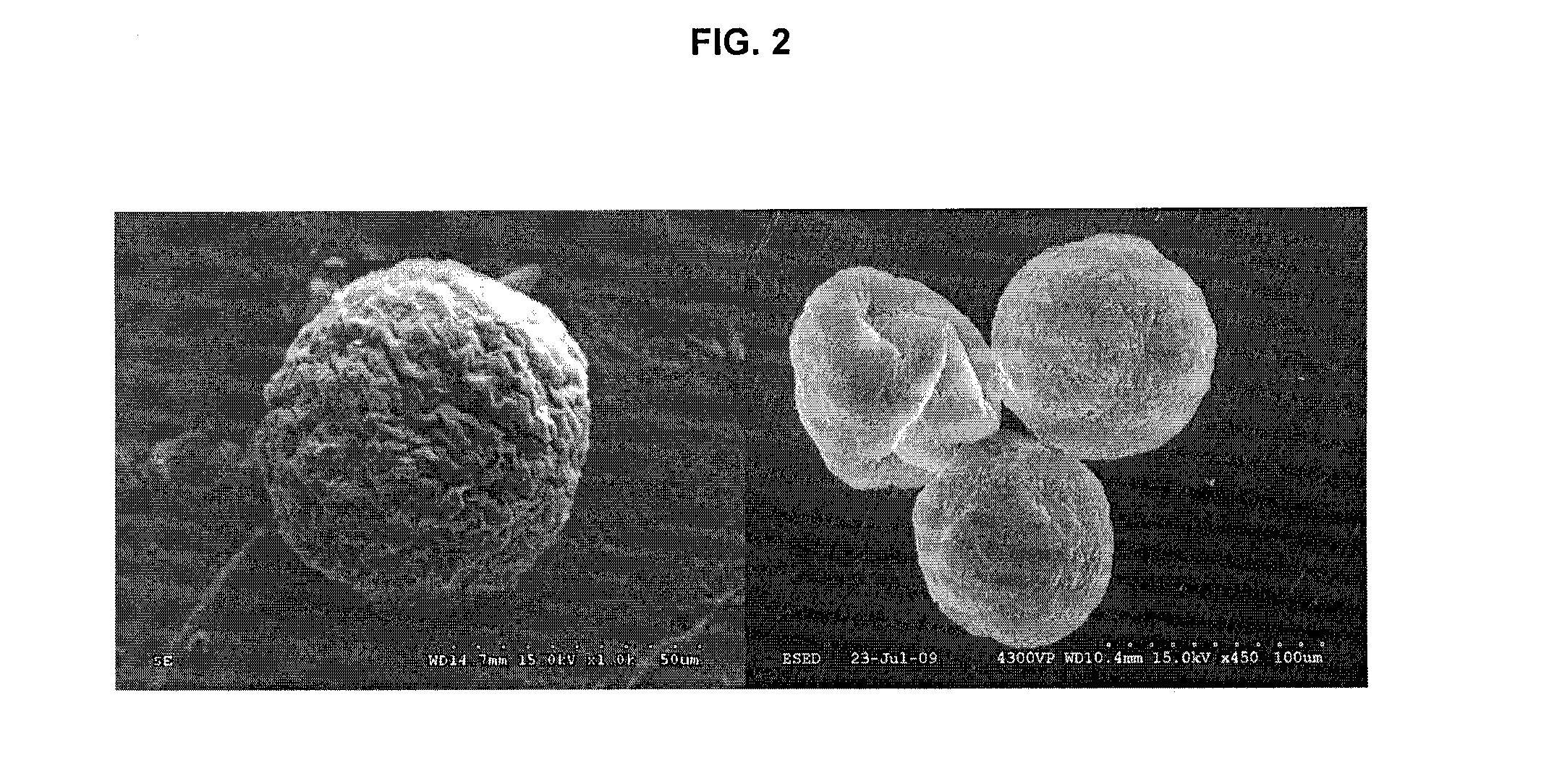
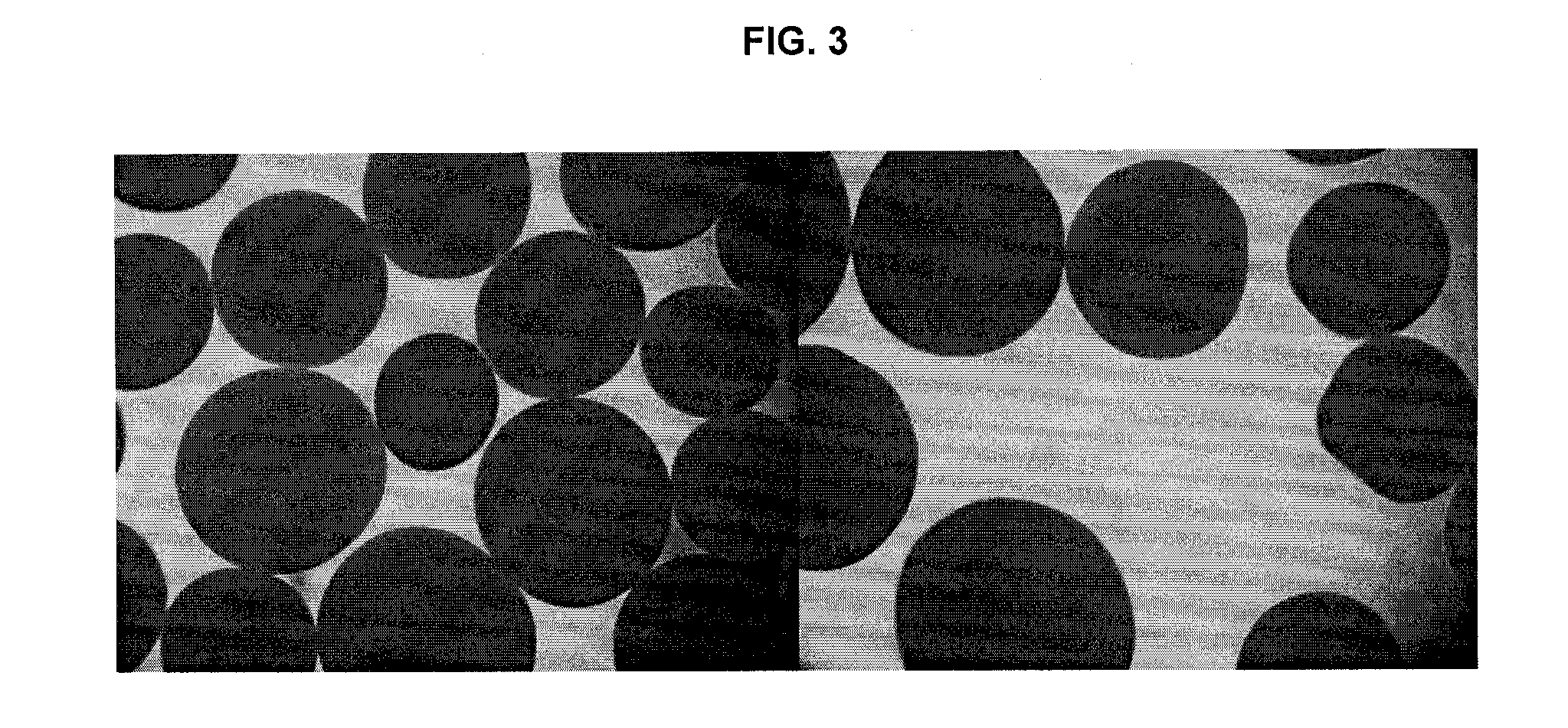
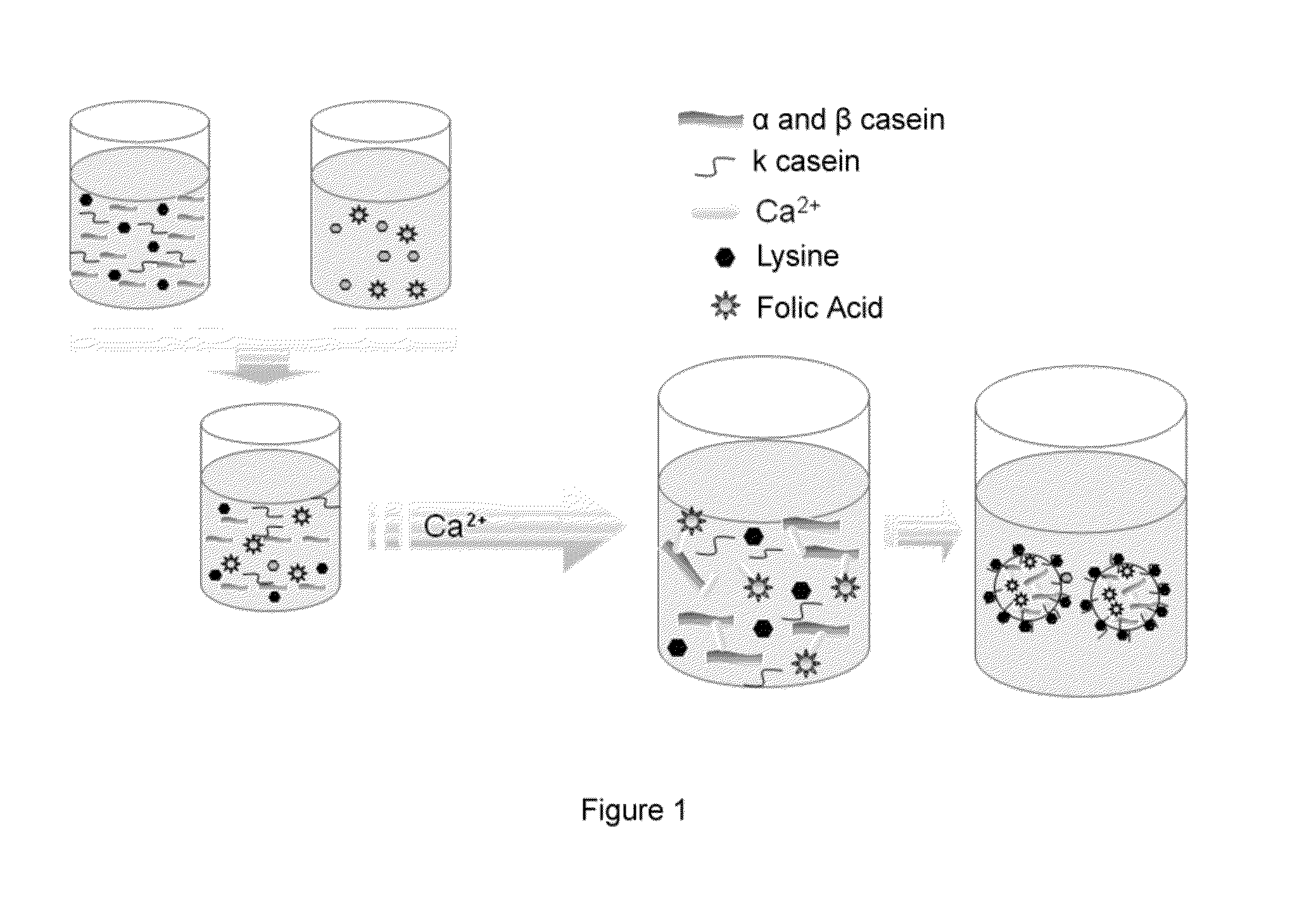
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38results about "Granular delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Apatite-reinforced resin composition
Owner:ASAHI KASEI KK
Compositions and methods for treating conditions of the nail unit
InactiveUS20060275230A1Improve blood supplyPromote circulationBiocideCosmetic preparationsMicroparticlePhase change
Owner:TALIMA THERAPEUTICS INC
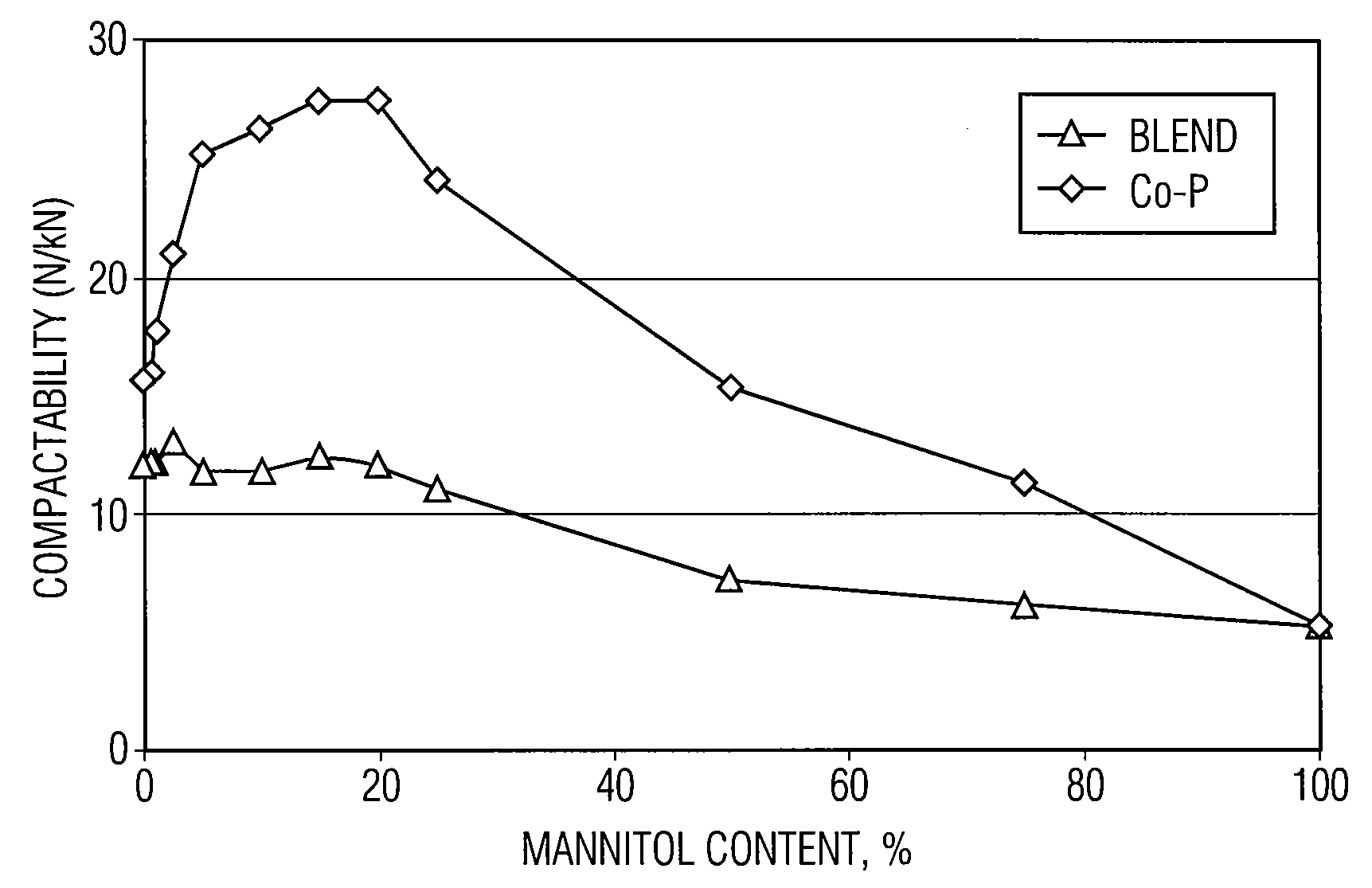
Co-processed microcrystalline cellulose and sugar alcohol as an excipient for tablet formulations
ActiveUS20080131505A1Improve the compaction effectWood working apparatusPill deliveryParticulatesMANNITOL/SORBITOL
Owner:DUPONT NUTRITION USA INC
Ocular nanoformulation and method of use in angiogenesis-mediated disorders
An ophthalmic formulation that includes nanoparticles. Each nanoparticle includes a shell which encapsulates sulfated non-anticoagulant heparin (SNACH), with or without hydrophobic anti-angiogenesis Tyrosine Kinase inhibitors. The SNACH is ionically or covalently bonded to the shell. The shell includes a polymer selected from the group consisting of poly (lactic-co-glycolic acid) (PLGA), chitosan, chitosan-alginate, and NIPAAM-APMAH-AA, wherein NIPAAM is N-isopropyl acrylamide, APMAH is N-3-aminopropylmethacrylamide hydrochloride, and AA is acrylic acid. A method for treating an eye disease of a subject includes: administering to an eye of the subject a therapeutically effective amount of the ophthalmic formulation for treating the eye disease. The eye disease involves an ocular angiogenesis-mediated disorder.
Owner:MOUSA SHAKER A
O type foot and mouth disease virus-like particle vaccine as well as preparation method and application thereof
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
Controlled release formulation for treating sleep disorders
The invention relates to a controlled-release formulation for preventing and / or treating sleep disorders comprising Zaleplon or a pharmaceutically acceptable salt thereof in immediate release form and Zolpidem or a pharmaceutically acceptable salt thereof in sustained release form, wherein Zaleplon or a pharmaceutically acceptable salt thereof and Zolpidem or a pharmaceutically acceptable salt thereof are released in two phases where the first phase is a immediate release phase of Zaleplon or a pharmaceutically acceptable salt thereof and the second phase is a sustained release phase of Zolpidem or a pharmaceutically acceptable salt thereof.
Owner:TAIWAN BIOTECH
Drug delivery compositions and methods
InactiveUS20050129751A1Snake antigen ingredientsPharmaceutical non-active ingredientsControlled releaseDrug release
Contemplated drug delivery systems allow controlled release of a drug in a manner that is independent of the physicochemical parameters of both the drug and its carrier. In one preferred aspect, the drug and the carrier are released from a second carrier that has a predefined release characteristics, which is predominantly determined by the physicochemical properties of the second carrier.
Owner:BIOTECH PHARMA ADVISORY
Composition and method for treatment of metabolic disorders
ActiveUS20180228863A1Safely eliminated through excretionFast dissolutionDispersion deliveryPeptide/protein ingredientsTherapeutic effectEndocrine system
The present invention provides compositions and methods for treatment of metabolic syndromes. Namely, the presently disclosed compositions and methods are provided for affecting the function of the gastrointestinal endocrine system in key regions of the gut, thereby, producing therapeutic effects on obesity, diabetes and other metabolic disorders. The compositions include components for forming luminal barriers within the gastrointestinal tract of a subject where the barrier is created in-situ via interaction of resident mucin with the mucin-interacting agent.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Layered double hydroxides
The invention relates to layered double hydroxide (LDH) materials and in particular to new methods of preparing improved LDH materials which have intercalated active anionic compounds (improved LDH-active anion materials). The improved LDH-active anion materials are characterised by their high degree of robustness, demonstrated by their high Particle Robustness Factor values, and by their ability to retain substantially all of the intercalated active anionic compound, in the absence of ion exchange conditions and / or at pH>4.
Owner:OXFORD PHARMASCI
Cactus freshly-squeezed spray dried granule decoction piece and preparation process thereof
Owner:WUHU TIANCHENG PUYANG TRADITIONAL CHINESE MEDICINE TECH CO LTD
Zinc oxide-based nano-drug composition, and preparation method and application thereof
ActiveCN105853373AIncrease the difficultyIncrease costOrganic active ingredientsZinc oxides/hydroxidesSynthesis methodsPotassium hydroxide
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Capsules capable of activating blood and stopping pains and preparation method of capsules
InactiveCN106074658AIncreased yield of ferulic acidSimple structureHeavy metal active ingredientsHydroxy compound active ingredientsAlcohol contentGround beetle
Owner:南京多宝生物科技有限公司
Epsilon-poly-lysine capsules
InactiveUS20120258047A1Protection attackIncrease buoyancyPowder deliveryBiocideMedicineGreek letter epsilon
Owner:MICROPHARMA
Grape seed extract micropills and preparation thereof
InactiveCN101214322AGood dispersionQuick effectAntinoxious agentsGranular deliveryExtracted grape seedsMedicine
Owner:JF PHARMALAND TECH DEV
Method and Device for Restoring Spinal Disk Functions
InactiveUS20120283601A1Minimally invasiveSimple compositionOrganic active ingredientsPerson identificationSurgical instrumentationIntervertebral disk
The present invention relates to medical devices, systems and methods for restoring spinal disc function. The invention provides a curved path through the vertebra into the disc through which the disc can be filled with an augmenting substance, balloon, or pellets. Further, the delivery device can be used to access the disc with surgical instrumentation. The invention further discloses a responsive disc augmentation system.
Owner:DAUM WOLFGANG R
"nanoparticles for the encapsulation of compounds, preparation thereof and use of same"
ActiveUS20130209530A1Improve bioavailabilityReduced bioavailabilityPowder deliveryBiocideNanoparticleDivalent metal
Owner:CENT NACIONAL DE TECHA Y SEGURIDAD ALIMENTARIA LAB DEL EBRO +1
Traditional Chinese medicine bath antibacterial liquid/granules for regulating Yang-deficiency constitution and preparation and using method
InactiveCN109646489AFunction increaseFully dissolvedAntibacterial agentsAntimycoticsDiseaseYang deficiency
Owner:黄巧明
Preparation method for Ganhaiweikang preparation
Owner:SHAANXI DONGKE PHARMA
Highly dispersible granulate for the preparation of formulations of high dosage active substances and procedure for obtaining high dosage active substances thereof
InactiveUS20130108699A1Reduce manufacturing costIncrease chanceBiocideGranular deliveryActive principlePharmacological action
Owner:SMART PHARMA SOLUTIONS
Preparation method of traditional Chinese medicine granules for treating pig wind-heat type common cold
InactiveCN111588750AGreat tasteImprove solubilityPharmaceutical non-active ingredientsGranular deliveryBiotechnologyCommon cold
The invention discloses a preparation method of traditional Chinese medicine granules for treating pig wind-heat type common cold. The preparation method comprises the following steps of 1) performingmixing on a traditional Chinese medicine extract and auxiliary materials according to a ratio of 1: (4.5-6.5), performing grinding and crushing until the mixture is uniformly mixed to obtain a mixture, wherein the traditional Chinese medicine extract comprises the following components: 60-68g of honeysuckle extract; 27 to 35 g of scutellaria baicalensis extract; 40 to 46 g of licorice extract; 2)adding a wetting agent into the mixture obtained in the step 1), performing mixing, and uniformly stirring to obtain formed particles, wherein the use amount of the wetting agent is 15-25% of the mass of the mixture; and 3) performing drying on the formed particles obtained in the step 2) at the temperature of 50-60 DEG C to obtain the traditional Chinese medicine particles. According to the scheme, proper auxiliary materials are added for taste correction, and the traditional Chinese medicine particles with better water solubility are prepared through the wetting agent, so that clinical application is facilitated.
Owner:GUANGDONG RONGDA BIOENG CO LTD
Traditional Chinese medicinal composition capable of relieving cough and asthma and preparation method of traditional Chinese medicinal composition
PendingCN107029099AMildNo side effectsPharmaceutical non-active ingredientsGranular deliveryTreatment effectSide effect
Owner:魏增强
Formula and production process of granules for treating gout
InactiveCN112957435ALower blood uric acidSlightly sweet tasteSkeletal disorderPharmaceutical non-active ingredientsBiotechnologyCoix
The invention discloses a formula and a production process of granules for treating gout, the granules comprise raw materials and auxiliary materials, the raw materials comprise sunflower heads, dandelion, fresh lalang grass rhizome, coix seeds and hawthorn, and the auxiliary materials comprise maltodextrin and xylitol. Compared with the prior art, the sunflower heads are processed into the food solid beverage, so that patients with gout diseases can timely use the product taking the sunflower heads as the main raw material, the product is matched with the dandelion, the hawthorn, the lalang grass rhizome and the coix seeds which are homologous in medicine and food, the effects of reducing blood uric acid and further treating gout are achieved, auxiliary materials are matched, so that the taste is slightly sweet and cool, and the popularization and application values are realized.
Owner:SICHUAN KANGTAIJIA BIOTECHNOLOGY CO LTD
Feed additive enteric-coated sustained-release pellet with function of preventing and treating poultry respiratory syndromes and preparation method for feed additive enteric-coated sustained-release pellet
InactiveCN104739936AImprove conversion rateGrowth-promotingAntibacterial agentsHydroxy compound active ingredientsSustained release pelletsFeed conversion ratio
Owner:天津市静海县科杰制剂技术创新研究中心
HIV-1 integrase inhibitor phospholipid complex, preparation method and applications thereof
InactiveCN108434145AEasy to manufactureFast absorptionOrganic active ingredientsAntiviralsIn vivo absorptionPhospholipid complex
Owner:TIANJIN INT JOINT ACADEMY OF BIOTECH & MEDICINE
Nanocomposite formulation for use in hemostasis
The present invention relates to a nanocomposite formulation for use in hemostasis comprising at least one calcium-silicate and at least one polysaccharide. The invention also relates to solvent-free process for preparation of the nanocomposite formulation.
Owner:INDIAN INSTITUTE OF TECHNOLOGY BOMBAY
Method for preparing adhesive solution in wet granulation process
PendingCN113599529AInorganic non-active ingredientsGranular deliveryMeglumineAqueous sodium hydroxide
Owner:上海合全医药有限公司
Deamorphization of spray-dried formulations via spray-blending
Owner:NOVARTIS AG
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap