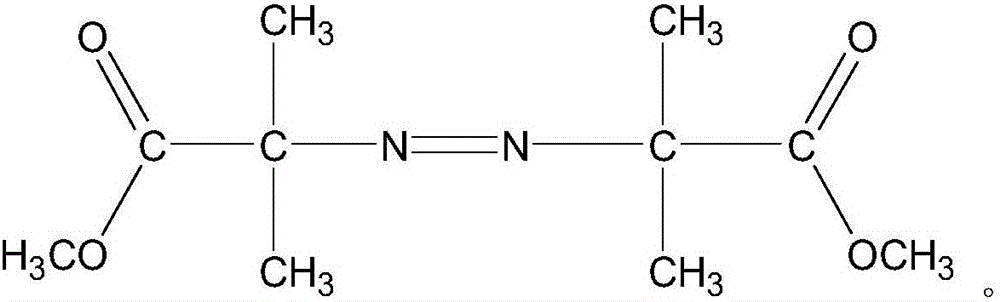Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58 results about "Toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell (cytotoxicity) or an organ such as the liver (hepatotoxicity). By extension, the word may be metaphorically used to describe toxic effects on larger and more complex groups, such as the family unit or society at large. Sometimes the word is more or less synonymous with poisoning in everyday usage.
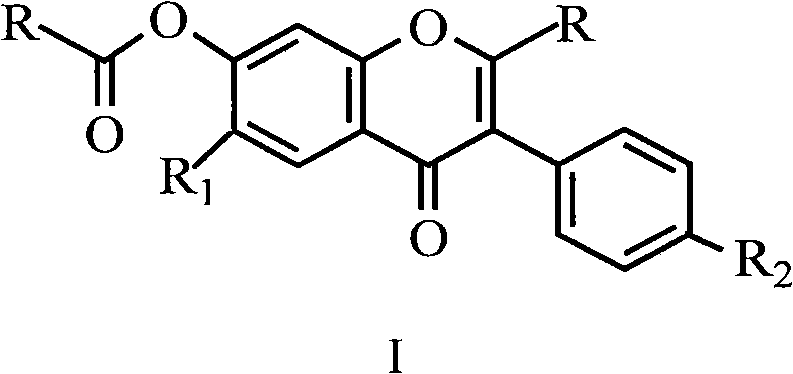
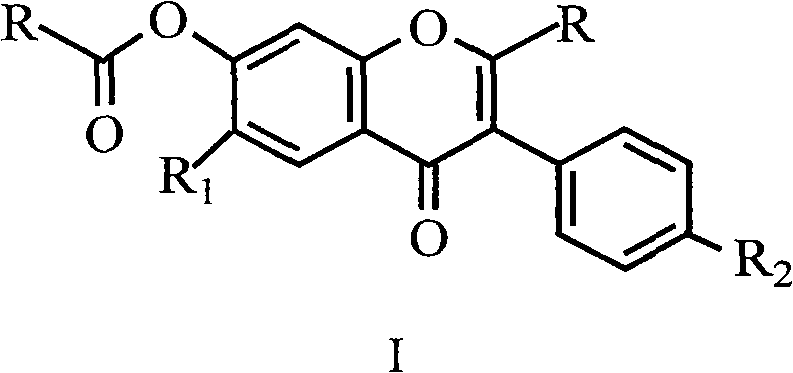
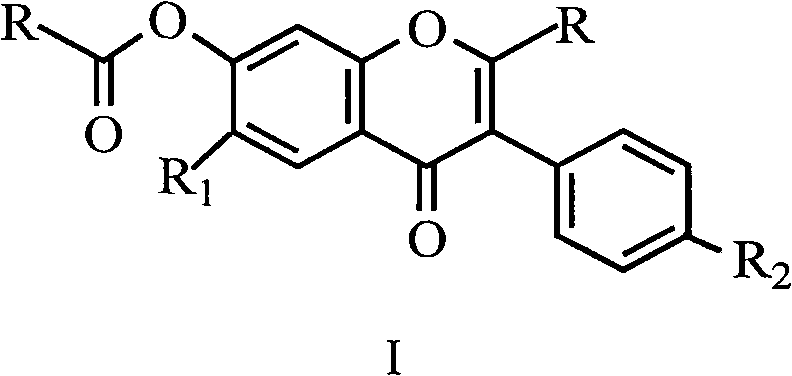
Uses for and article of manufacture including her2 dimerization inhibitor pertuzumab
InactiveUS20130095172A1Prolong progression-free survivalReduce riskOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMetastatic gastric cancerHER2 Positive Breast Cancer
The present application describes uses for and articles of manufacture including Pertuzumab, a first-in-class HER2 dimerization inhibitor. In particular, the application describes methods for extending progression free survival in a HER2-positive breast cancer patient population; combining two HER2 antibodies to treat HER2-positive cancer without increasing cardiac toxicity; treating early-stage HER2-positive breast cancer; treating HER2-positive cancer by co-administering a mixture of Pertuzumab and Trastuzumab from the same intravenous bag; treating HER2-positive metastatic gastric cancer; treating HER2-positive breast cancer with Pertuzumab, Trastuzumab and Vinorelbine; treating HER2-positive breast cancer with Pertuzumab, Trastuzumab and aromatase inhibitor; and treating low HER3 ovarian, primary peritoneal, or fallopian tube cancer. It also describes an article of manufacture comprising a vial with Pertuzumab therein and a package insert providing safety and / or efficacy data thereon; a method of making the article of manufacture; and a method of ensuring safe and effective use of Pertuzumab related thereto. In addition the application describes an intravenous (IV) bag containing a stable mixture of Pertuzumab and Trastuzumab suitable for administration to a cancer patient.
Owner:GENENTECH INC
Preparation method of submicron CuS (copper sulphide) classification ball
The invention discloses a preparation method of submicron CuS (copper sulphide) classification balls. The method comprises the following steps: adding polymer into a good solvent to dissolve and remove big gel particles; adding copper source solution to the good solvent and stirring; adding sulfur source solution and then stirring; reacting the reaction liquid under 100-1,000 KPa at 100-200 DEG C; naturally cooling to the room temperature to obtain black precipitate; and washing and drying the precipitate to obtain the classification balls. The classification balls has the advantages of cheap and readily available templates, environmental friendliness, safety without toxicity, renewability and high water solubility, the contents of raw materials are abundant in nature and the operation of the reaction system is simple; the size and structure of the prepared classification ball are adjustable: the diameter can be controlled by adjusting the molar weight of the added precursor, the template concentration, the reaction temperature and time and the like; the operation is simple; and the prepared classification balls have wide application value in the fields of catalyst, catalyst carrier, optical equipment, sensor, lithium-ion rechargeable battery cathode material, superconductor and the like.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI +1
External-use medicine for treating neck, shoulder, waist and leg diseases and osteoproliferation, and preparation method of medicine
InactiveCN103006861AEasy to carryEasy to use for treatmentMedical devicesSkeletal disorderDiseaseTreatment effect
Owner:WUHAN ZHENGXINGHE BIOTECH
Glycerol derivative, device and pharmaceutical composition
InactiveUS6020317AToxic reductionBiocideGroup 5/15 element organic compoundsGlycerol DerivativesPhospholipid
Owner:NIPPON SHINYAKU CO LTD
Water-based anti-doodling paint and preparation method thereof
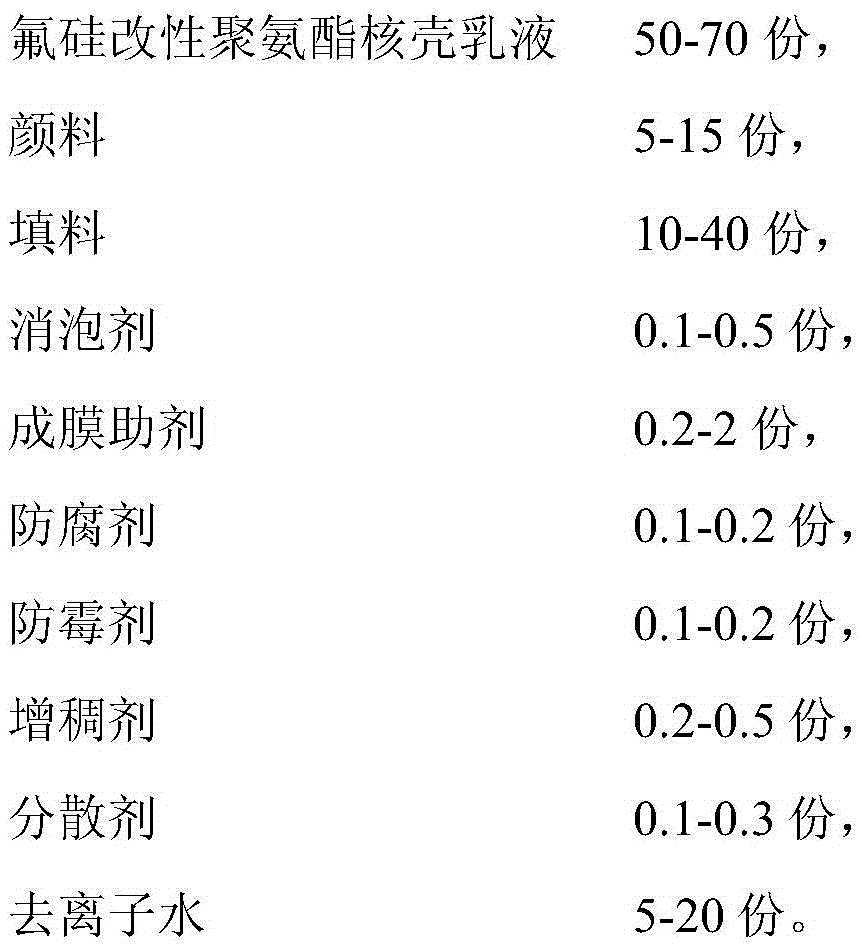
ActiveCN105482604ALow VOC emissionsLow weather resistanceAntifouling/underwater paintsPaints with biocidesWater basedDefoaming Agents
Owner:HANGZHOU JIHUA POLYMER MATERIAL CO LTD
Anti-tumor activity polypeptide and application thereof
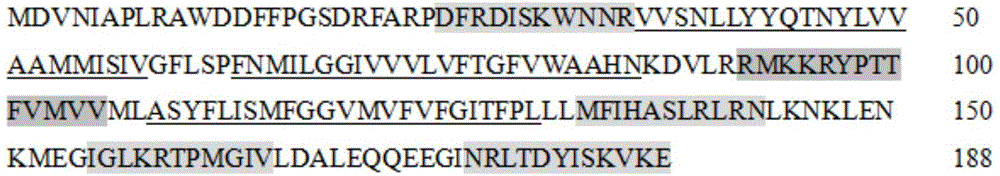
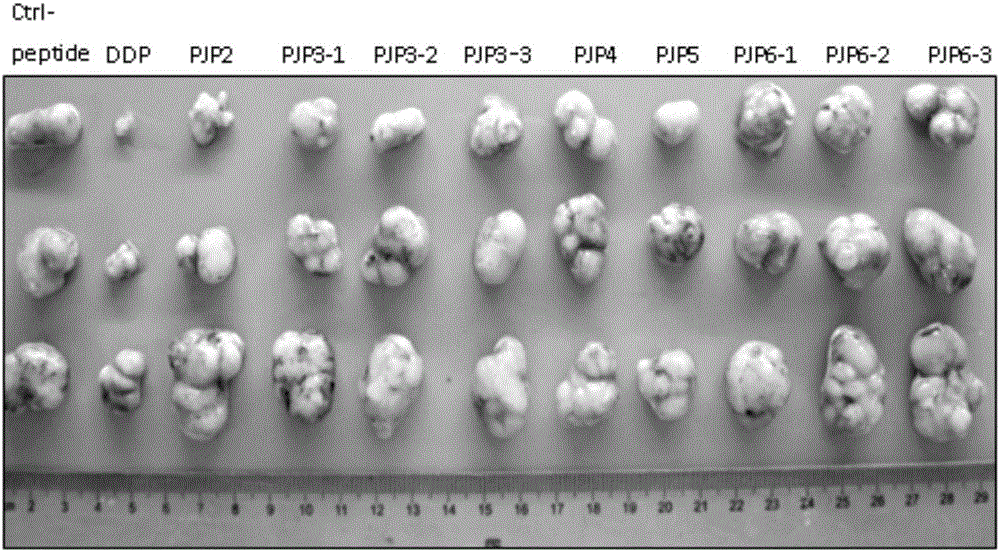
ActiveCN105820230ANo toxicityShort peptide sequencePeptide/protein ingredientsAntineoplastic agentsPhosphorylationNormal tissue
Owner:SUZHOU MINGREN PHARM BIOTECHNOLOGY CO LTD
Chinese patent medicine for treating idiopathic edema
InactiveCN102366617AEdema disappearsReduce puffinessBlood disorderPlant ingredientsDiseaseSide effect
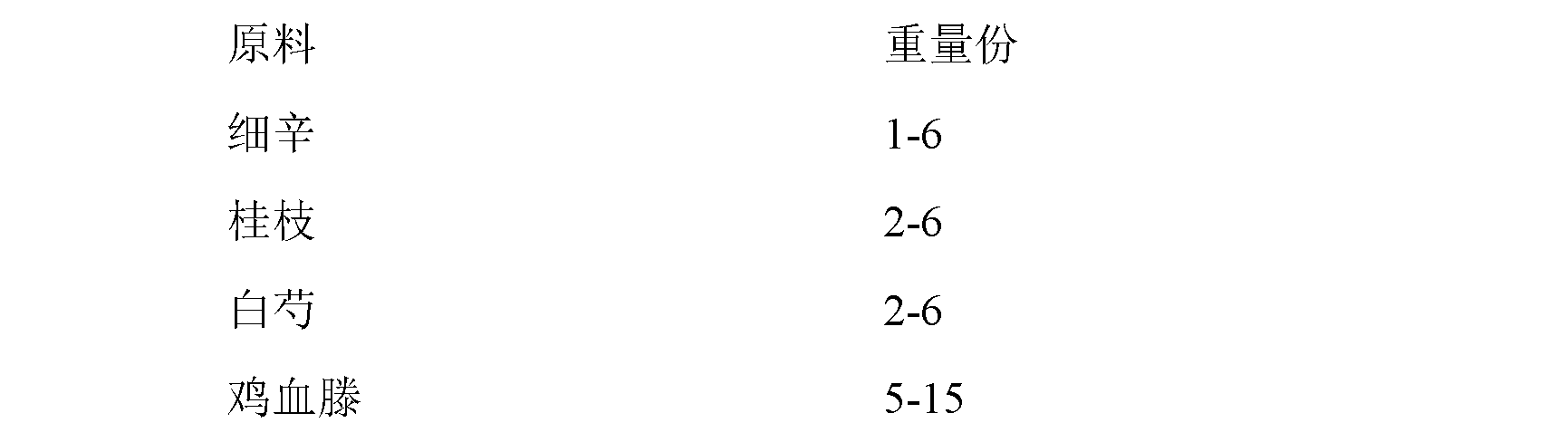
The invention provides a Chinese patent medicine for treating idiopathic edema, which comprises the following components by weight part: 9-15 parts of tribulus terrestris, 8-12 parts of semen psoraleae, 6-10 parts of radix angelicae, 4-8 parts of placenta hominis, 4-8 parts of fleece flower root, 3-5 parts of Maytenus hookeri, 1-3 parts of gen-seng and 1-3 parts of spatholobus stem. Compared with the prior art, the Chinese patent medicine of the present invention aims at internal disease of idiopathic edema, is capable of performing therapies with syndrome differentiation, and has the advantages of fast curative effect, low cost, no toxicity, no side-effect and substantial effect. The Chinese patent medicine for treating idiopathic edema treats both principal and secondary aspect of disease.
Owner:CHENGDU LYUDI TECH
Traditional Chinese medicine decoction formula for treating female acne concomitant with irregular menstruation
InactiveCN104784573AAnthropod material medical ingredientsDermatological disorderToxicityLigusticum chuanxiong
Owner:GENERAL HOSPITAL OF PLA
Efficient anaerobic and aerobiotic continuous use industrial wastewater treatment method
ActiveCN109626717AAvoid toxicityAvoid inhibitionWater treatment parameter controlWater treatment compoundsFenton reactionContinuous use
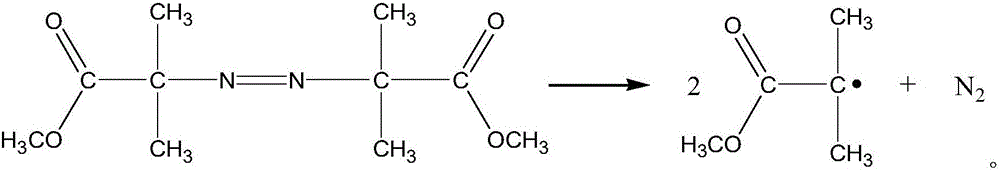
The invention discloses an efficient anaerobic and aerobiotic continuous use industrial wastewater treatment method. Fenton oxidation is adopted for preprocessing wastewater, hydrazine hydrate and other reducibility contaminants in wastewater are removed, the purpose of lowering a COD value can be achieved, and toxicity and inhibition of microorganisms in follow-up biochemistry can be avoided. A fenton reaction catalyst is adopted, the catalyst has the high catalytic activity in the aspect of organic contaminant degradation, and iron digestion and loss in the reaction process can be avoided; the preprocessed wastewater enters the efficient anaerobic and aerobiotic continuous use industrial wastewater treatment technology section, and COD in water can be efficiently removed; compared with the traditional technology, and the method is the treatment technology which is low in cost and high in efficiency and can effectively treat HPPO epoxypropane production wastewater.
Owner:LIAONING ZHONGZHOUDESHUI ENVIRONMENTAL PROTECTION TECH CO LTD
Poultry feed additive and preparation method thereof
Owner:沈阳耘垦饲料有限公司
Metal paint remover and method for preparing same
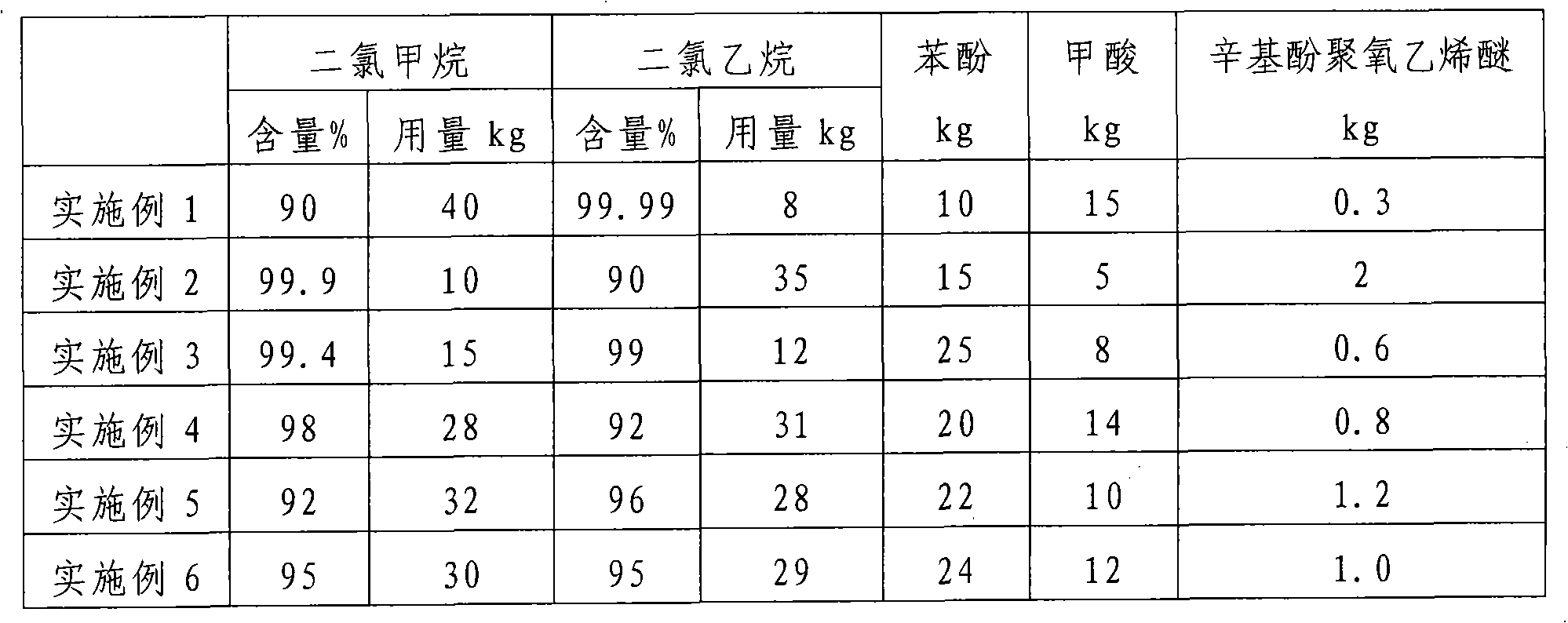
InactiveCN101284961AFast paint removalGood paint removal effectChemical paints/ink removersMethylene DichlorideEthane Dichloride
Owner:JIANGSU DEMEI TECH
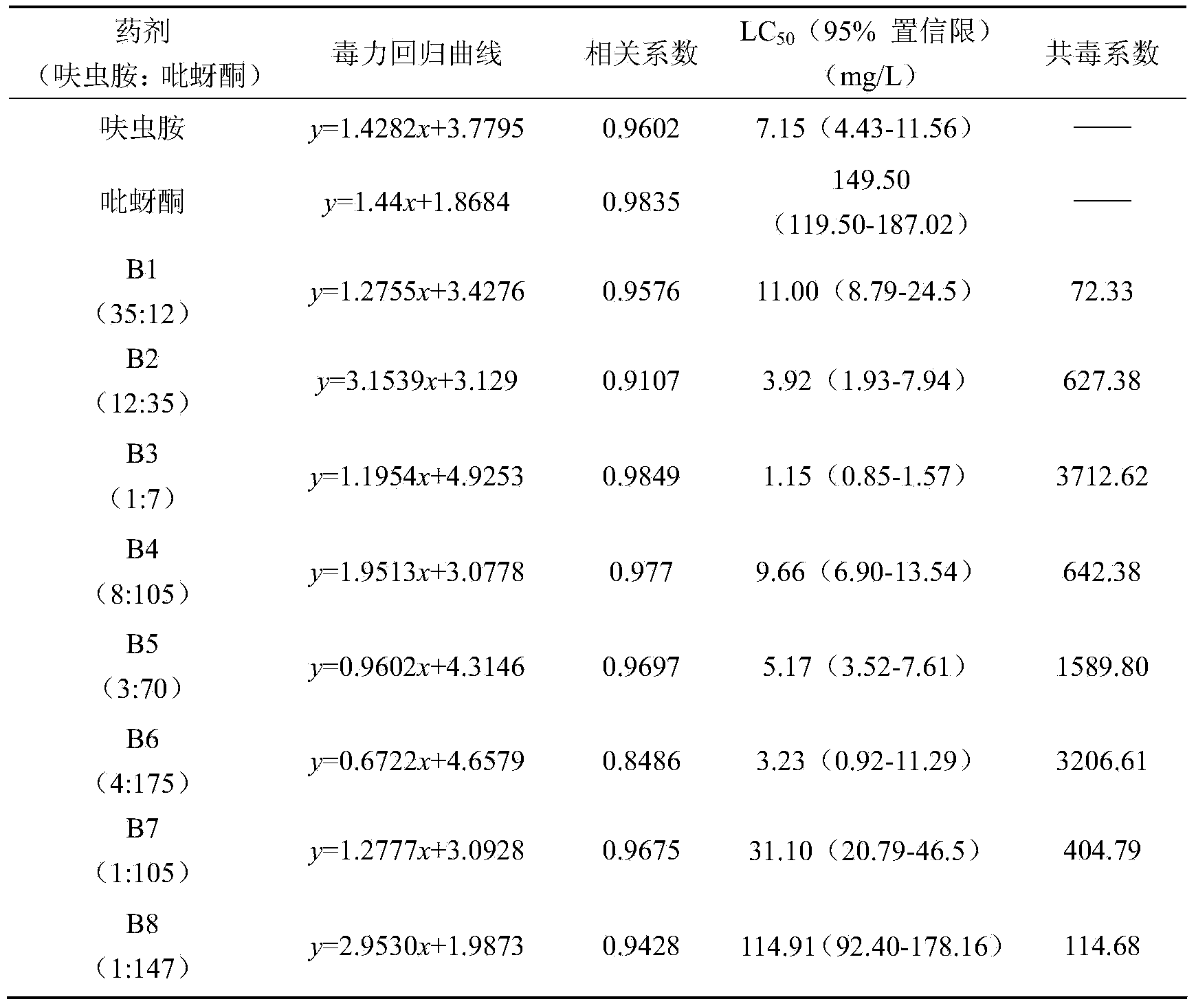
Compound insecticide containing dinotefuran and pymetrozine
Owner:NANJING AGRICULTURAL UNIVERSITY
Ink cleaning agent with no damage to equipment and preparation method thereof
InactiveCN103613978AImprove craftsmanshipReduce pollutionChemical paints/ink removersLimoneneCleansing Agents
Owner:东莞梵拓熙实业有限公司
Making method of high-protein fermented feed
InactiveCN106509363AFull of nutritionImprove palatabilityFood processingAnimal feeding stuffFlavonoid glycosidesFermentation
Owner:HARBIN WEIPING TECH DEV
Enrofloxacin water-soluble powder and preparation method thereof
InactiveCN106727333ASimple production processLow costPowder deliveryOrganic active ingredientsSide effectToxic material
Owner:SHANDONG BINZHOU ZHIYUAN BIO TECH CO LTD
Rheumatism plaster and thread gluing type ventilating bagged rheumatism plaster and production method thereof
InactiveCN101214330AGood treatment effectSimple structureHeavy metal active ingredientsAntipyreticAdditive ingredientPinellia
Owner:谢焕青
Composition with effect of treating gout
InactiveCN102000159AHeat-clearing and detoxifyingWith swelling and pain reliefAntipyreticAnalgesicsDiseaseAfter treatment
Owner:TIBET UNIV
Water-soluble hypocrellin PLGA nanoparticle and preparation method thereof
InactiveCN103933568AGood biocompatibilitySmall particlesPowder deliveryEnergy modified materialsSolubilitySide effect
The invention relates to the technical field of medicines, and particularly relates to a water-soluble hypocrellin PLGAnanoparticle and a preparation method thereof. The preparation method comprises the following steps: by taking poly(lactic-co-glycolic acid) (PLGA) as a carrier, dissolving PLGA and hypocrellin into an organic solvent to prepare an oil phase, adding into freeze-drying excipient-containing water phase under high-speed stirring, and finally obtaining the hypocrellin nanoparticle by adopting an emulsification freeze-drying method. By using a biodegradable medical polymer material PLGA used in the preparation method and approved by the food and drug administration (FDA) of the United States, the toxic and side effects of a common drug-carrying material can be reduced, the water solubility of the drug can be improved, the particle size of the prepared nanoparticle ranges from 20-200nm, the drug has red shift, the absorption of the nanoparticle in a phototherapy window (600-900nm) can be increased, and the dark toxicity of the drug can be reduced. The preparation method is simple in raw materials and easy to operate.
Owner:NANJING NORMAL UNIVERSITY
Use of isoflavone with substituent at intermedium ring 2 position as animal feed additives
ActiveCN101331913AImprove biological activityImprove qualityAnimal feeding stuffAccessory food factorsAntibiotic YAnimal product
Owner:广东新南都饲料科技有限公司
Method for establishing animal model for senile dementia, special liquid medicine and dosing device
InactiveCN103284980ATypical behaviorTypical pathological symptomsHydroxy compound active ingredientsMedical devicesSterile environmentSenile dementia
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Lung caring pill
InactiveCN102579785ADefinite curative effectImprove securityRespiratory disorderPlant ingredientsCapillary networkSide effect
Owner:郭纯
Method of applying nontoxic deforming agent to liquid edible fungus fermentation tank
InactiveCN104073446ALight weightHigh market priceFungiFoam dispersion/preventionManufacturing cost reductionBiotechnology
The invention discloses a method of applying a nontoxic deforming agent to a liquid edible fungus fermentation tank; the liquid edible fungus fermentation tank must be cleaned thoroughly and inspected after every use or before reuse; the method comprises the following steps: boiling the tank to disinfect; adding sucrose, bean flour and a monopotassium phosphate main ingredient to the liquid edible fungus fermentation tank; adding water and stirring; injecting a deforming agent; selecting a proper cultivating process, wherein soybean oil serves as the defoaming agent, and is added and adjusted depending on a volume of 3-8%; the soybean oil, which is lower than polyoxypropylene-polyoxyethylene glycerol ether in specific gravity, is easier to float on surface in a cultivating environment that the fermentation tank is strenuous and is longer to act on an effective area of foam; scientific name of the polyoxypropylene-polyoxyethylene glycerol ether is polyether, and has low toxicity although being under edible grade, while the soybean oil, as edible oil, contains a great amount of linoleic acid which is fatty acid essential to body, free from toxicity and harm and is more suitable for massive use of food-grade fermentation tank products; and market price of the soybean oil is lower than that of the polyoxypropylene-polyoxyethylene glycerol ether, so as to reduce manufacturing cost.
Owner:XUZHOU LVWEI MODERN AGRI TECH CO LTD
Degradable capacitor plastic shell and preparation method thereof
Owner:TONGLING XINTAI ELECTRIC APP & CAPACITOR
Oxidative degradation method for cyanide in cyaniding tailings
InactiveCN107890622AReduce consumptionPromote decomposition and releaseChemical protectionVacuum extractionHigh pressure
Owner:FUJIAN SHUANGQISHAN MINING +1
Polyvinyl acetate polymerization method
InactiveCN106008773ANarrow molecular weight distributionQuality improvementPolyvinyl acetateDecomposition
Owner:ANHUI WANWEI UPDATED HIGH TECH MATERIAL CO LTD
Drug for treating rheumatoid arthritis
ActiveCN106880789AGood curative effectShorten the timeAnthropod material medical ingredientsAntipyreticAngelica Sinensis RootClinical manifestation
The invention discloses a drug for treating rheumatoid arthritis. The drug is prepared from radix aconiti kusnezoffi preparata, radix aconiti preparata, radix angelicae, asarum, lumbricus, rhizoma arisaematis, flos carthami, yam rhizome, semen coicis, radix angelica sinensis, placenta and ants according to a proper ratio. The medicines are ground into fine powder, and the fine powder is coated with raw honey to form pellets. Due to the fact that the toxicity of the formula is high, the drug needs to be stopped for two days to observe the pharmacodynamic function after being used for one week. The drug has a remarkable curative effect for rheumatoid arthritis, especially when clinical manifestations include symmetrical pain and swelling of multiple joints in the whole body, symmetrical stiffness or tightness of joints, even deformation to different degrees, alternate chills and fever, limb joint and muscular ache, unsmooth flexion and extension, or unstable pain, and even severe pain, swelling, hardness and deformation of joints. Treatment of 200 patients confirmed through blood and modern medical equipment proves that 166 patients are cured, 11 patients are improved, the effective rate is 88.5%, and the cure rate is 83%.
Owner:SHAANXI UNIV OF CHINESE MEDICINE
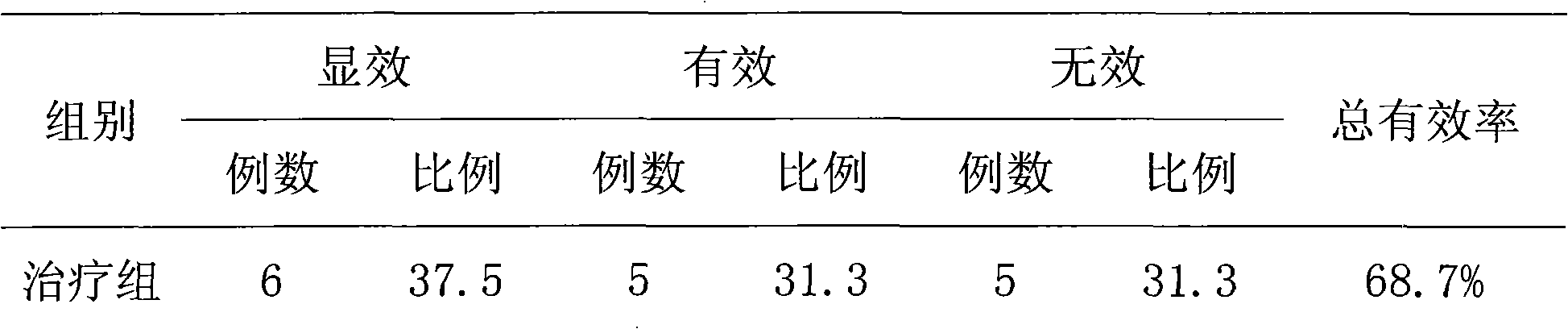
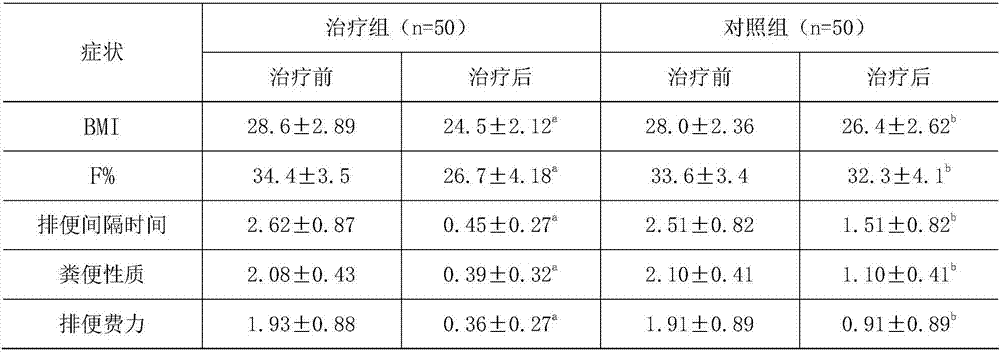
Nutritional food for treating constipation and reducing internal fat and preparation method thereof
Owner:哈尔滨瑞康源生物科技有限公司
Syrup capable of relieving cough and reducing sputum
InactiveCN106387536AHealth care and anti-agingPromote circulationConfectionerySweetmeatsLicorice rootsRhizome
Owner:陈宇
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap