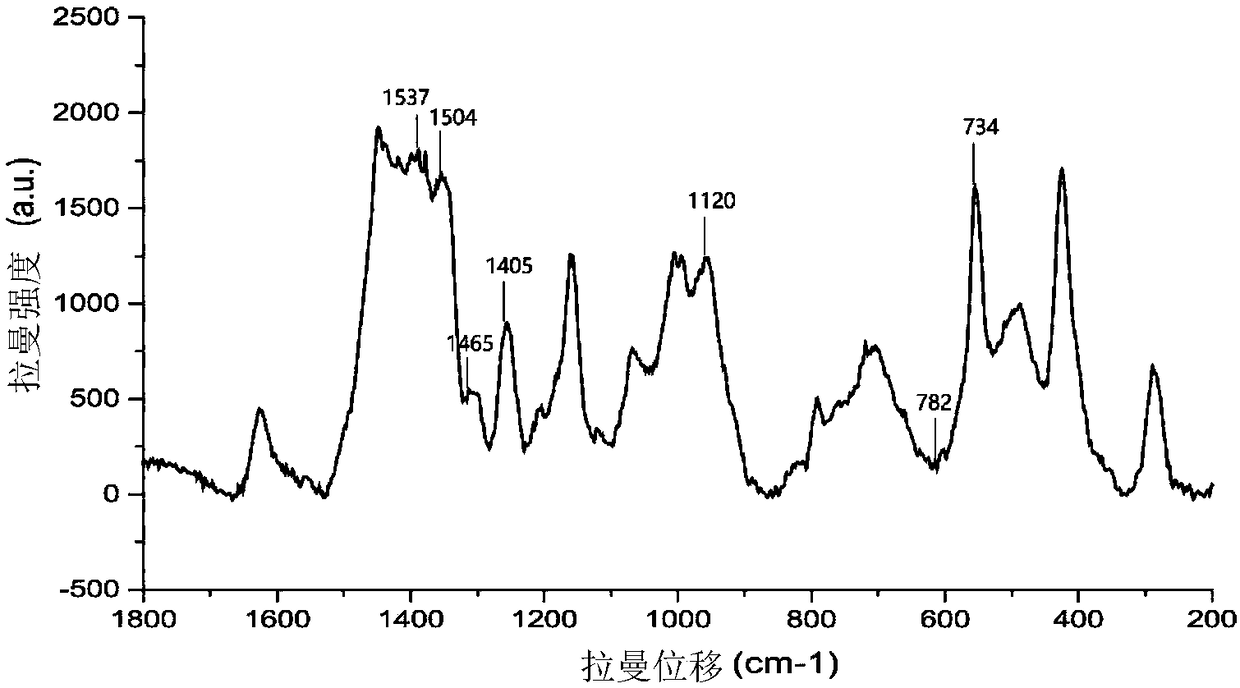
InactiveCN109307669AImprove adsorption stabilityIncrease the number of "hot spots"Raman scatteringSingle strand dnaBiology
Owner:SHANGHAI OCEAN UNIV
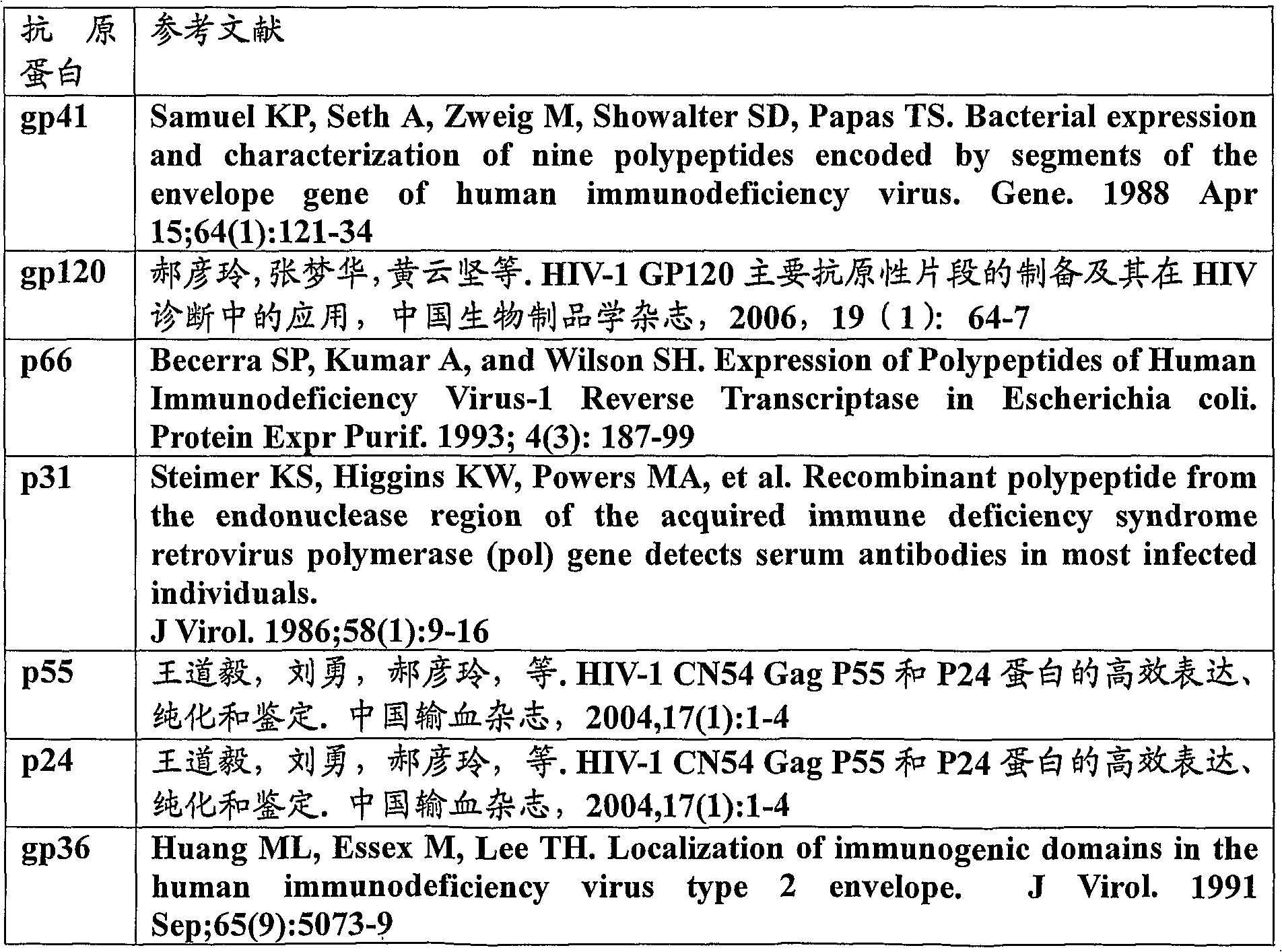
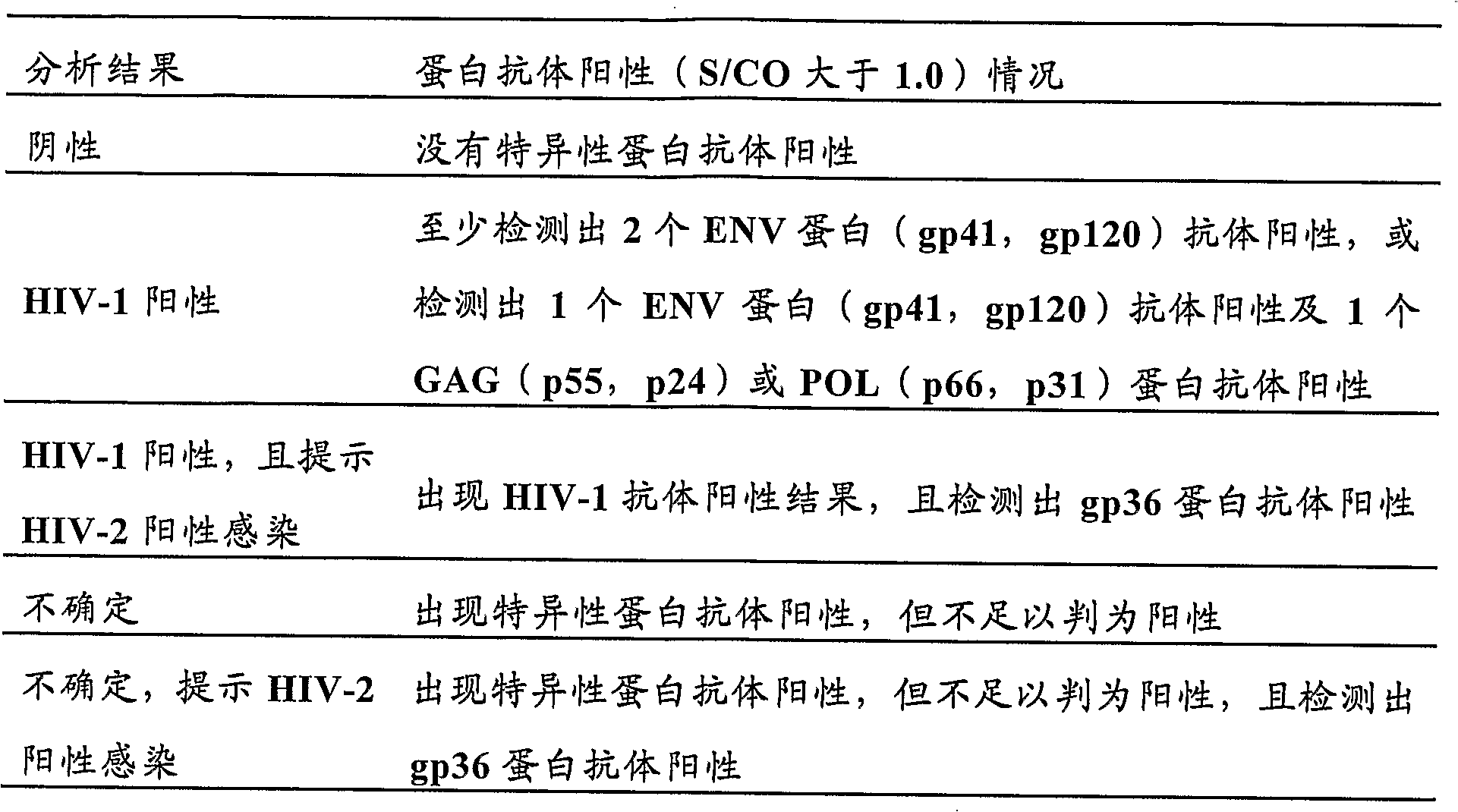
Method of detecting antibodies against a series of human immunodeficiency virus proteins
Owner:BEIJING YUANDE BIO MEDICAL ENG
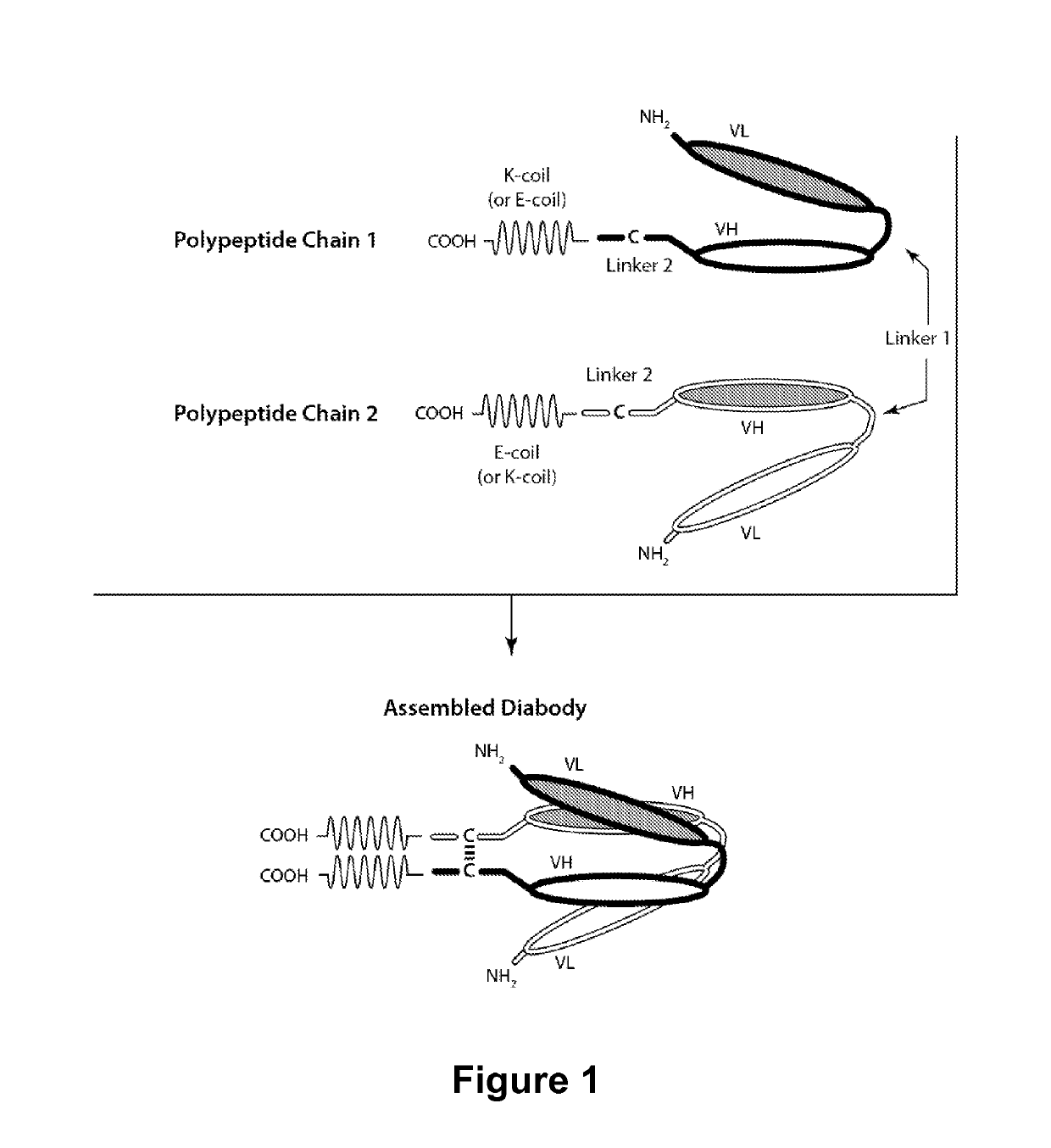
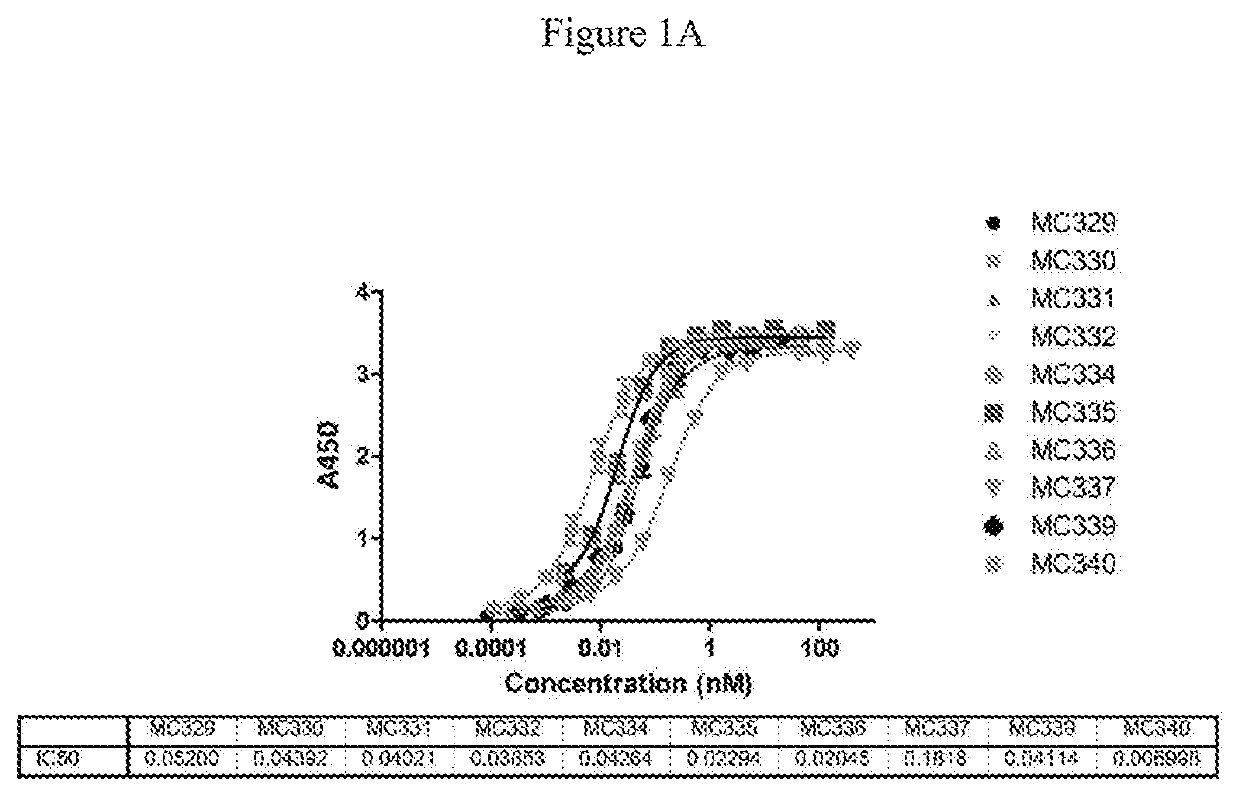
Multivalent molecules comprising DR5-binding domains
ActiveUS10501552B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsChemistryAntibody
Owner:MACROGENICS INC
Antibodies specific for cancer associated antigen SM5-1 and uses thereof
The invention concerns antibodies which is specific for SM5-1 antigen expressed in melanoma, breast cancer and hepatocellular carcinoma, and polynucleotides encoding the antibodies. The invention further concerns use of such antibodies and / or polynucleotides in diagnosing and treating malignancies.
Owner:ONCOMAX ACQUISITION CORP
Traditional Chinese medicine health preservation medicine bag with effects of invigorating stomach and promoting digestion
InactiveCN107375782AGood composition compatibilityPromote digestionPowder deliveryAntipyreticAdditive ingredientDigestion
Owner:重庆信首科技有限公司
Reagent for bioconjugation via irreversible rebridging of disulfide linkages
PendingUS20220024904A1Well formedOrganic chemistryRadioactive preparation carriersChemical synthesisSynthon
Owner:RES FOUND THE CITY UNIV OF NEW YORK +1
Anti-s100a8 for treating leukemia
ActiveUS20180256710A1Inhibit cell proliferationOrganic active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsLeukemiaAntibody
Owner:UNIV LAVAL
Magnetic MOF@aptamer and method for detecting food-borne pathogenic bacteria by using same
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Cotton swab detection device for rapidly collecting human immunodeficiency viruses
Owner:深圳市美迪科生物医疗科技有限公司
AL-1 neurotrophic factor treatments
The present invention provides nucleic acids encoding AL-1 protein, as well as AL-1 protein produced by recombinant DNA methods. Such AL-1 protein is useful in preparing antibodies and in diagnosing and treating various neuronal disorders.
Owner:GENENTECH INC
Specific target, primer and detection method for detecting Escherichia coli O157:H7, and application of specific target and primer
PendingCN112301139AStrong specificityReduce false positivesMicrobiological testing/measurementDNA/RNA fragmentationAntibodyHigh concentration
The invention provides a specific target, primer and detection method for detecting Escherichia coli O157:H7, and an application of the specific target and primer. Compared with a conventional detection target, the novel target provided by the invention has relatively good specificity, so that the generation of false-positive results in the detection is reduced; and an Eva Green third-generation saturated fluorescent dye is adopted, the dye has a relatively high fluorescence signal value, and therefore the detection sensitivity is improved. Meanwhile, even if the dye is used at a high concentration, the dye does not show the PCR inhibition effect, so that the resolution of a melting curve during detection is greatly improved, and therefore the detection accuracy is further improved; the detection method is convenient and fast, the observation is convenient, tedious electrophoresis operations are not needed, and the detection time is shortened within 12 h; and no antibody is involved inthe detection process, the detection cost is low, and therefore detection requirements of general laboratories can be met.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method For Preparation of Quick Dissolving Thin Films Containing Bioactive Material With Enhanced Thermal Stability
InactiveUS20210322537A1Improve stabilityThe process is stable and efficientViral antigen ingredientsInorganic non-active ingredientsActive agentPolymer thin films
Owner:ARIDIS PHARMA INC
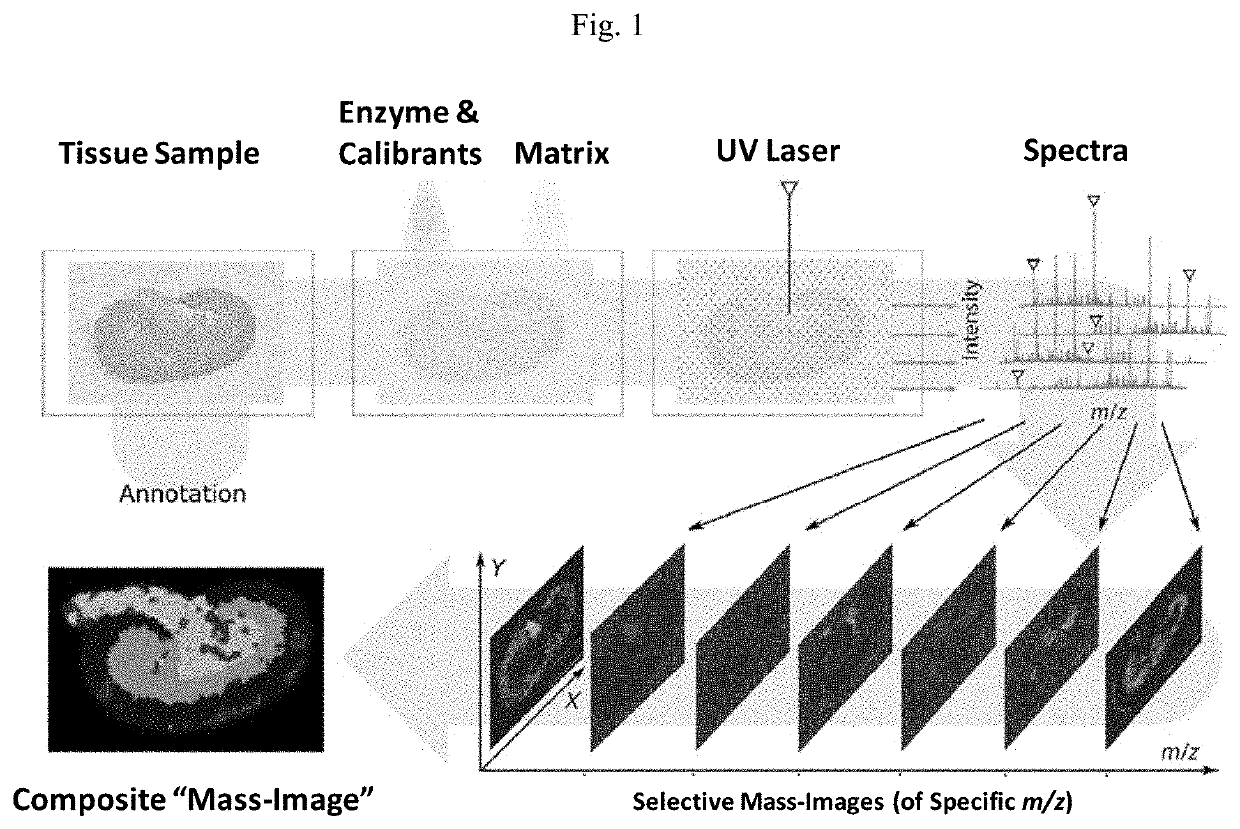
Novel photocleavable mass-tags for multiplexed mass spectrometric imaging of tissues using biomolecular probes
PendingUS20220137064A1Avoid artifactsOrganic chemistryMicrobiological testing/measurementMass spectrometry imagingPathology diagnosis
Owner:AMBERGEN
Composite antibody extract, preparation method and applications thereof
InactiveCN108623680AImprove stabilityStrong antibody specificityCosmetic preparationsAntibacterial agentsYolkPathogenic bacteria
Owner:蒙永祥
Preparation method of electrochemiluminescence biosensor for detecting organochlorine pesticides
ActiveCN106198500AEasy to makeEasy to operateChemiluminescene/bioluminescenceAntigenElectrochemical response
Owner:河南安必诺检测技术有限公司
Chemiluminescence immune detection kit of anti-trophoblast cell membrane antibody and preparation method of chemiluminescence immune detection kit
Owner:SHENZHEN YHLO BIOTECH
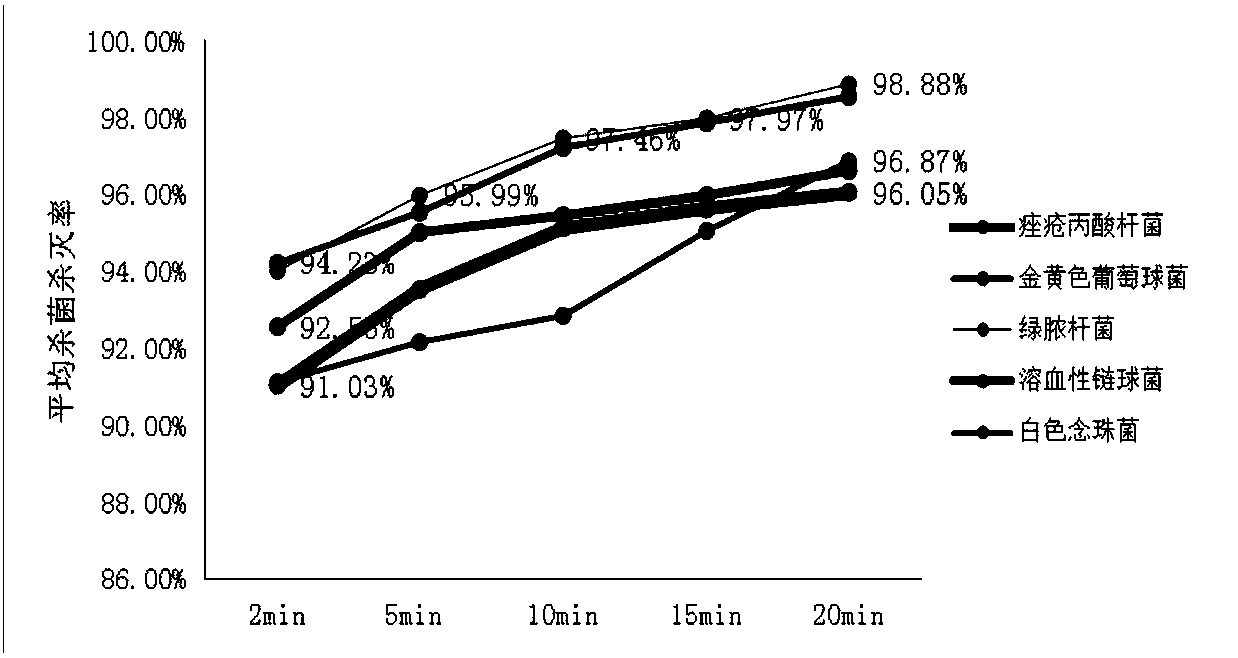
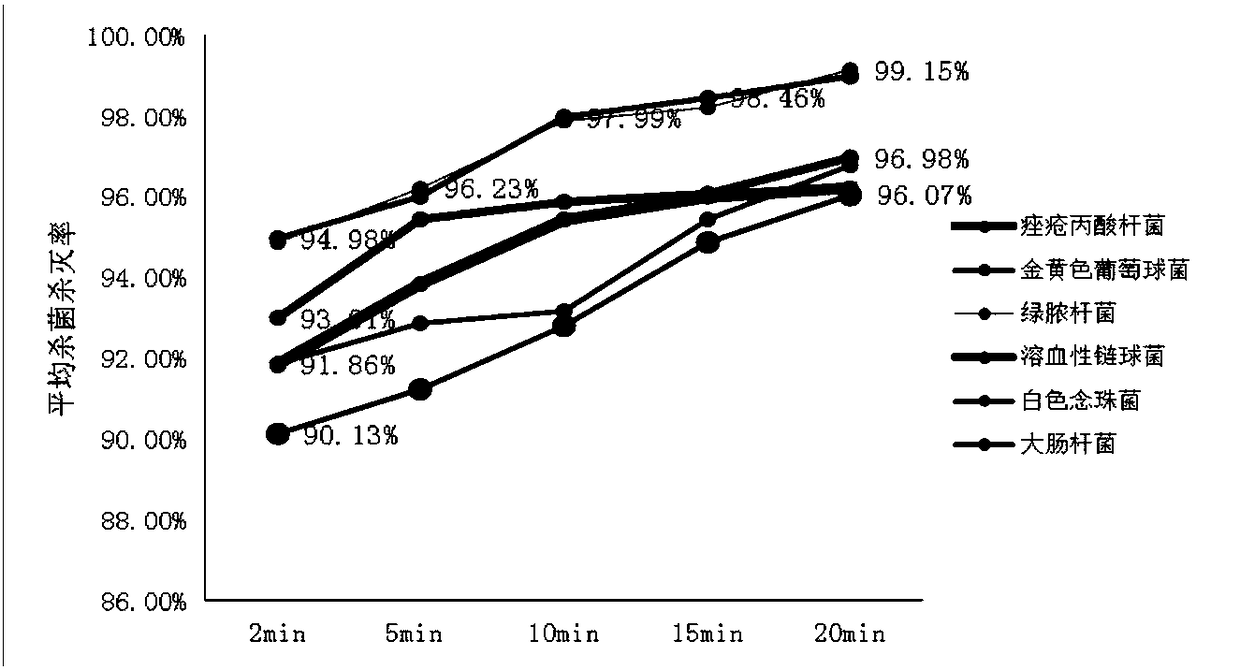
Method for screening anaerobic bacterium traditional Chinese medicine inhibitor based on FeSiCo nano probe
The invention discloses a method for screening an anaerobic bacterium traditional Chinese medicine inhibitor based on a FeSiCo nano probe, and belongs to the technical field of traditional Chinese medicine development application. The method depends on a method for making a traditional Chinese medicine formula for preparing a FeSiCo nano probe and screening inhibiting anaerobic bacteria. According to the method, by virtue of the characteristic that a paramagnetic FeSiCo nano probe wrapped by an antibody is specifically combined with a target bacterium, whether a sample comprises a target bacterium or not can be detected by virtue of the influence of the paramagnetic property of FeSiCo to the relaxation time of magnetic resonance imaging. The paramagnetic FeSiCo nano probe has a linear relationship with the relaxation time of magnetic resonance imaging within a certain range, that is, the larger the content of nano FeSiCo is, the smaller the values of magnetic resonance imaging spinning-crystal lattice relaxation time and spinning-spinning relaxation time of the sample are, target bacteria can be quantitatively detected within a certain range, and thus the bacterium resistance effects of traditional Chinese medicine formulae can be indirectly evaporated. By adopting the method, formulae of traditional Chinese medicines with an inhibiting function on anaerobic bacteria can be screened, and the development speeds of traditional Chinese medicines can be increased.
Owner:NANCHANG UNIV
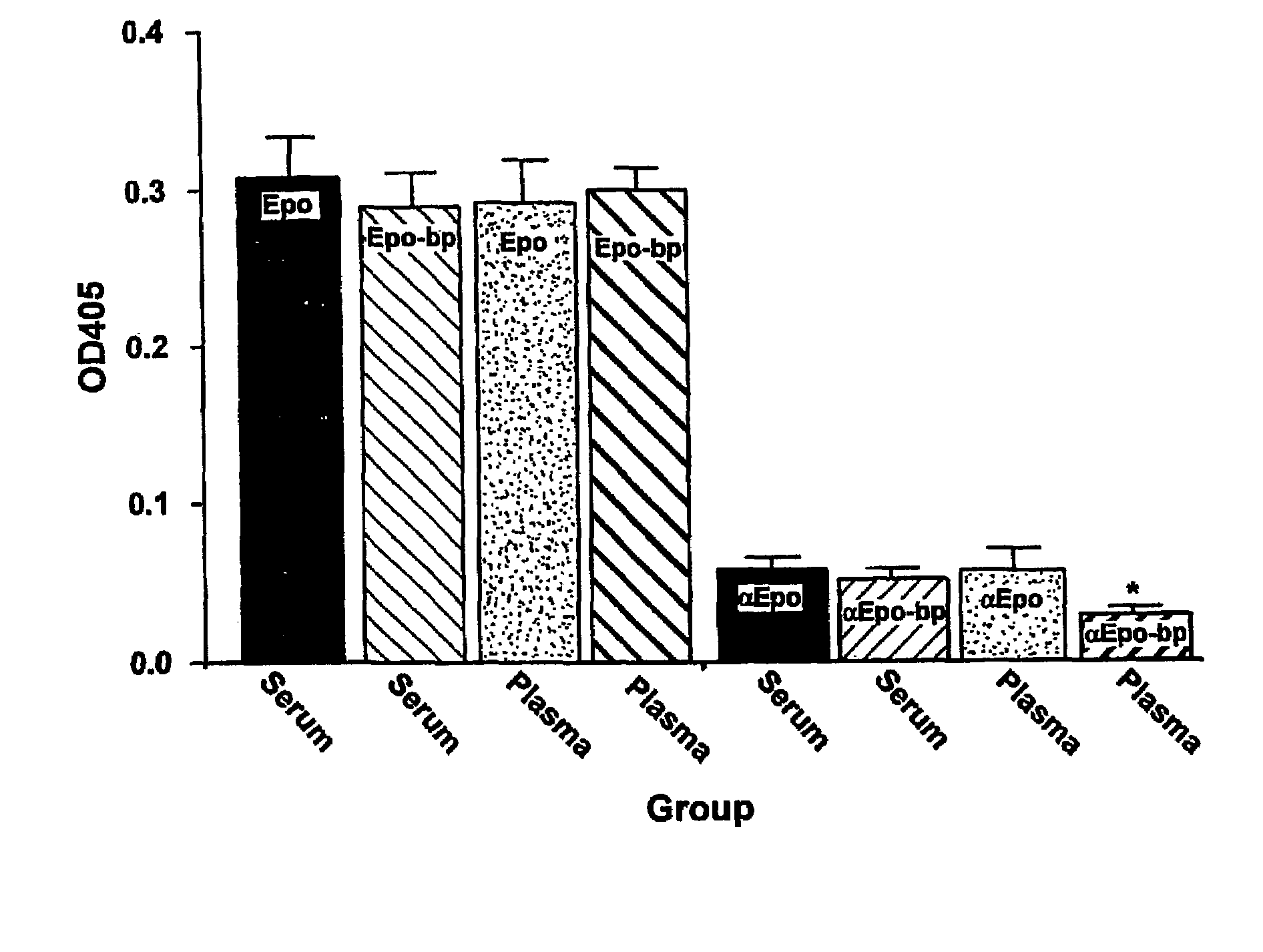
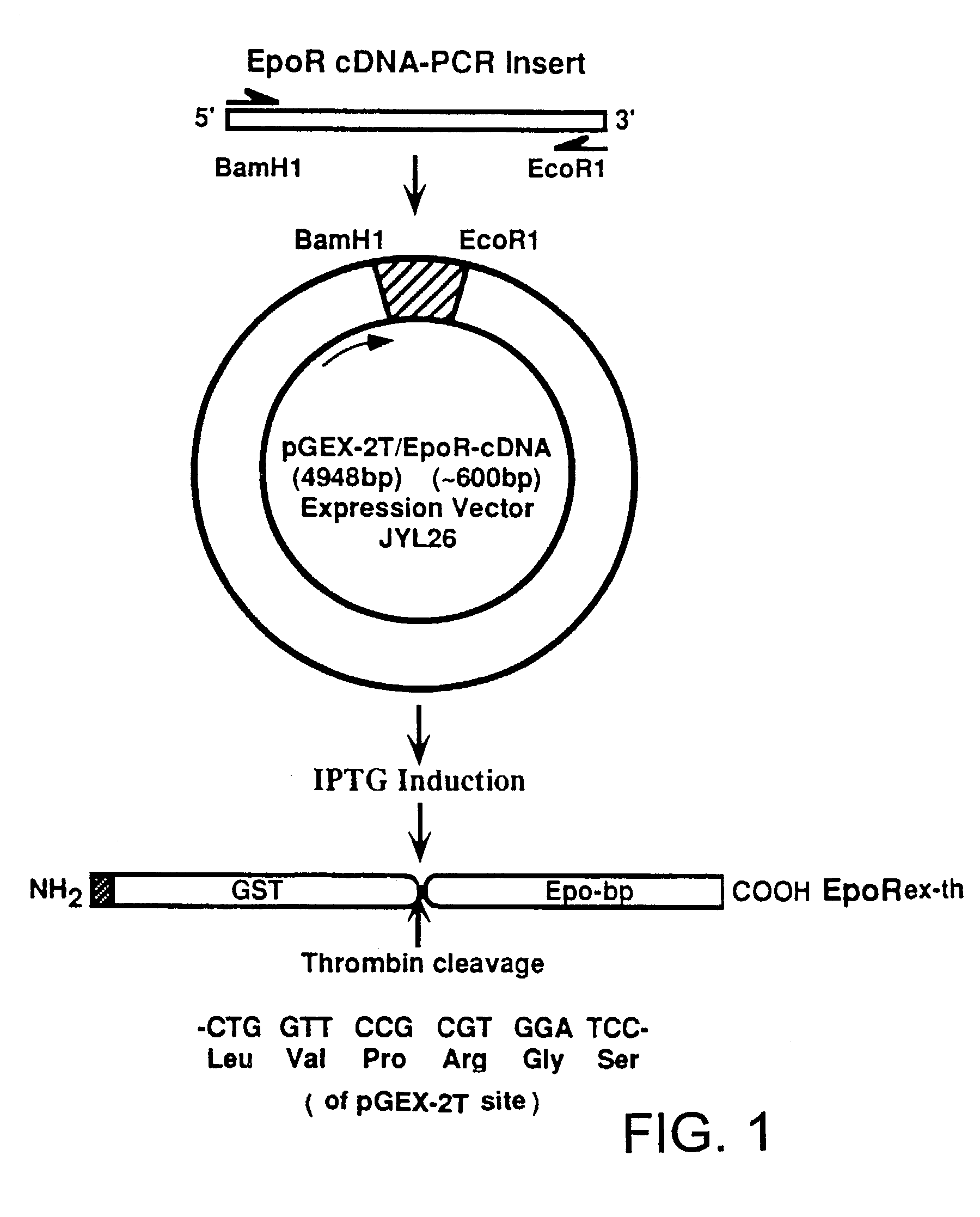
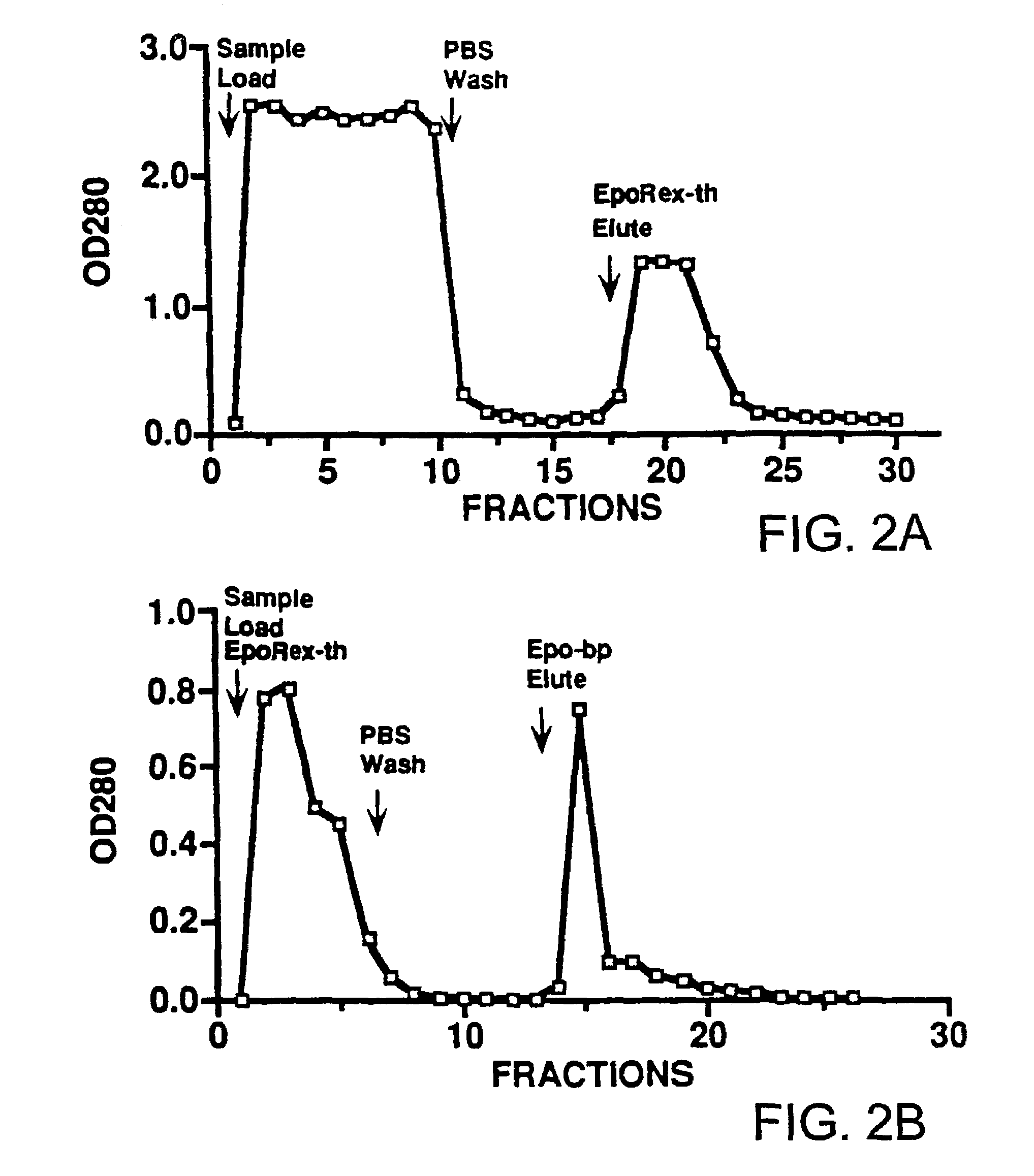
Detection of erythropoietin and erythropoietin receptor
InactiveUS7312089B2Bioreactor/fermenter combinationsBiological substance pretreatmentsAntibodyErythropoietin receptor
Owner:LEE JONG Y
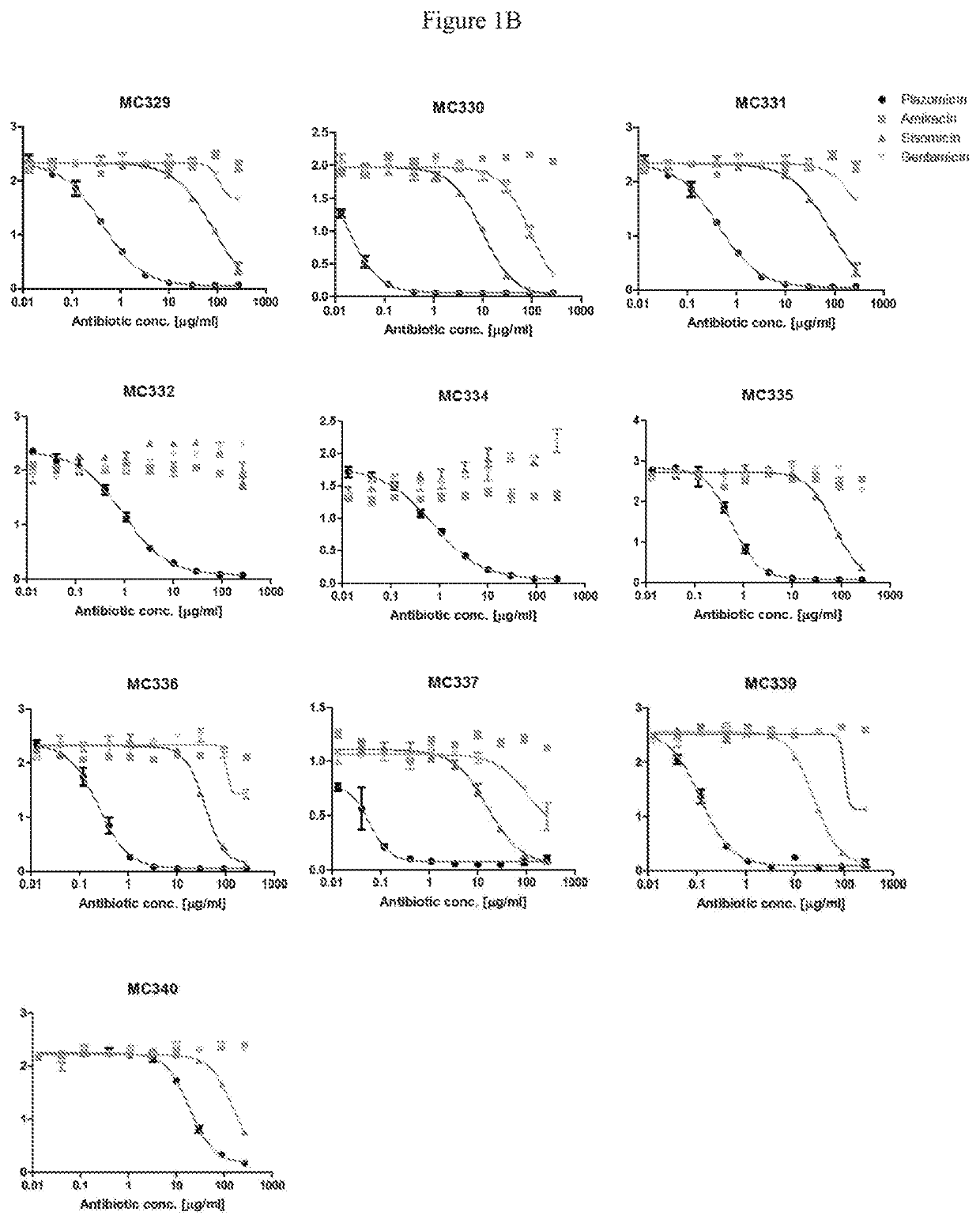
Plazomicin antibodies and methods of use
Owner:CIPLA USA INC
Test strip for detecting CD2v and MGF360 mucous membrane antibodies of African swine fever viruses and application of test strip
ActiveCN111912984AReduce the differenceImprove uniformityBiological testingImmunoassaysNitrocelluloseAfrican swine fever
The invention provides a test strip for detecting African swine fever virus CD2v and MGF360 mucous membrane antibodies and application of the test strip, and belongs to the field of production and technology of veterinary biological diagnosis products. The test strip comprises a nitrocellulose membrane provided with a detection line and a quality control line, wherein the detection line is coatedwith a mouse anti-swine SC protein monoclonal antibody; the quality control line is coated with purified protein of positive serum of African swine fever. the sample diluent contains quantum dot microsphere labeled recombinant protein MGF360 and recombinant protein CD2v; the sequence of the recombinant protein MGF360 is as shown in SEQ ID NO:4; the sequence of the recombinant protein CD2v is shownas SEQ ID NO:2. The test strip and the sample diluent are used for detecting the African swine fever virus CD2v and MGF360 mucous membrane antibodies, the test strip can be used for early detection of African swine fever infection, and have the advantages of strong specificity, high sensitivity and good stability.
Owner:JIANGSU ACAD OF AGRI SCI +1
Popular searches
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap