Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
117results about "Powder delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
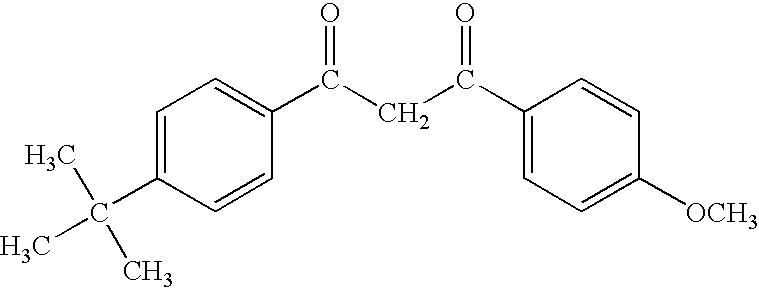
Particles of cross-linked proteins and polysaccharides with hydroxamic groups for chelating metals and their uses notably in cosmetics
Owner:BASF BEAUTY CARE SOLUTIONS FRANCE SAS
Methods and compositions related to peptides and proteins with c-terminal elements
ActiveUS20090226372A1Powder deliveryMicrobiological testing/measurementCell selectivityPeptide sequence
Disclosed are compositions and methods useful for targeting and internalizing molecules into cells of interest and for penetration by molecules of tissues of interest. The compositions and methods are based on peptide sequences that are selectively internalized by a cell, penetrate tissue, or both. The disclosed internalization and tissue penetration is useful for delivering therapeutic and detectable agents to cells and tissues of interest.
Owner:SANFORD BURNHAM MEDICAL RES INST
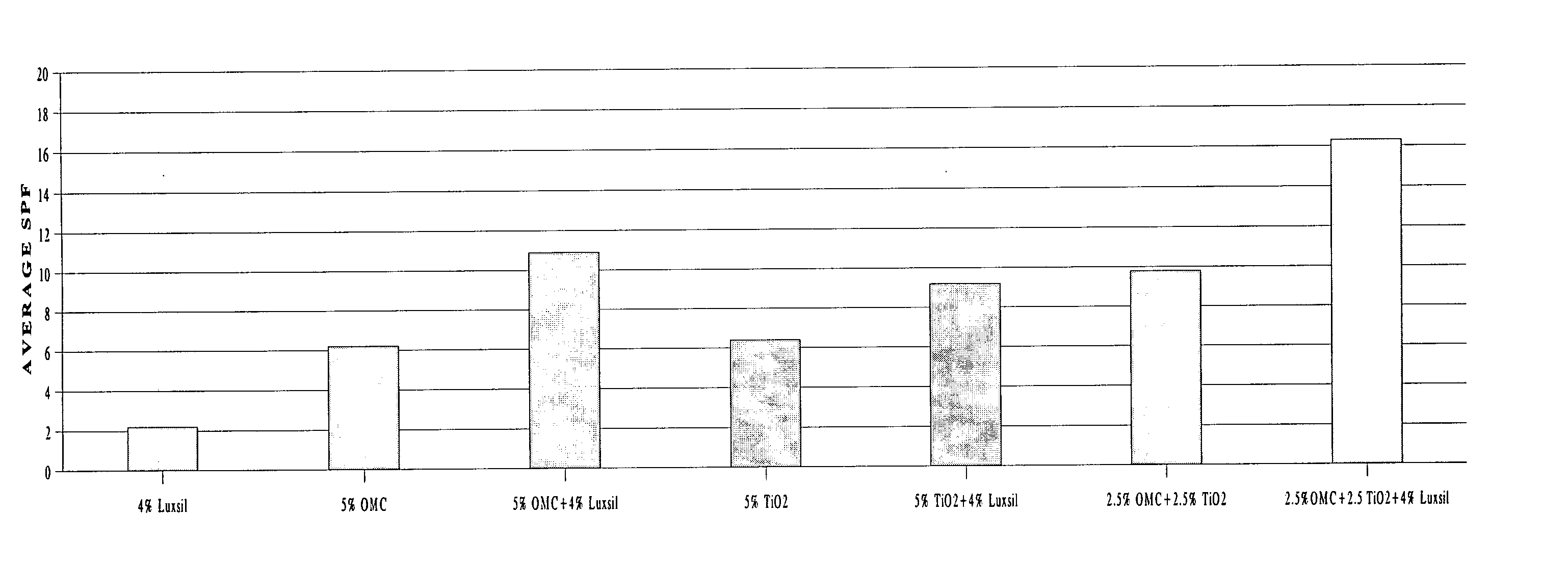
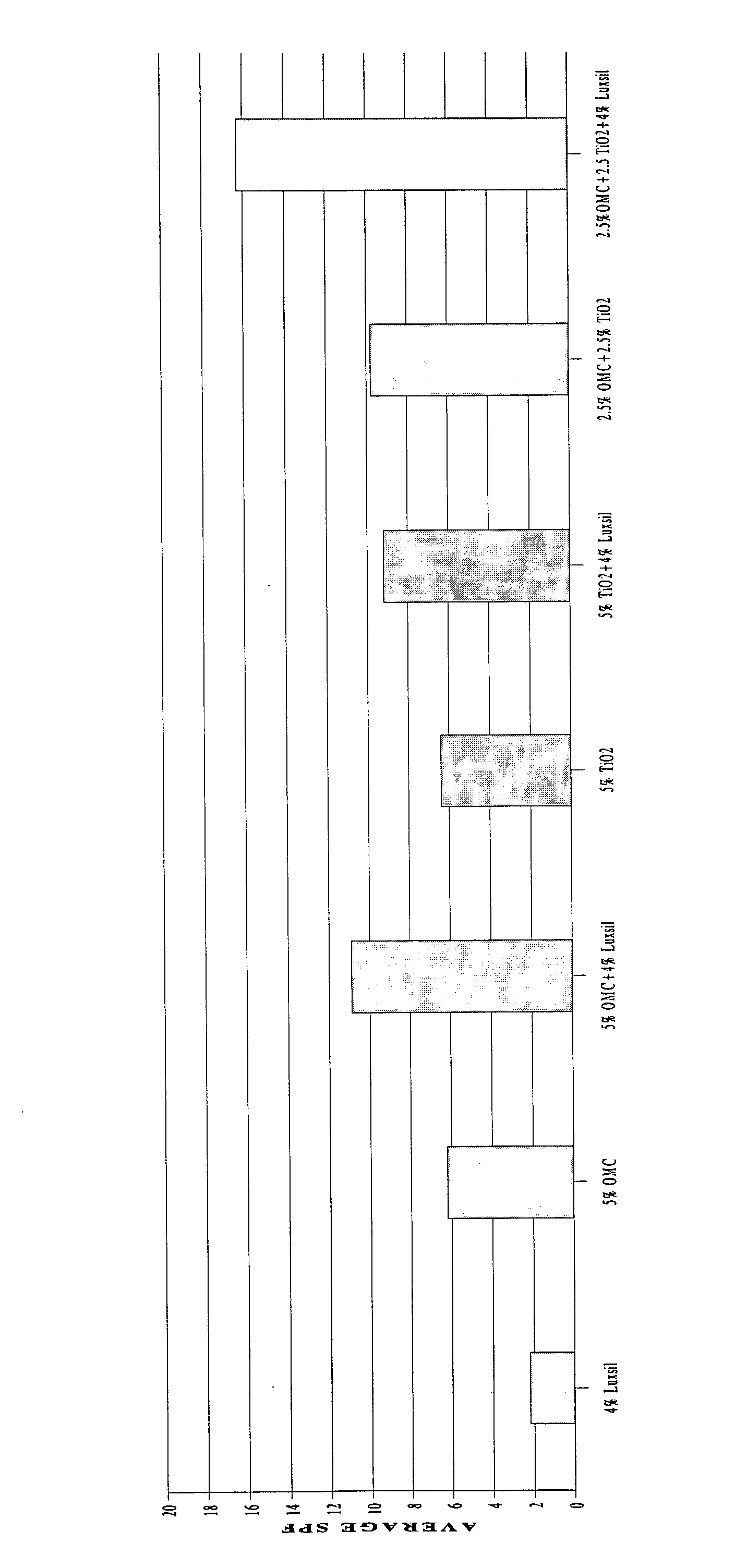
Aesthetically and SPF improved UV-sunscreens comprising glass microspheres
InactiveUS20050036961A1Soft and nongreasy and nontacky feelIncreased in vitro SPFPowder deliveryCosmetic preparationsPhotoprotectionGlass microsphere
Owner:LOREAL SA
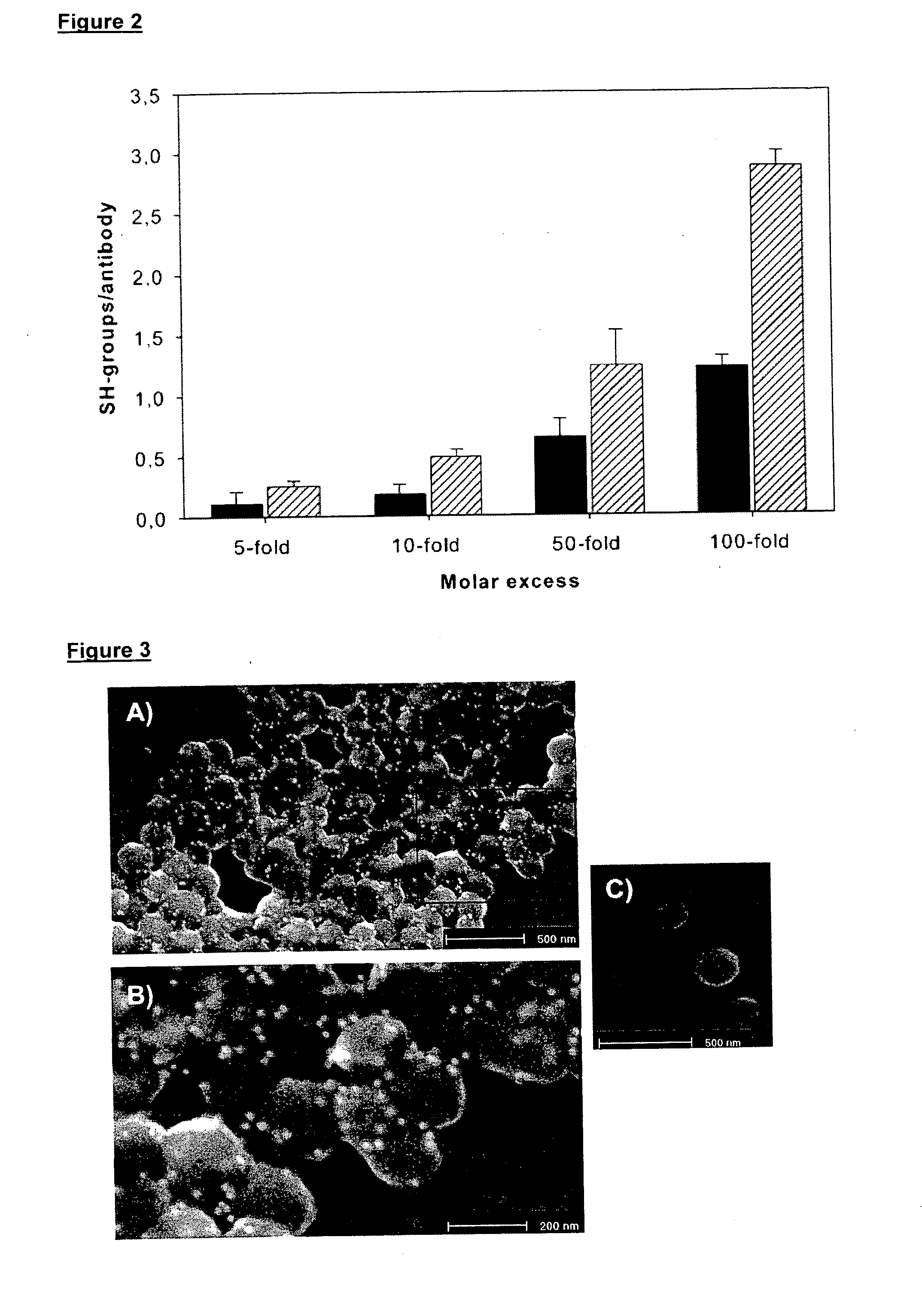
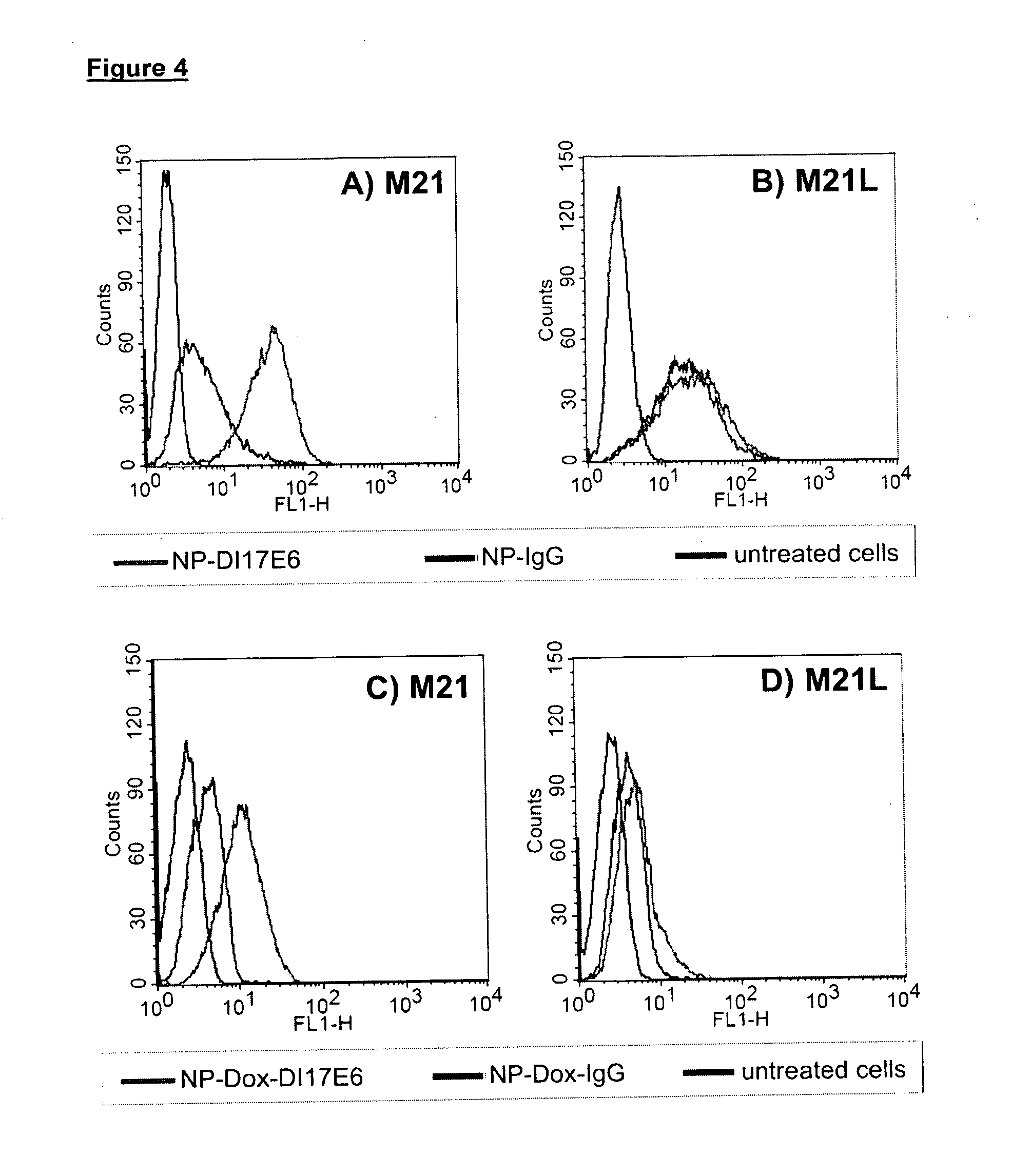
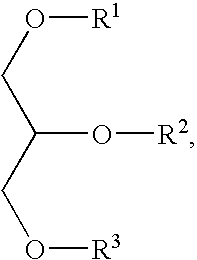
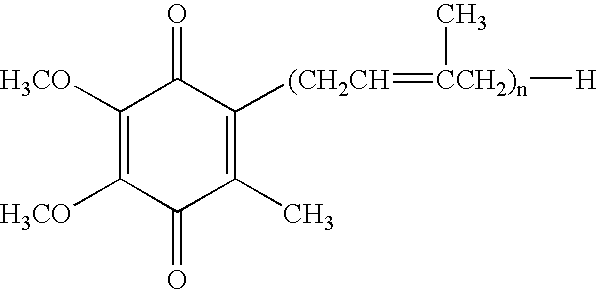
Anti integrin antibodies linked to nanoparticles loaded with chemotherapeutic agents
InactiveUS20120263739A1Good curative effectGood effectPowder deliveryNanomedicineDoxorubicinAntibody
Owner:MERCK PATENT GMBH
Methods and compositions that enhance bioavailability of coenzyme-Q10
Owner:THE NATURES BOUNTY CO
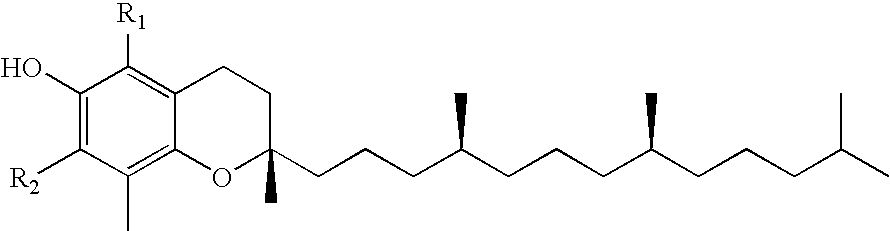
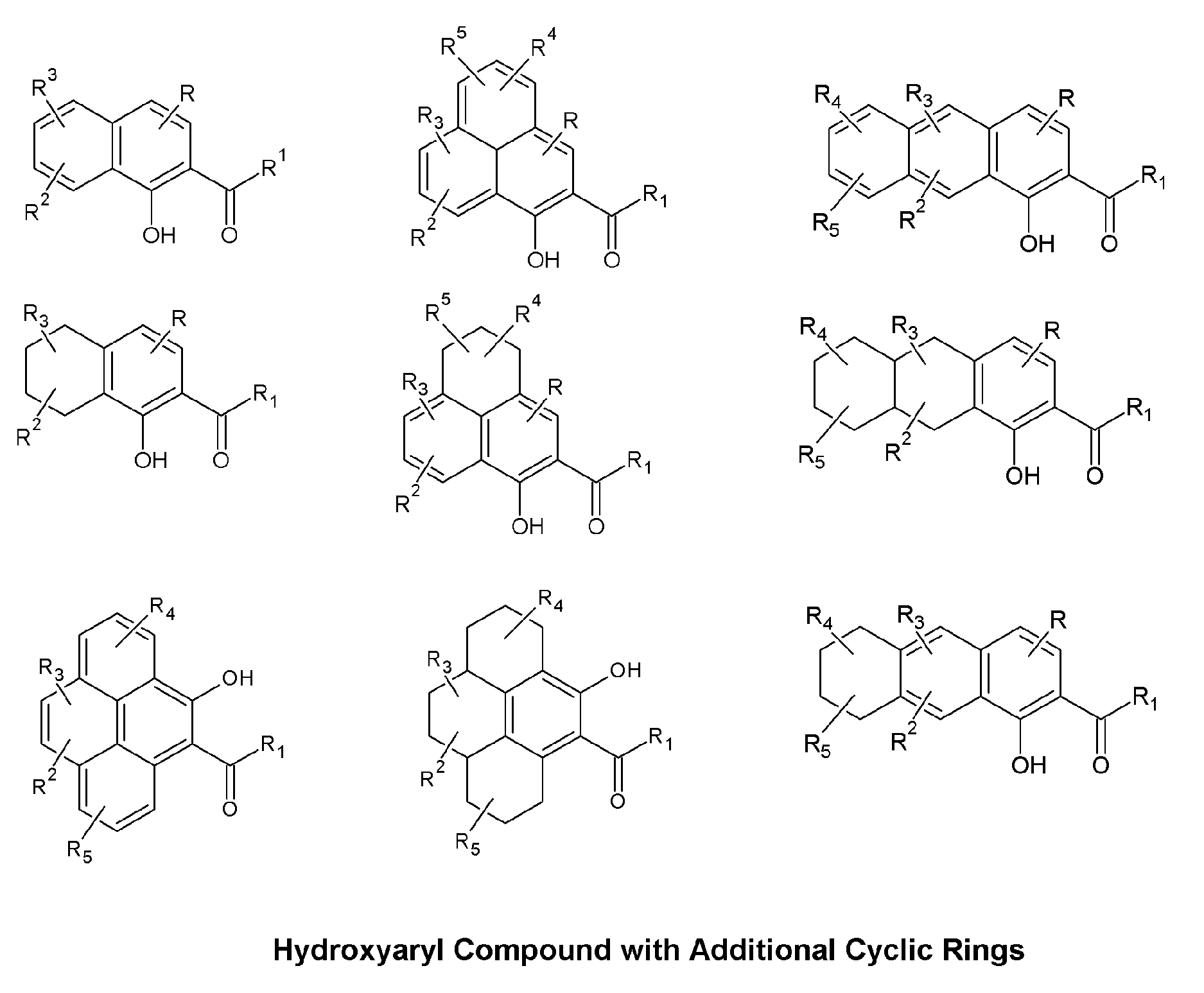
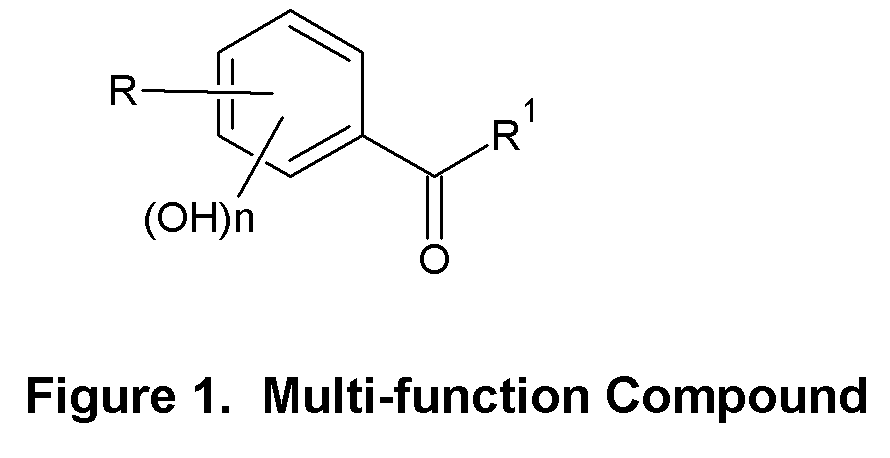
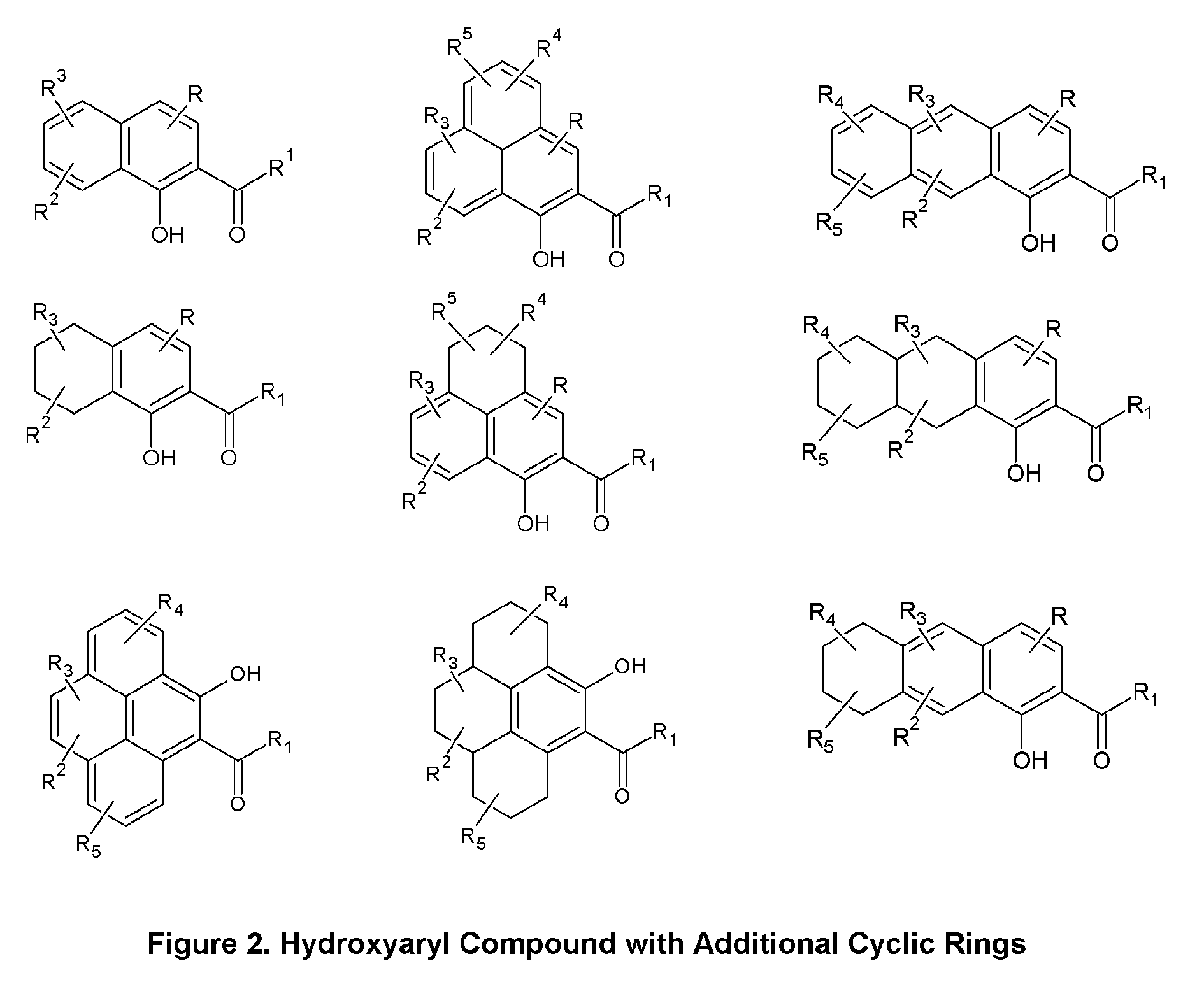
Skin Antiaging & Brightening via Multi-function Treatment of Enzyme Dysfunction
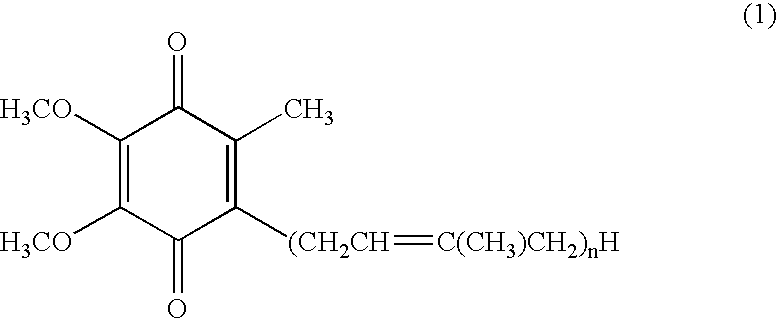
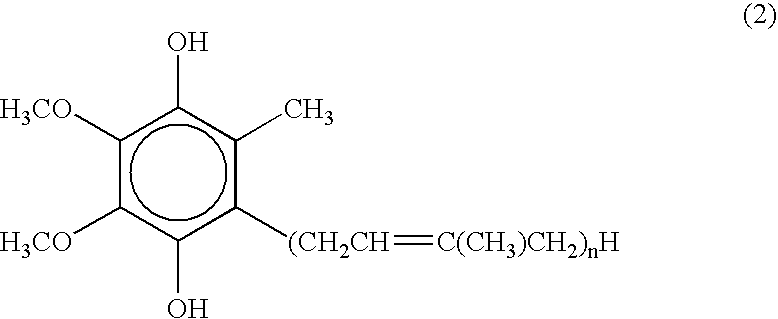
The present invention relates to a topical method of treatment for dysfunction of certain dermal enzymes, and the treatment of skin condition or disorder caused by said dysfunction. The said method of treatment consists of (i) an extra-cellular, matrix metalloprotease regulating agent, and (ii) an intra-cellular ubiquitin—proteasome regulating agent, and (iii) an epidermal melanocyte-regulating agent; and, wherein, said extra-cellular agent, said intracellular agent, and said epidermal agent can, surprisingly and unexpectedly, be a single multi-function compound having chemical formula (I). Additionally, the method of the present invention provides treatment of skin condition or disorder caused by dysfunction of said dermal enzymes; wherein said skin disorder is skin aging, skin wrinkles, dark skin, age spots, acne, skin inflammation, loss of cellular antioxidants, loss of collagen, loss of skin pliability, loss of skin suppleness, oily skin, or a combination thereof:
Owner:BIODERM RES
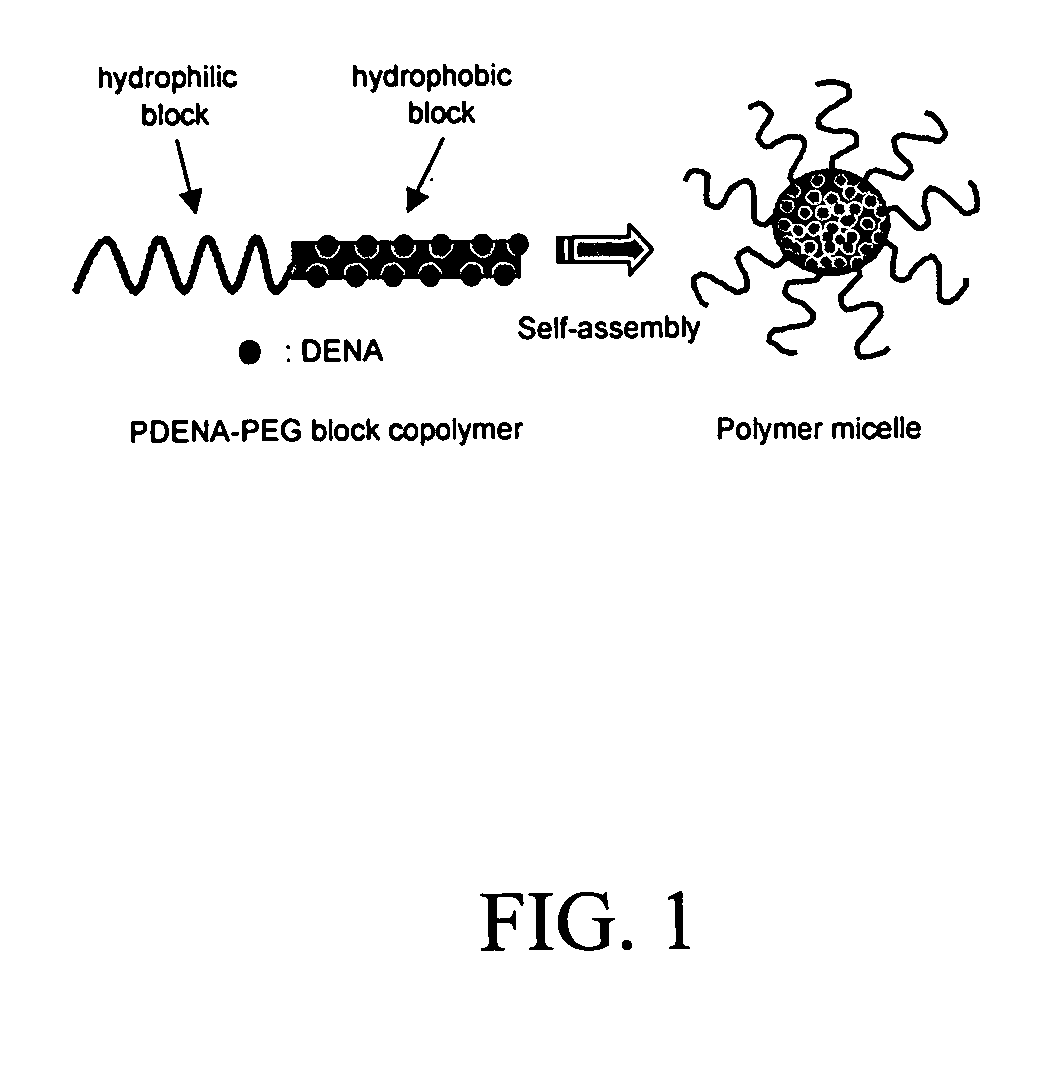
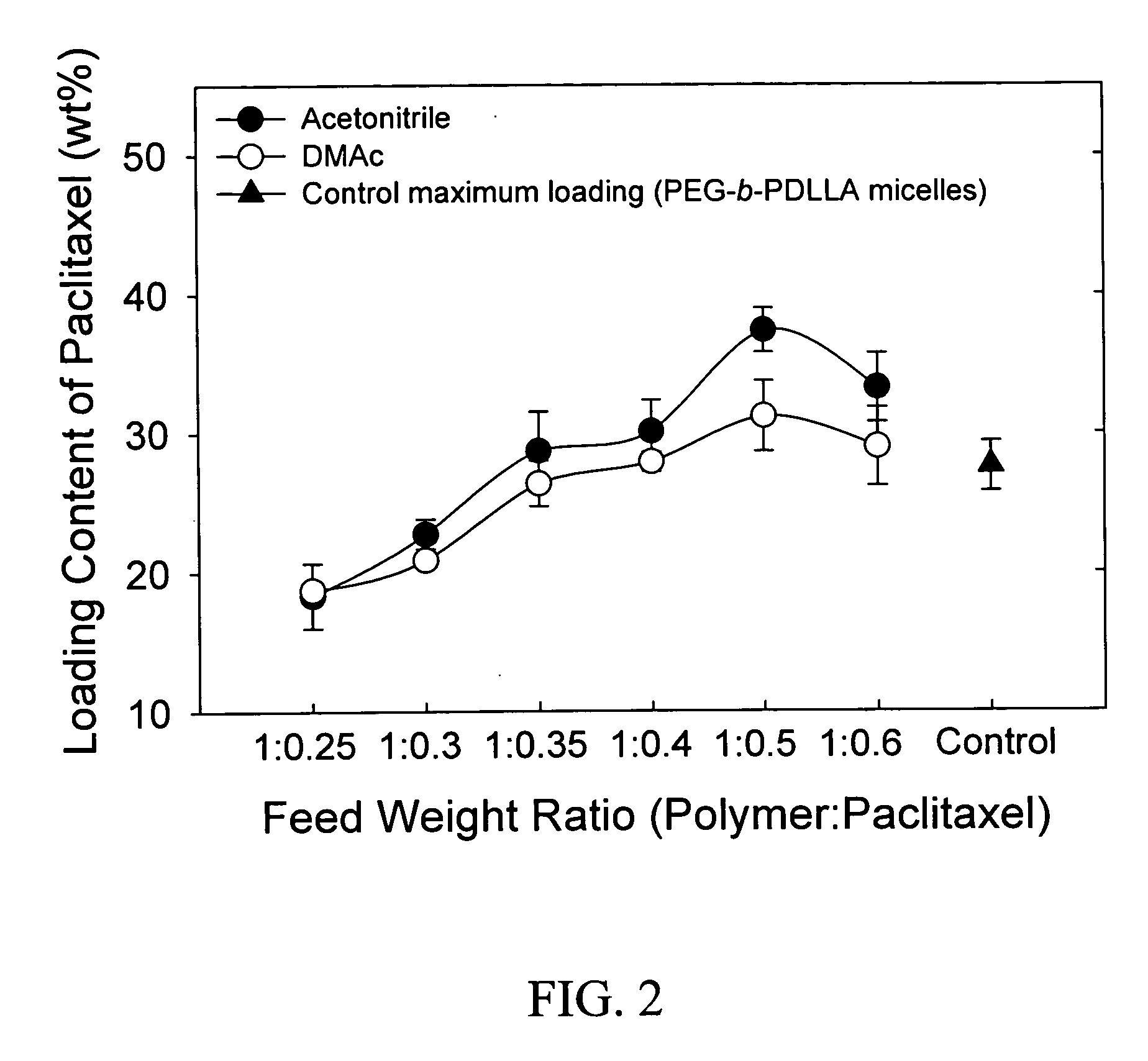
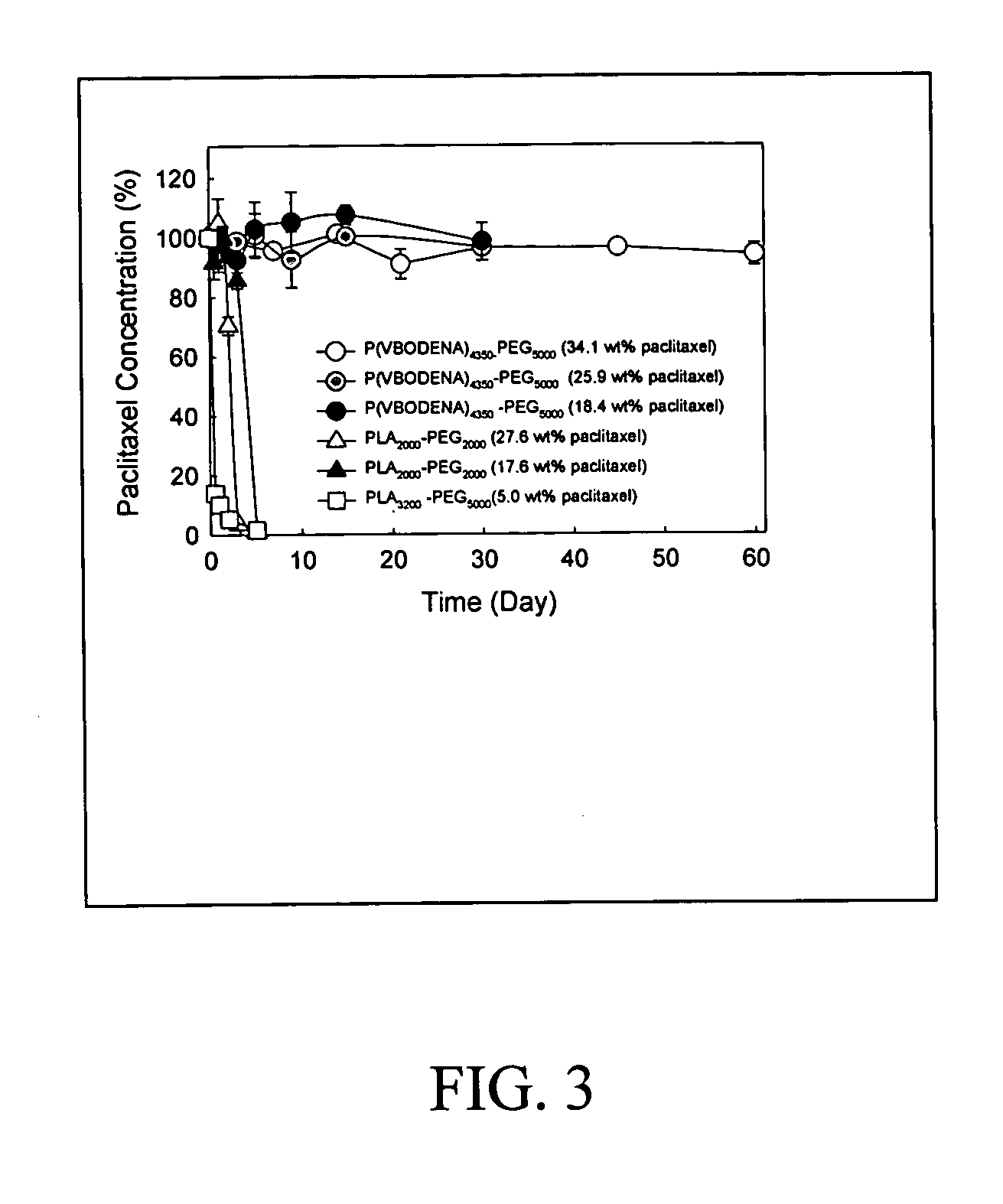
Pharmaceutical applications of hydrotropic polymer micelles
InactiveUS20050158271A1Good water solubilityPowder deliveryOrganic active ingredientsWater soluble polymersWater soluble
Owner:AKINA INC
Controlled-release formulations, method of manufacture, and use thereof
Owner:SUN PHARMA IND INC
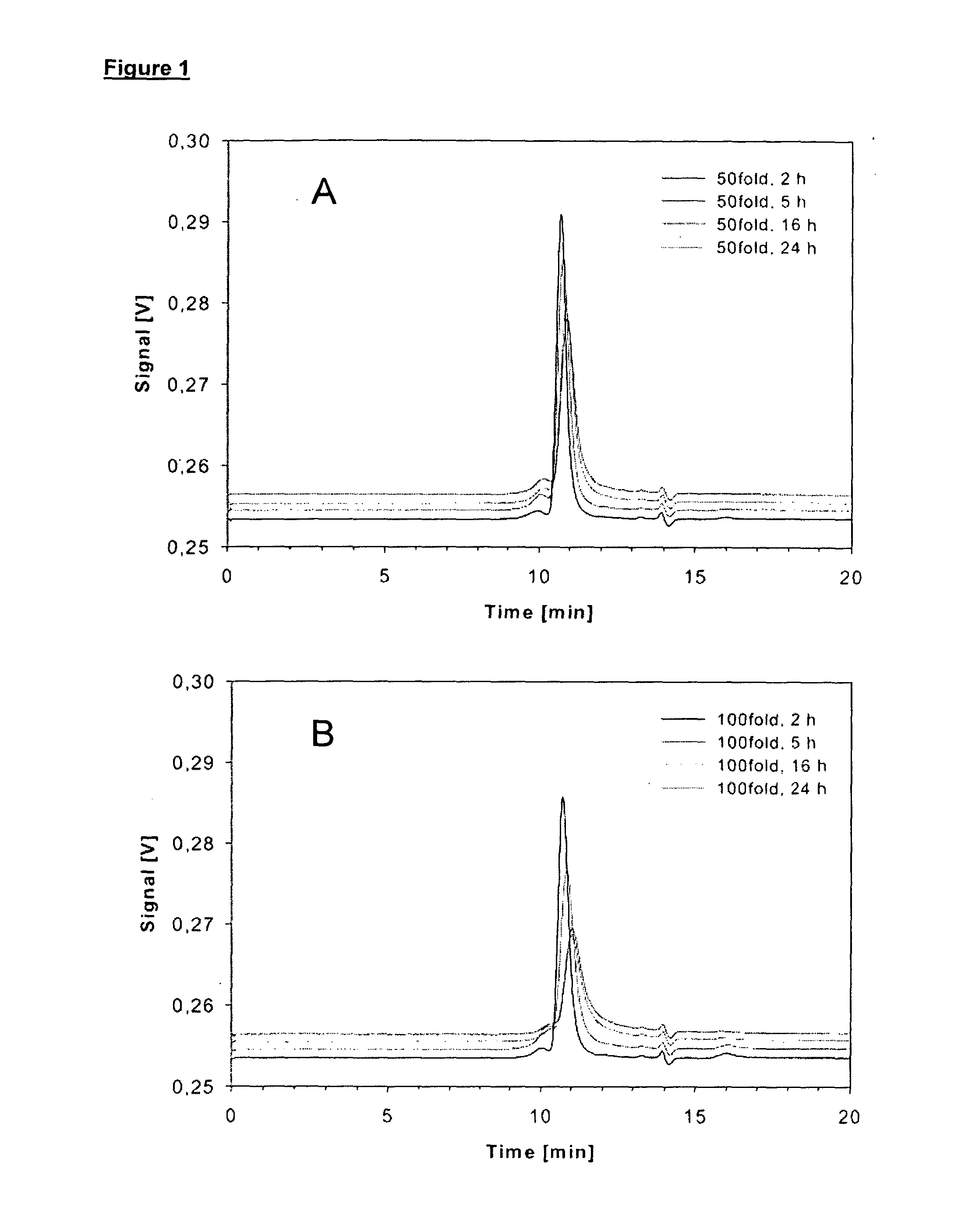
Modulating charge density to produce improvements in the characteristics of spray-dried proteins
InactiveUS20050123509A1High net chargeIncrease the differencePowder deliveryOrganic active ingredientsDrugAqueous solution
Owner:NOVARTIS FARMA
Pharmaceutical compositions for poorly soluble drugs
Owner:MAYNE PHARMA INT
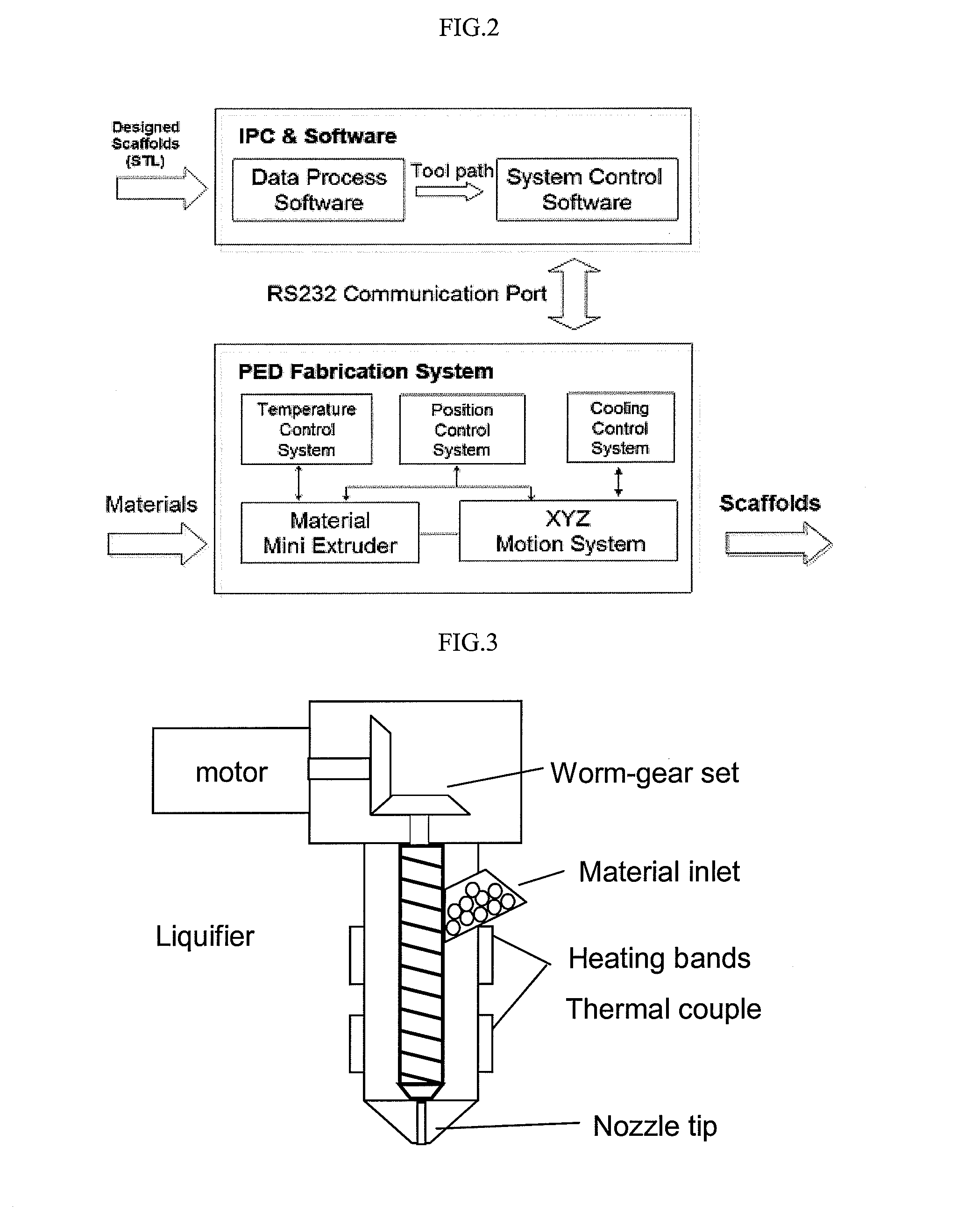
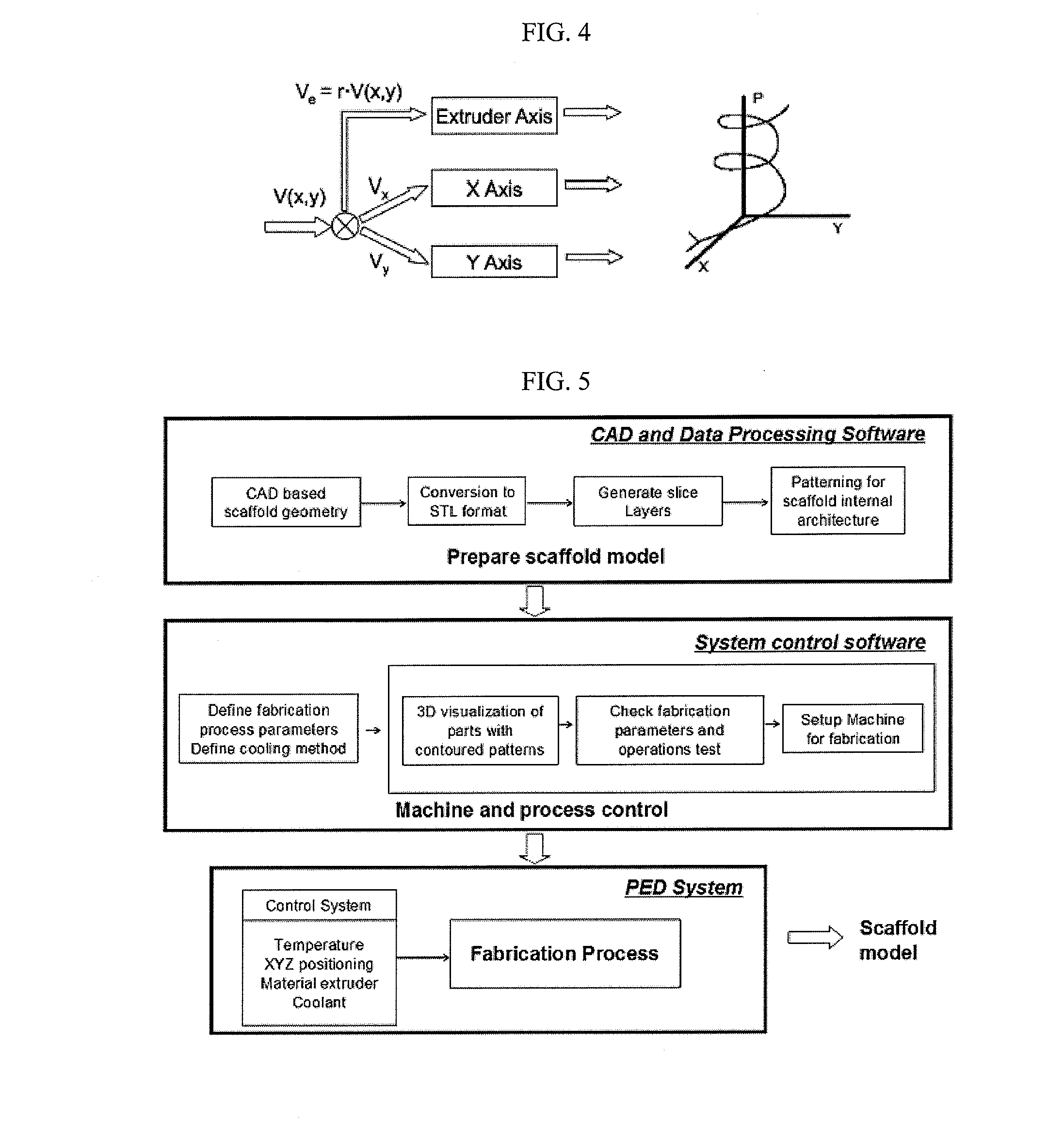
Super-sparger microcarrier beads and precision extrusion deposited poly-epsilon-caprolactone structures for biological applications
Owner:DARLING ANDREW +3
Health-care food with functions of improving immunocompetence and protecting damaged gastric mucosa and its preparing method
Owner:吉林云尚保健食品有限公司
Nimodipime nanometer suspension freeze-dried composition, its preparing method and use
Owner:SHANGHAI INST OF PHARMA IND
Medicine for trenting flooding and spotting, haematemesis and homafecia
A Chinese medicine in the form of capsule, tablet, powder, dripping pill, or aerosol for treating uterine bleeding, hematemesis and hematochezia is prepared from 11 Chinese-medicinal materials including rhubarb, coptis root, notoginseng, donkey-hide gelatin, etc.
Owner:XIAN CHIHO PHARMA
Gold-core-composite nano-carrier as well as preparation method and application thereof
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Composition comprising bioactive substance
Owner:KANEKA CORP
Ocular nanoformulation and method of use in angiogenesis-mediated disorders
An ophthalmic formulation that includes nanoparticles. Each nanoparticle includes a shell which encapsulates sulfated non-anticoagulant heparin (SNACH), with or without hydrophobic anti-angiogenesis Tyrosine Kinase inhibitors. The SNACH is ionically or covalently bonded to the shell. The shell includes a polymer selected from the group consisting of poly (lactic-co-glycolic acid) (PLGA), chitosan, chitosan-alginate, and NIPAAM-APMAH-AA, wherein NIPAAM is N-isopropyl acrylamide, APMAH is N-3-aminopropylmethacrylamide hydrochloride, and AA is acrylic acid. A method for treating an eye disease of a subject includes: administering to an eye of the subject a therapeutically effective amount of the ophthalmic formulation for treating the eye disease. The eye disease involves an ocular angiogenesis-mediated disorder.
Owner:MOUSA SHAKER A
Tilmicosin soluble powder and preparation method thereof
ActiveCN104473876AImprove performanceCompliancePowder deliveryOrganic active ingredientsAdditive ingredientCombinatorial chemistry
Owner:SHANGHAI TONGREN PHARM CO LTD
Self-assembly starch nanoparticle and preparation method thereof
Owner:SOUTH CHINA UNIV OF TECH
Bifidobacterium colonic-dissolving soft capsule and preparation method thereof
InactiveCN105997926AEasy to storeEasy to transportPowder deliveryDigestive systemBifidobacteriumSolubility
Owner:HEILONGJIANG UNIV
Treatment of a hematologic malignancy with 2-(4-chlorophenyl)-n-((2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-5-yl)methyl)-2,2-difluoroacetamide
Owner:CELGENE CORP
Methods for treating urinary incontinence in mammals
InactiveUS20020127197A1Reducing urinary incontinencePowder deliveryGeneral/multifunctional contrast agentsUrethraSolvent
Owner:TYCO HEALTHCARE GRP LP
Heparin-modified adriamycin liposome preparation and preparation method thereof
InactiveCN103720658AEasy to prepareHeparin has good hydrophilicityPowder deliveryOrganic active ingredientsSide effectCholesterol
The invention relates to the field of medicinal preparations, and in particular relates to a heparin-modified adriamycin liposome (Hep-DOX-Lip) preparation. The preparation is characterized by consisting of 1 part of adriamycin, 1-4 parts of heparin, 5-30 parts of soybean lecithin, 0.5-4 parts of cholesterol and 0.5 part of a cationic material. The invention further discloses a preparation method of the heparin-modified adriamycin liposome preparation. The heparin-modified adriamycin liposome preparation has an effect similar to pegylation, and can remarkably enhance the stability of an adriamycin liposome, prolong the in-vivo half-life period of a medicament, and enhance the bioavailability of the medicament. Meanwhile, the heparin-modified adriamycin liposome preparation can remarkably lower the toxic and side effects of chemotherapeutic drugs and enhance the compliance of patients.
Owner:CHINA PHARM UNIV
VLP Stabilized Vaccine Compositions
Owner:INVENTPRISE INC
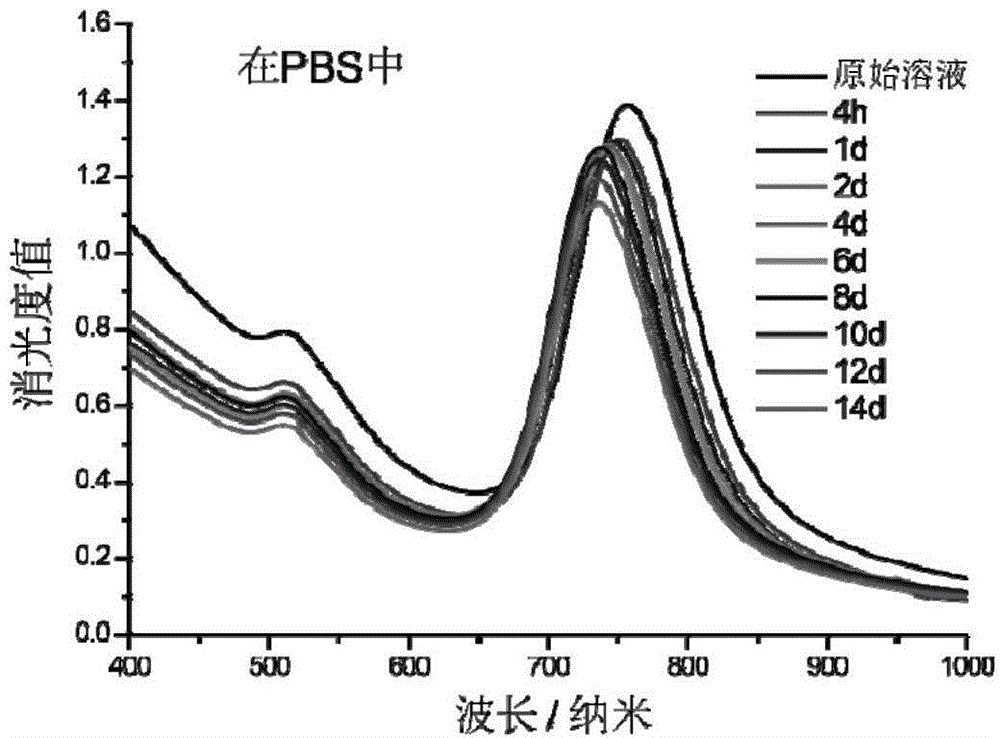
Preparation method of zif-8 nanosphere loaded SERS coded gold nanoparticles for intracellular photothermal therapy
InactiveCN110755385AInhibition of premature releaseImprove internalizationPowder deliveryOrganic active ingredientsCancer cellPolyethylene glycol
Owner:BEIJING FORESTRY UNIVERSITY
Layered double hydroxides
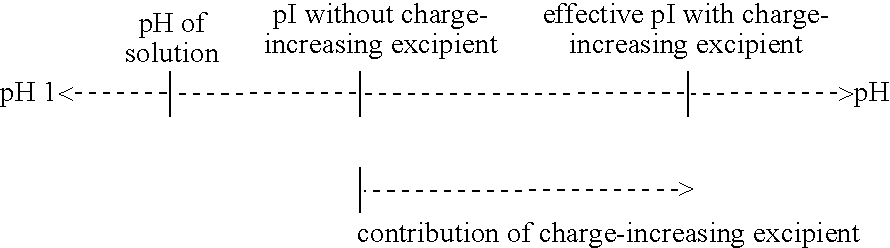
The invention relates to layered double hydroxide (LDH) materials and in particular to new methods of preparing improved LDH materials which have intercalated active anionic compounds (improved LDH-active anion materials). The improved LDH-active anion materials are characterised by their high degree of robustness, demonstrated by their high Particle Robustness Factor values, and by their ability to retain substantially all of the intercalated active anionic compound, in the absence of ion exchange conditions and / or at pH>4.
Owner:OXFORD PHARMASCI
Modified release formulation
Owner:NOVARTIS AG
Tumor-targeting nanoparticles, preparation method and application thereof
InactiveCN109172830AGood dispersionGood biocompatibilityPowder deliveryEnergy modified materialsTumor targetLiposome membrane
Owner:CHONGQING MEDICAL UNIVERSITY
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap