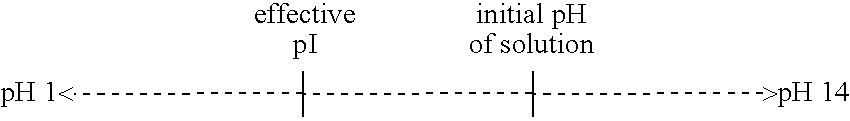Modulating charge density to produce improvements in the characteristics of spray-dried proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0115] A human growth hormone (hGH) formulation for pulmonary delivery was prepared. Methionyl-human growth hormone (Met-hGH, pI 5.2), obtained from BreSagen Limited (Adelaide, SA) was mixed at a concentration of 7 mg / mL (70% w / w) with trileucine (pI 5.9, Bachem Calif. Inc., Torrance, Calif.) at concentrations of 1.5 and 3 mg / mL, in separate solutions. Each solution was then divided and one solution was adjusted to a pH of 3.6 with an acid while the other was adjusted to a pH of 7.8 with a base. Individually, the solutions were spray dried to form particles using a Buchi 190 laboratory scale drier (Buchi, Switzerland) under the following conditions: feed rate: 5 ml / min; outlet temperature: 60° C.; and atomization pressure: 80 psi (0.55 MPa). The dispersibility of these Met-hGH particles are listed in Table 3 below:
TABLE 3Emitted Dose (ED) Results for Spray-Dried Met-hGH Formulationsat Two pH and Two Trileucine Levels15% trilecuine30% trileucinepH 3.692% ED88% EDpH 7.875% ED73
example 2
[0117] An interferon beta formulation for pulmonary delivery was prepared. Interferon beta, obtained from Biogen, Inc. (Cambridge, Mass.), at a concentration of 1 mg / mL was mixed with raffinose at a concentration of 9 mg / mL and titrated to pH 4.0 with HCl. A pH of 4.0 lies 2 to 2.5 pH units below the pI of the fully glycosylated interferon beta. The solution was spray dried to form particles using a Buchi 190 laboratory scale drier (Buchi, Switzerland) under the following conditions: feed rate: 5 ml / min; outlet temperature: 65° C.; and atomization pressure: 100 psi (0.69 MPa). The ED for this formulation was 67% (±8%). The ED for formulations at a higher pH (e.g., pH 5) could not be determined due to the presence of protein aggregates.
example 3
[0118] An interleukin-4 receptor formulation for pulmonary delivery was prepared. Soluble interleukin-4 receptor, obtained from Immunex, Inc. (Seattle, Wash.), was mixed with raffinose and citrate at pH 4 and 7. The mixture had a total solids content of 5 to 10 mg / mL. Excipient components represented 5 to 15% of the total solids content. Each solution was spray dried to form particles using a Buchi 190 laboratory scale drier (Buchi, Switzerland) under the following conditions: feed rate: 5 ml / min; outlet temperature: 70° C.; atomization pressure: 100 psi (0.69 MPa). The ED values of the pH 4.0 and 7.3 formulations were 71 and 66%, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap