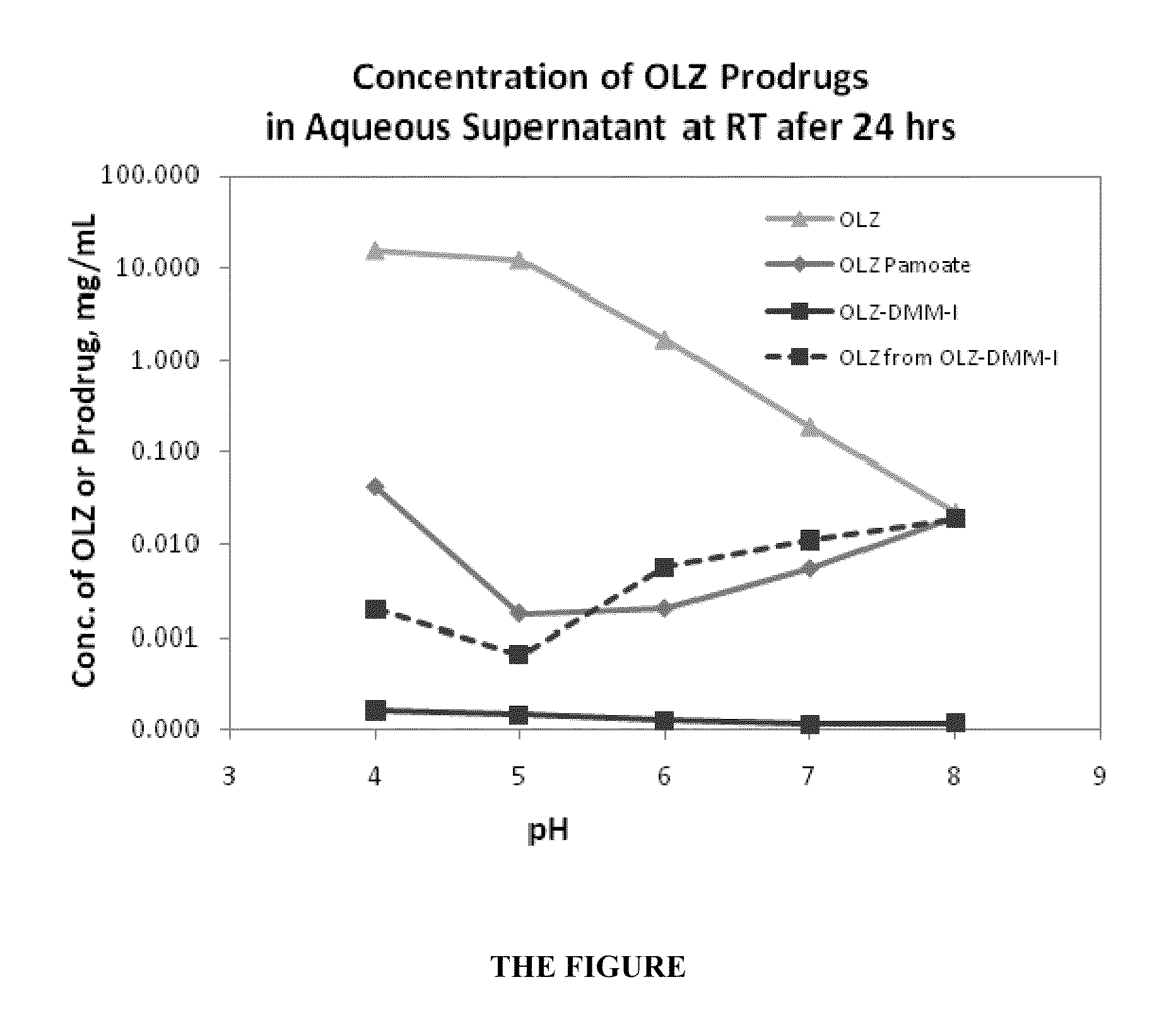
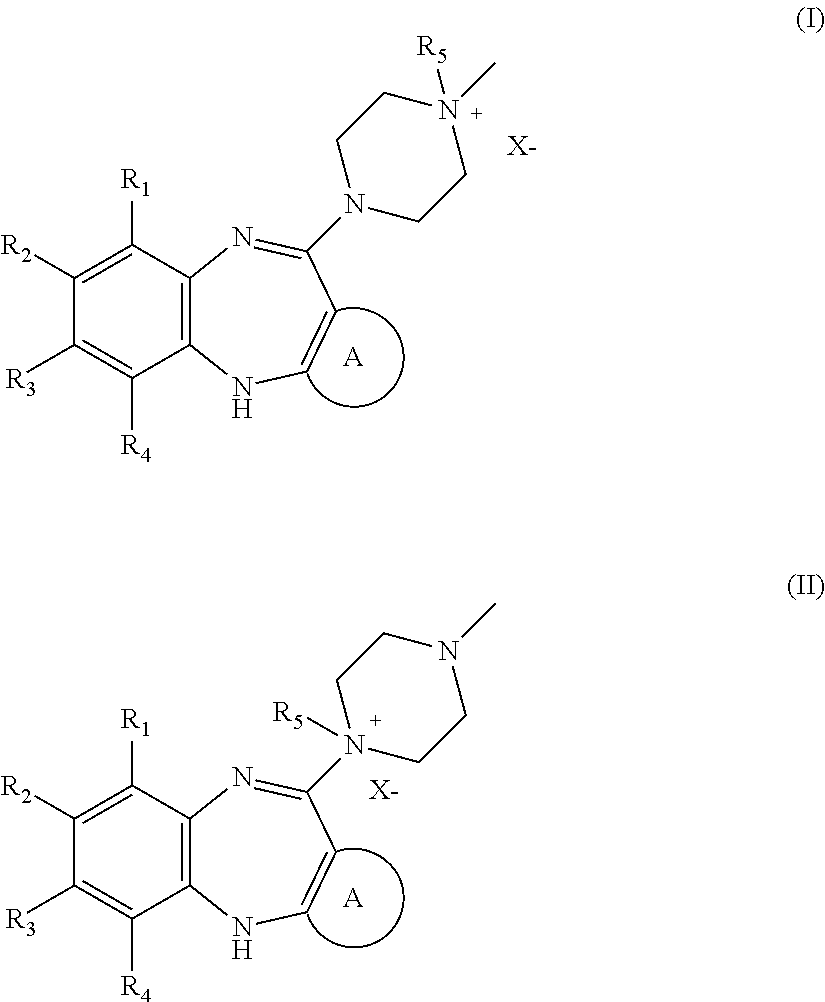
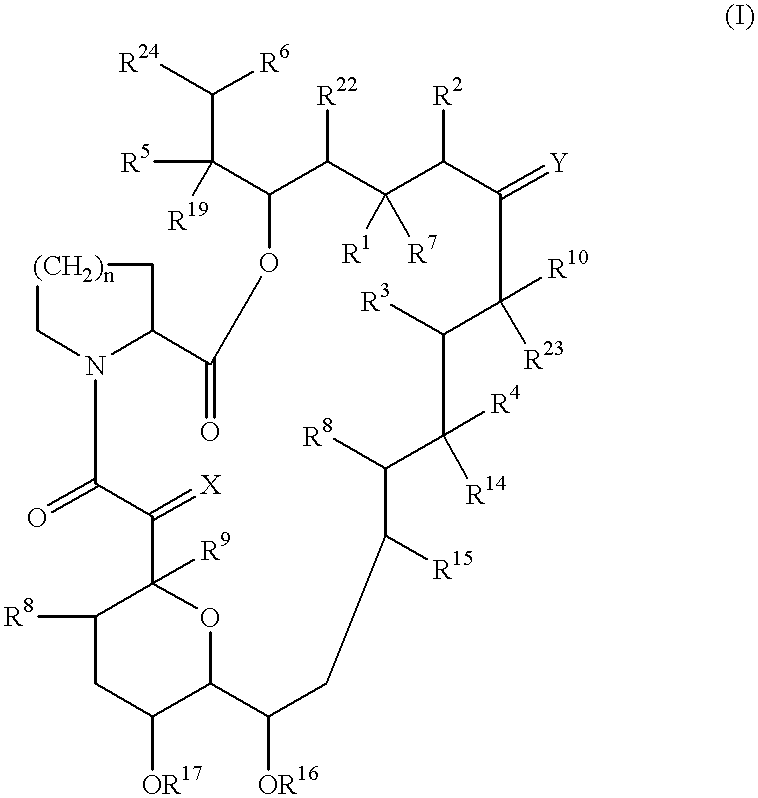
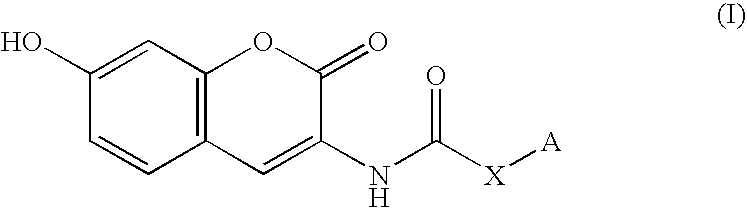
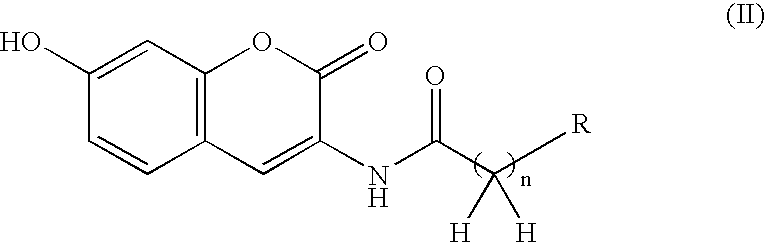
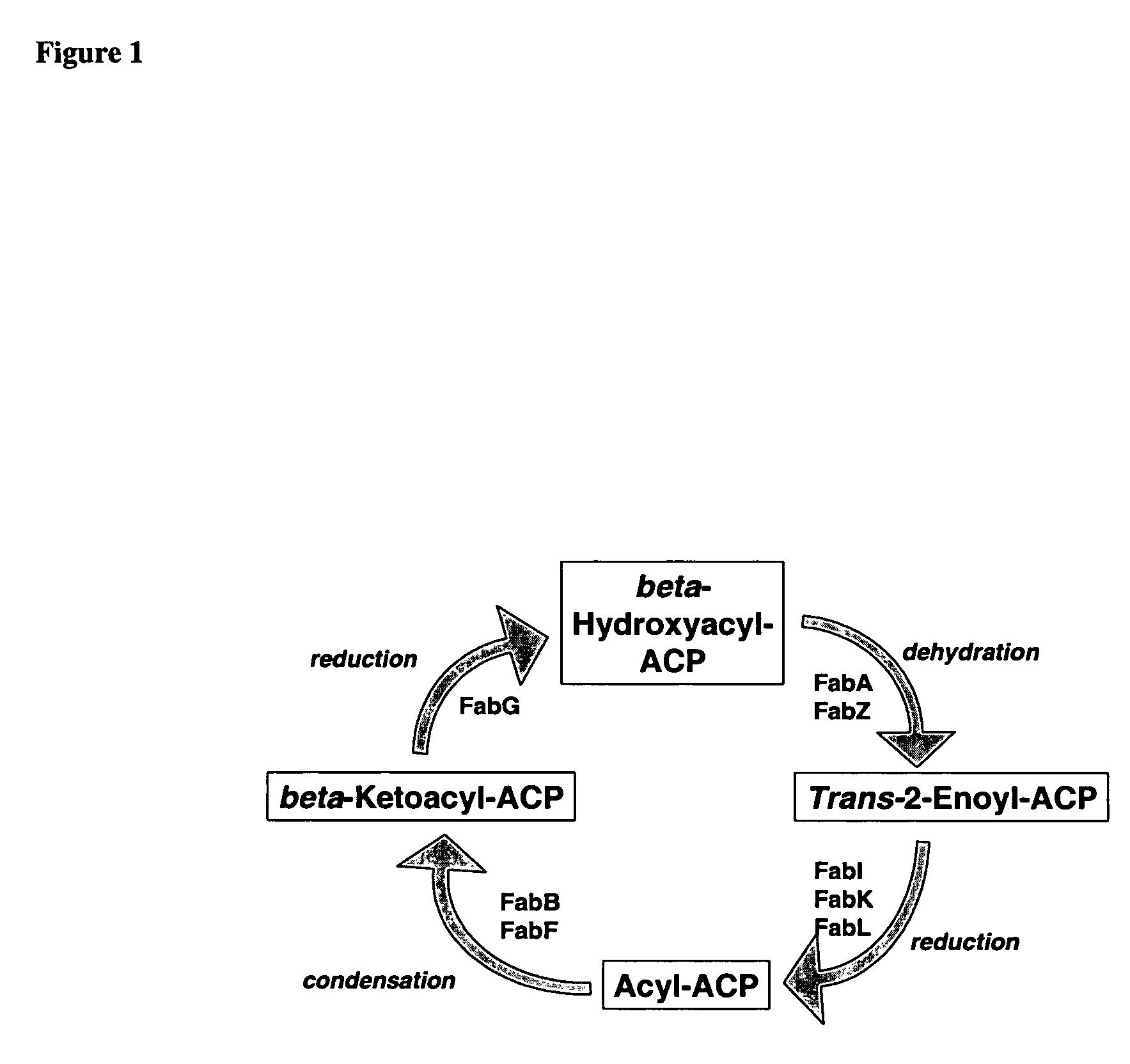
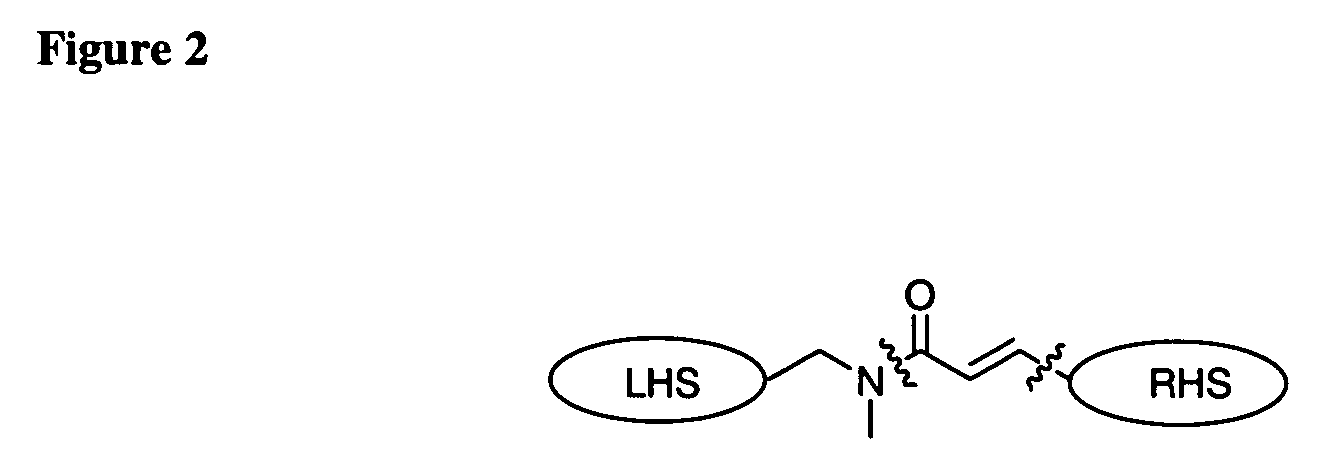
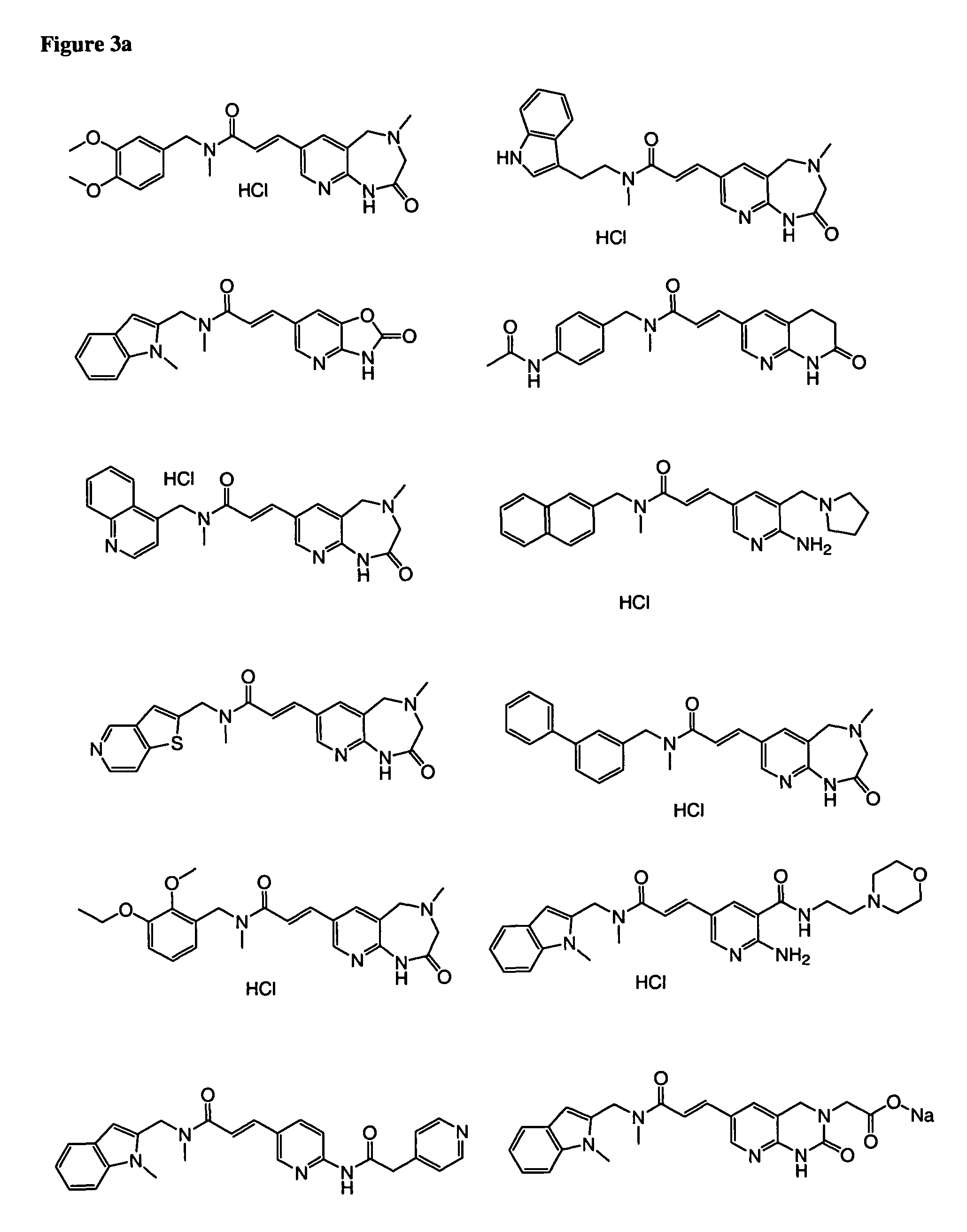
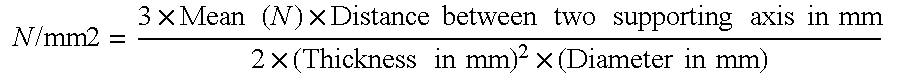Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
83results about "Heterocyclic compound active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diaryldiazepine Prodrugs for the Treatment of Neurological and Psychological Disorders
InactiveUS20110166128A1Long duration of actionReduce solubilityBiocideNervous disorderDrug compoundProdrug
Owner:ALKERMES INC
Materials and methods for treating neuropathies and related disorders including those involving a keystone nerve
InactiveUS20160030408A1Increase blood flowAlter perception of painBiocideElectrotherapyDiseaseMedicine
Methods, apparatus, compositions and kits for inhibiting a disorder in a human patient, including non-cerebral neurovascular disorder or muscular headache pain, or loss of motor or sensory function, sympathetic tone or range or fluidity of motion that affect a nerve pathway at more than one locus associated with the disorder to inhibit the disorder. Alternatively or in addition, neuropathy associated with a disorder is treatable by palpating to determine a Keystone nerve essential to the neuropathy, applying pressure to determine a point of maximum discomfort or trigger of increased symptoms to identify a Levin Sign as a locus of initial intervention, and intervening to treat the neuropathy at the location of the Levin Sign by administering a pharmaceutically active agent, internal implanted or external neuro stimulation affecting the nerve pathway to inhibit the neuropathy.
Owner:BHL PATENT HLDG
Modified nucleosides for the treatment of viral infections and abnormal cellullar proliferation
The disclosed invention is a composition for and a method of treating a Flaviviridae (including BVDV and HCV), Orthomyxoviridae (including Influenza A and B) or Paramyxoviridae (including RSV) infection, or conditions related to abnormal cellular proliferation, in a host, including animals, and especially humans, using a nucleoside of general formula (I)-(XXIII) or its pharmaceutically acceptable salt or prodrug.This invention also provides an effective process to quantify the viral load, and in particular BVDV, HCV or West Nile Virus load, in a host, using real-time polymerase chain reaction (“RT-PCR”). Additionally, the invention discloses probe molecules that can fluoresce proportionally to the amount of virus present in a sample.
Owner:GILEAD PHARMASSET LLC
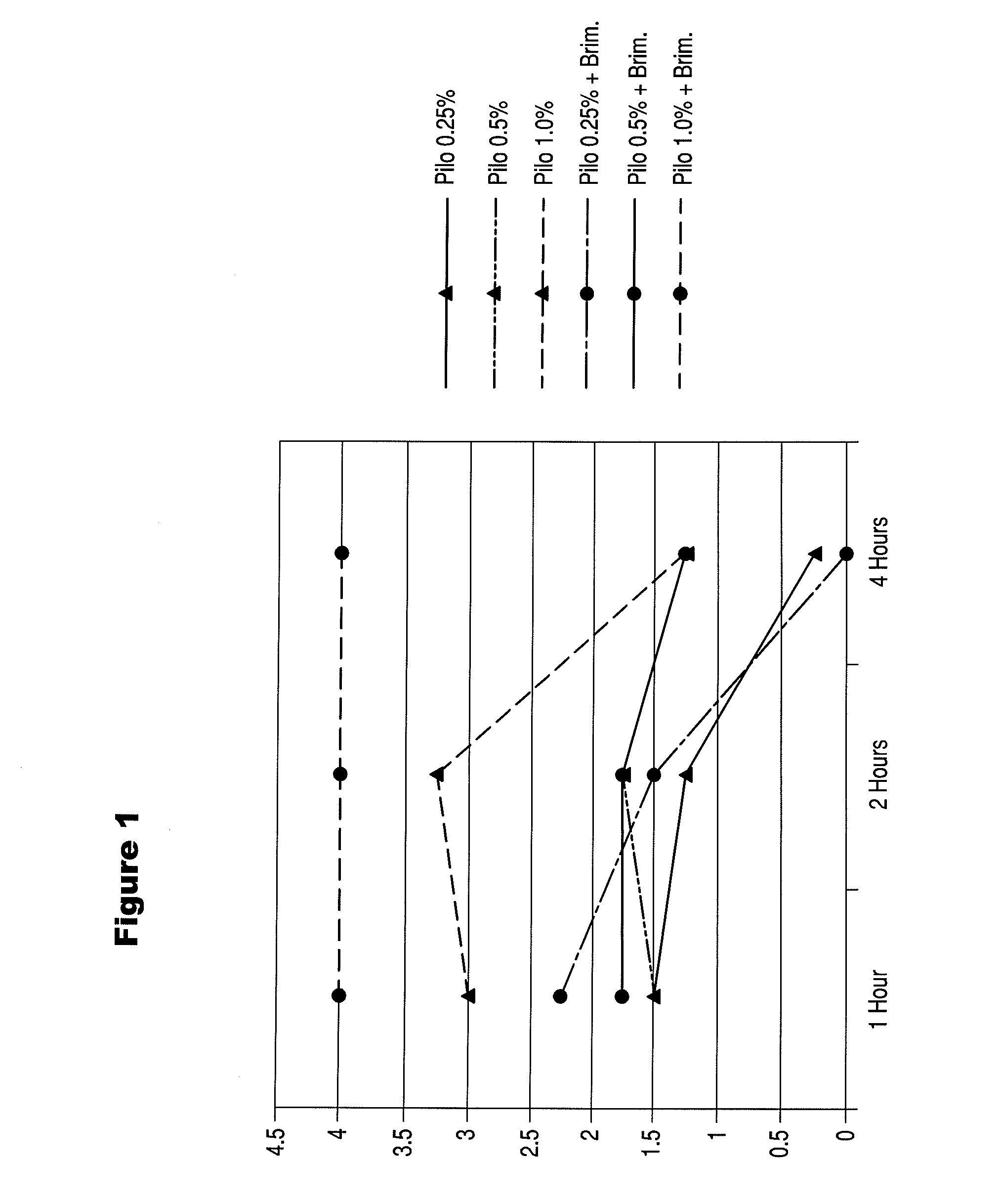
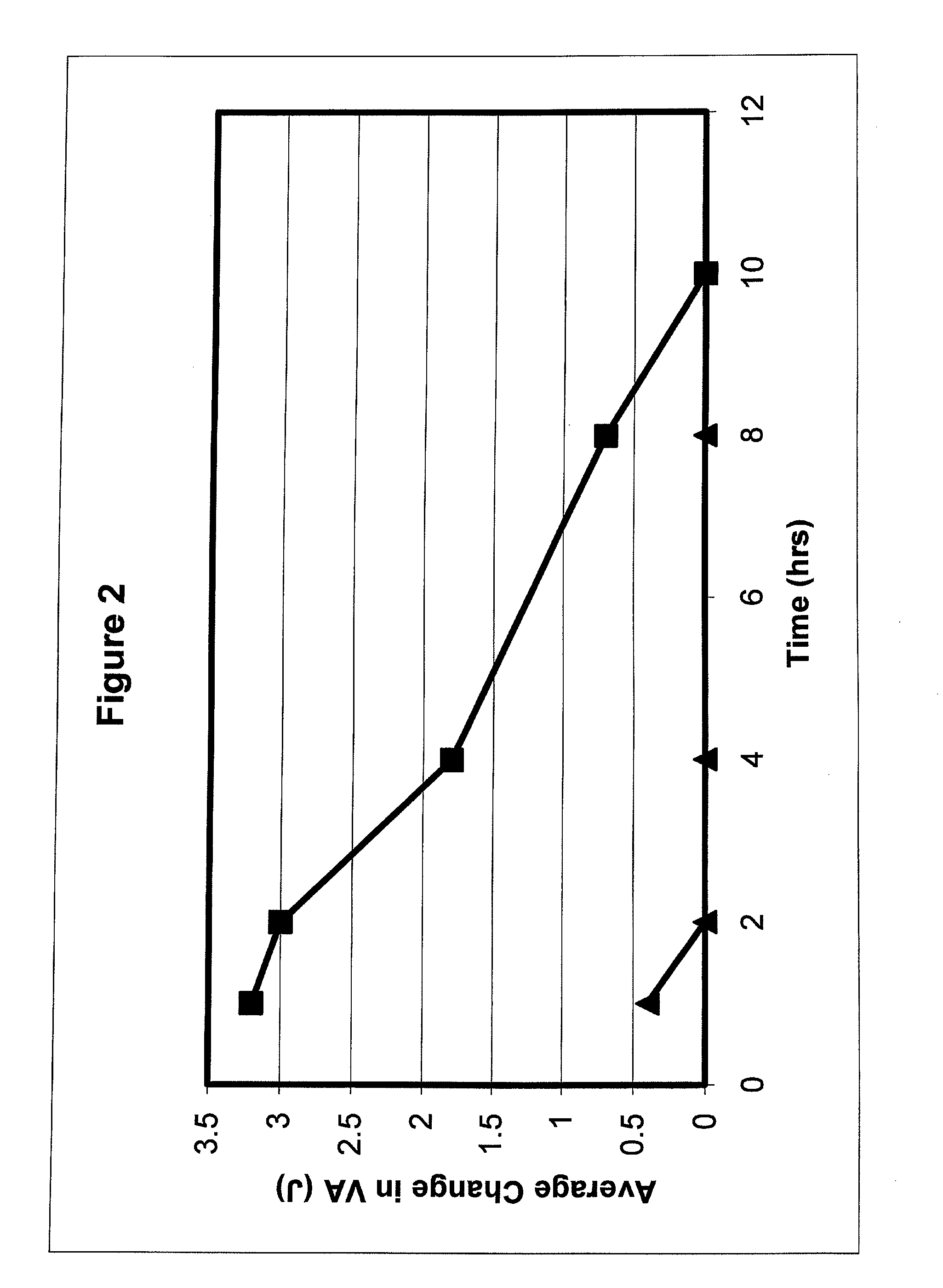
Use of macrolide compounds for treating glaucoma
Owner:ASTELLAS PHARMA INC
Uniform delivery of topiramate over prolonged period of time with enhanced dispersion formulation
InactiveUS20050058707A1Improve bioavailabilityPromote absorptionNervous disorderCapsule deliveryTopiramateControlled Release Dosage Form
Compositions and dosage forms for enhanced dispersion of topiramate in a controlled release dosage form delivered as a dry or substantially dry erodible solid at a uniform rate over a prolonged period of time are described.
Owner:ALZA CORP
Thioredoxin reductase inhibiter compounds and preparation method and application thereof
The invention discloses thioredoxin reductase inhibiter compounds, which have antitumor activity, particularly have growth inhibition activity for solid tumor, wherein the solid tumor is thyroid cancer, prostatic cancer, colon cancer, rectal cancer, melanoma, liver cancer, lung cancer, gastric cancer, carcinoma submaxilary gland, nasopharyngeal darcinoma or breast cancer.
Owner:KEAISE MEDICINE WUHAN
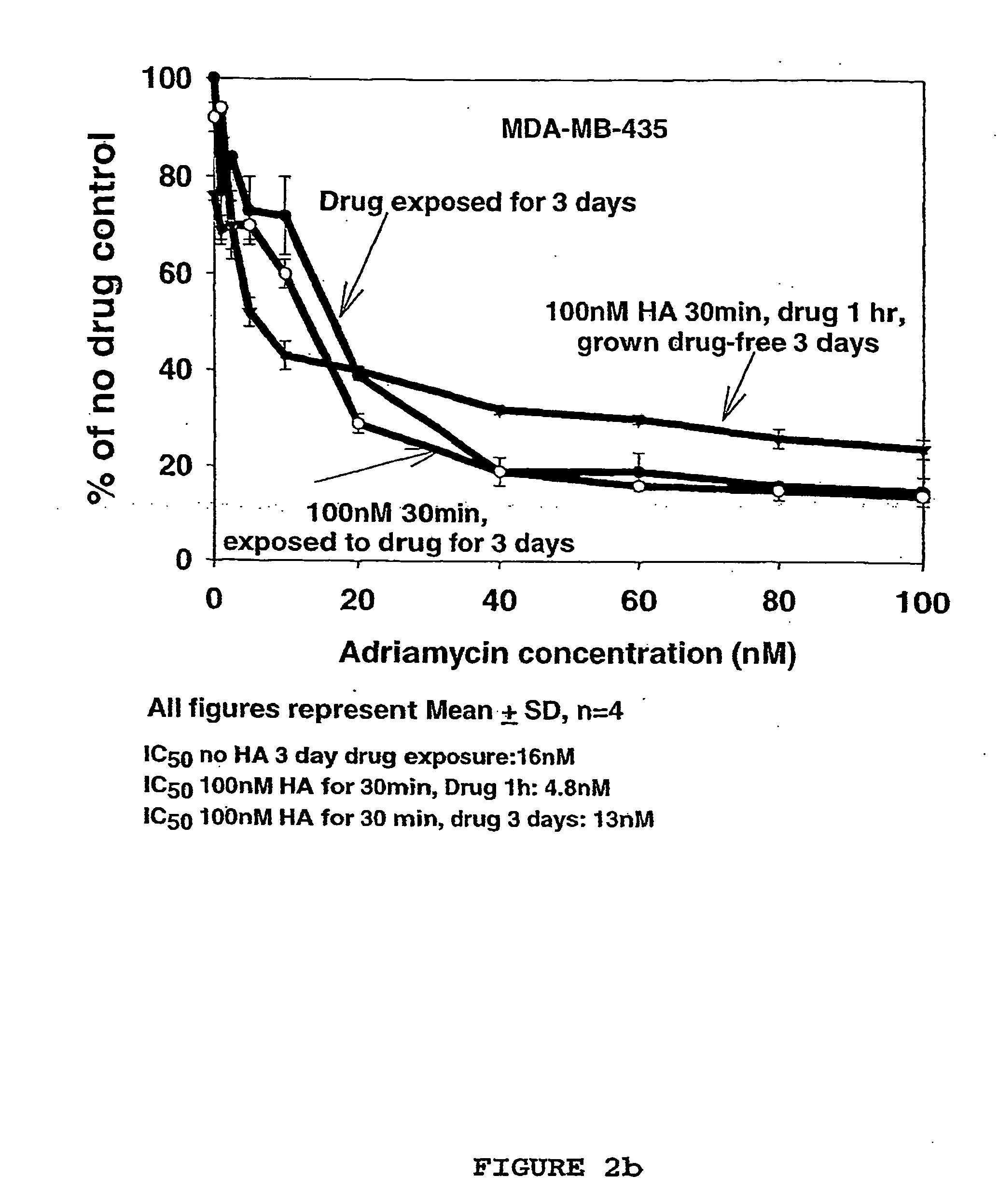
Hyaluronan as a cytotoxic agent, drug pre-sensitizer and chemo-sensitizer in the treatment of disease
InactiveUS20060178342A1Eliminate side effectsHeavy metal active ingredientsBiocideIrinotecanHyaluronic acid
Owner:ALCHEMIA ONCOLOGY PTY LTD
Compositions and methods for treating conditions of the nail unit
InactiveUS20060275230A1Improve blood supplyPromote circulationBiocideCosmetic preparationsMicroparticlePhase change
Owner:TALIMA THERAPEUTICS INC
Use Of Dipyridamole For Treatment Of Resistance To Platelet Inhibitors
InactiveUS20090048173A1Reduce decreaseBiocidePeptide/protein ingredientsDipyridamolePlatelet inhibitor
Owner:EISERT WOLFGANG +1
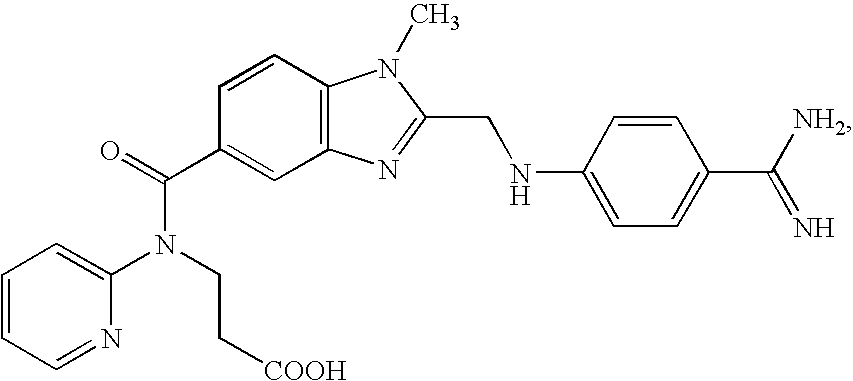
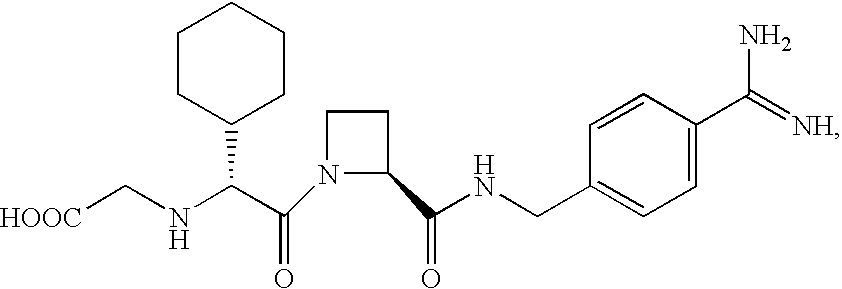
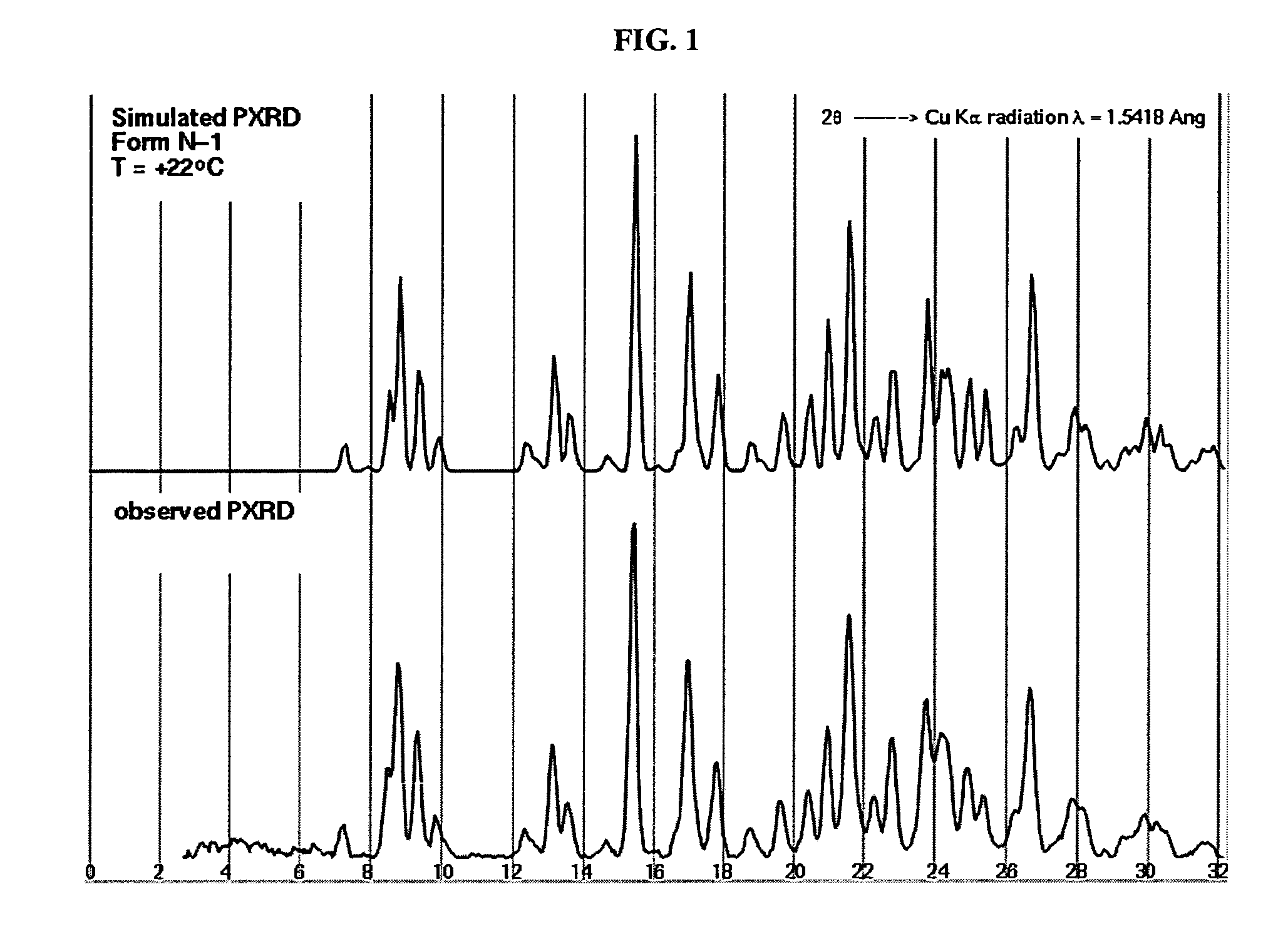
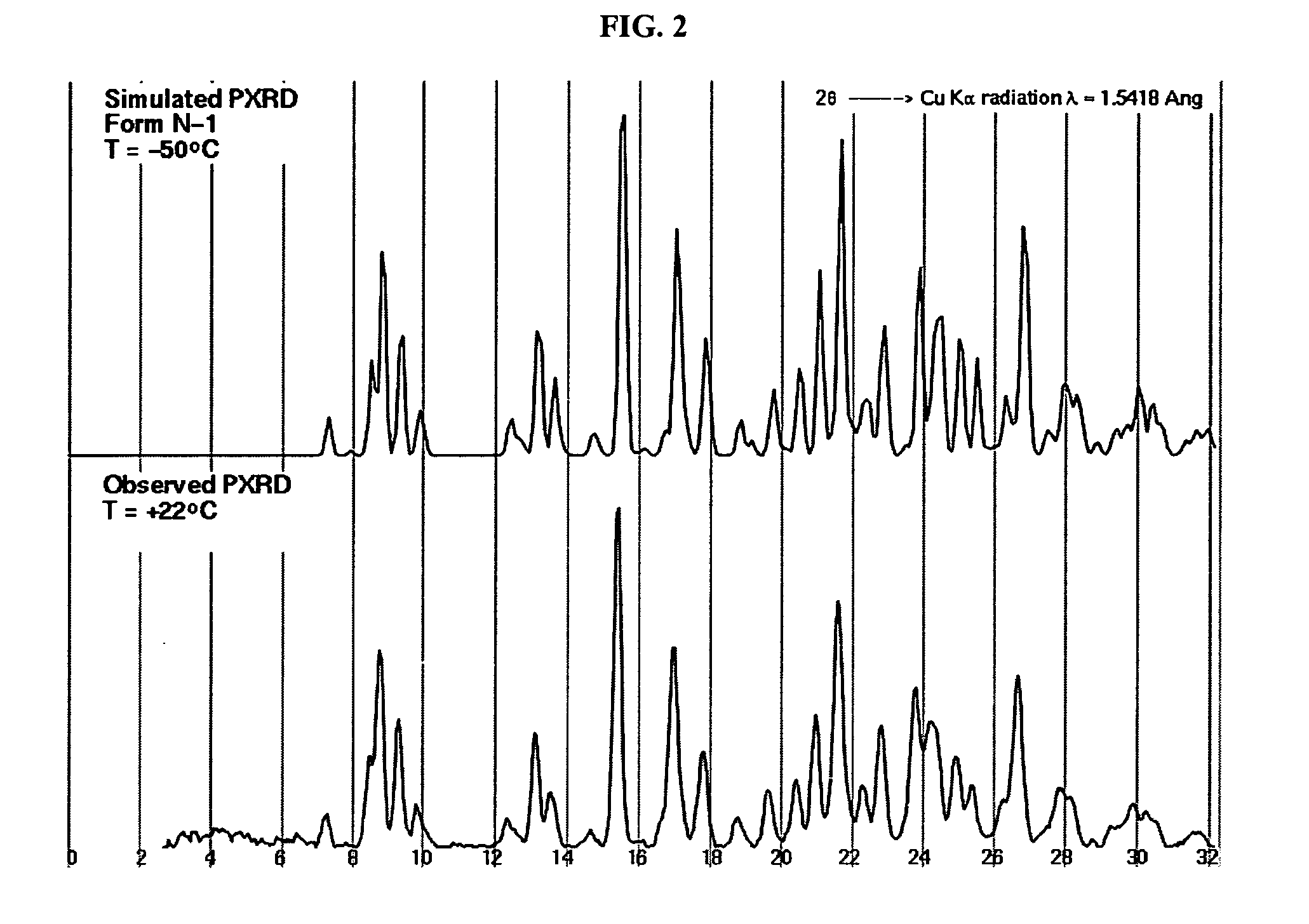
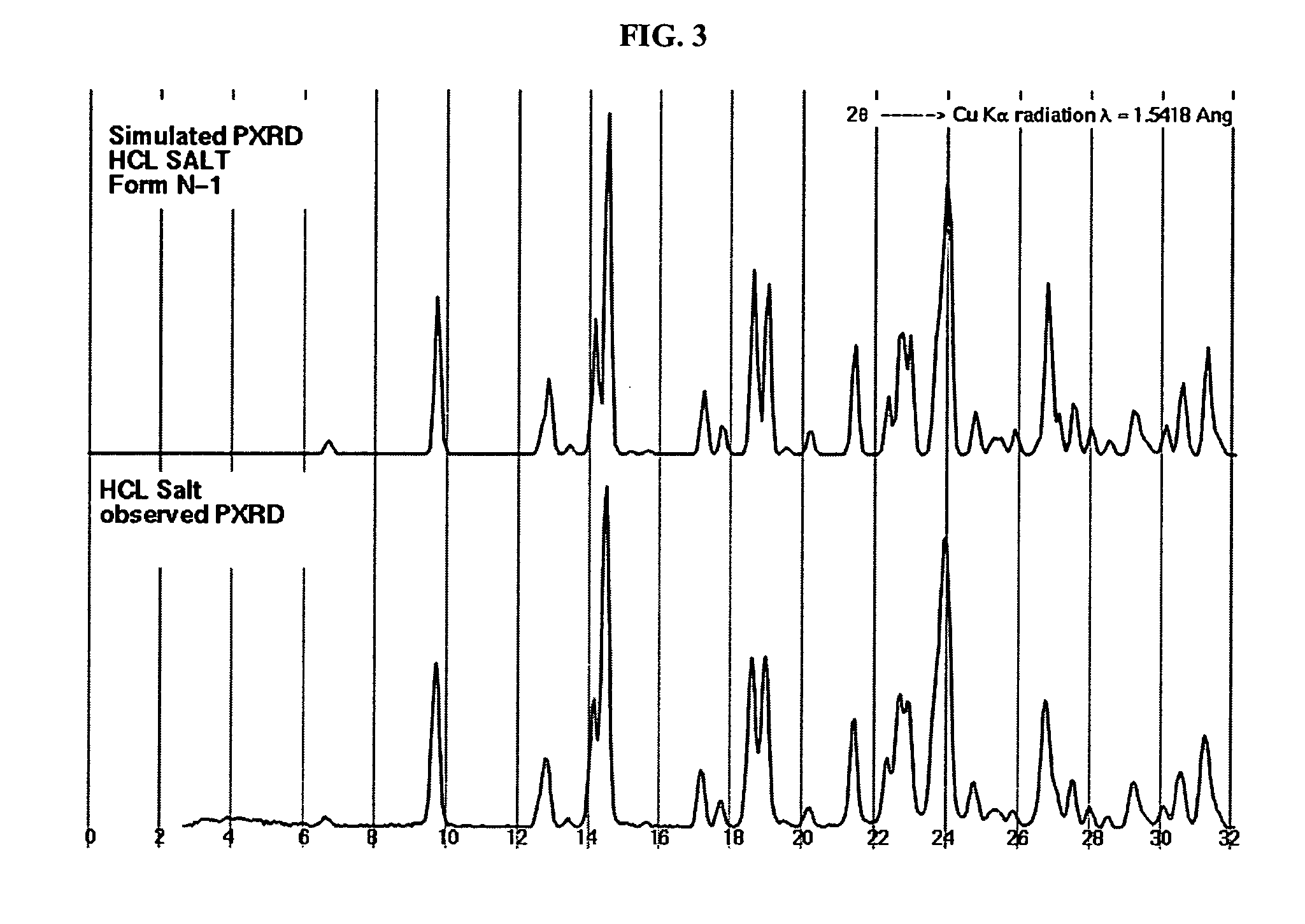
Crystalline forms and process for preparing spiro-hydantoin compounds
Owner:BRISTOL MYERS SQUIBB CO
Novel compounds as cannabinoid receptor ligands
Disclosed herein are cannabinoid receptor ligands of formula (I)wherein Y, X1, X2, X3, R1, and R2 are as defined in the specification. Compositions comprising such compounds, and methods for treating conditions and disorders using such compounds and compositions are also disclosed.
Owner:ABBVIE INC
Preparations and Methods for Ameliorating or Reducing Presbyopia
InactiveUS20100298335A1Ameliorating and reducing presbyopiaEfficient productionBiocideSenses disorderCholinesteraseAgonist
Owner:KAUFMAN HERBERT E
Amorphous and a crystalline form of genz 112638 hemitartrate as inhibitor of glucosylceramide synthase
The hemitartrate salt of a compound represented by the following structural formula: (Formula I Hemitartrate), which may be used in pharmaceutical applications, are disclosed. Particular single crystalline forms of the Formula (I) Hemitartrate are characterized by a variety of properties and physical measurements. As well, methods of producing crystalline Formula (I) Hemitartrate, and using it to inhibit glucosylceramide synthase or lowering glycosphingolipid concentrations in subjects to treat a number of diseases, are also discussed. Pharmaceutical compositions are also described.
Owner:GENZYME CORP
Gyrase Inhibitors and Uses Thereof
Owner:VERTEX PHARMA INC
Propargyl nitroxides and indanyl nitroxides and their use for the treatment of neurologic diseases and disorders
Owner:TEVA PHARMA IND LTD
USE OF PHTHALIMIDE AND/OR SULPHONAMIDE DERIVATIVES IN THE TREATMENT OF DISEASES WHICH REQUIRE REDUCING THE TNF-alpha LEVELS AND AN EXOGENOUS SOURCE OF NITRIC OXIDE, PHTHALIMIDE DERIVATIVES, SULPHONAMIDE DERIVATIVES, AND A METHOD FOR OBTAINING A SULPHONAMIDE DERIVATIVE
InactiveUS20100324107A1Improve the quality of lifeBiocideOrganic chemistryPhthalocyanine derivativesNitric oxide
Owner:UNIV ESTADUAL DE CAMPINAS UNICAMP +2
Mucosal Bioadhesive SLow Release Carrier for Delivering Active Principles
A mucosal bioadhesive slow release carrier comprising an active principle and devoid of starch, lactose, which can release the active principal for a duration of longer than 20 hours. This bioadhesive carrier contains a diluent, an alkali metal alkylsulfate, a binding agent, at least one bioadhesive polymer and at least one sustained release polymer, as well as a method for its preparation.
Owner:ONXEO SA
Compositions for the treatment of male erectile dysfunction
InactiveUS6482426B1Improved solubility profileLower requirementBiocideElcosanoid active ingredientsGlycinePenile Tumescence
Improved drug compositions and methods useful in the treatment of male erectile dysfunction. An optimized mixture of the drugs phentolamine mesylate, papaverine hydrochloride, and alprostadil in a buffer containing L-arginine and glycine is to be injected into the penile tissue to produce an erection in otherwise impotent men.
Owner:REPROS THERAPEUTICS
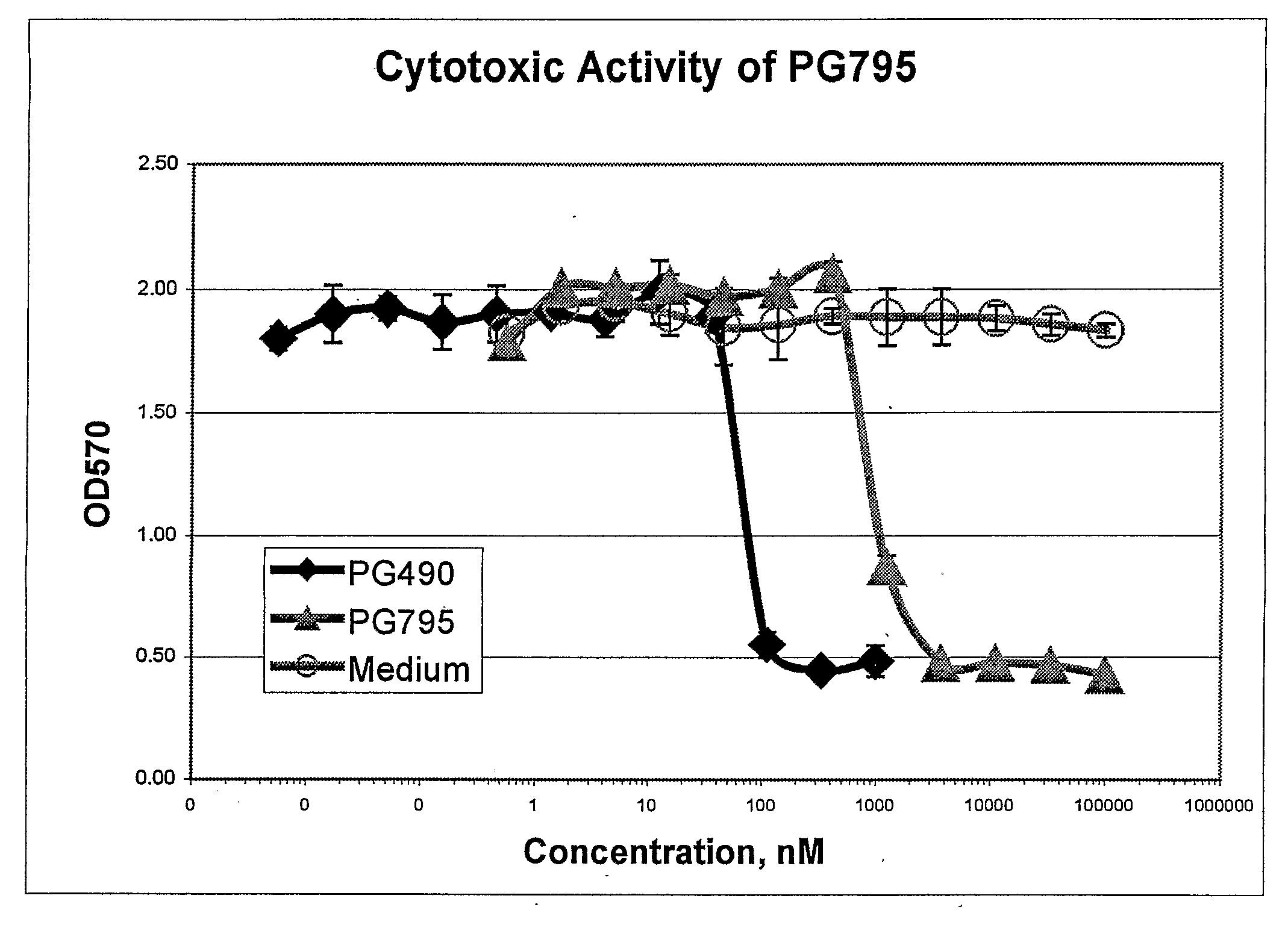
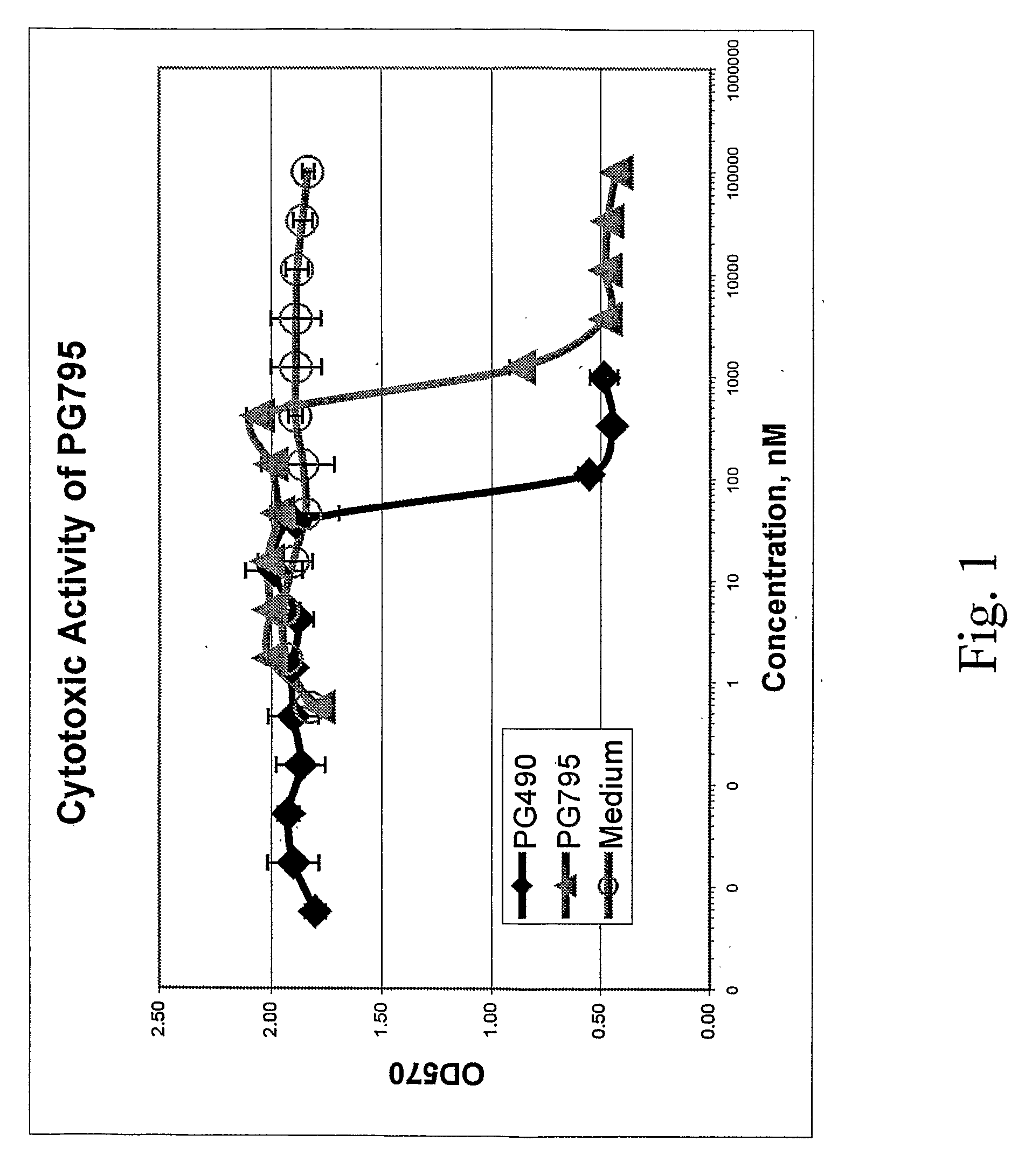
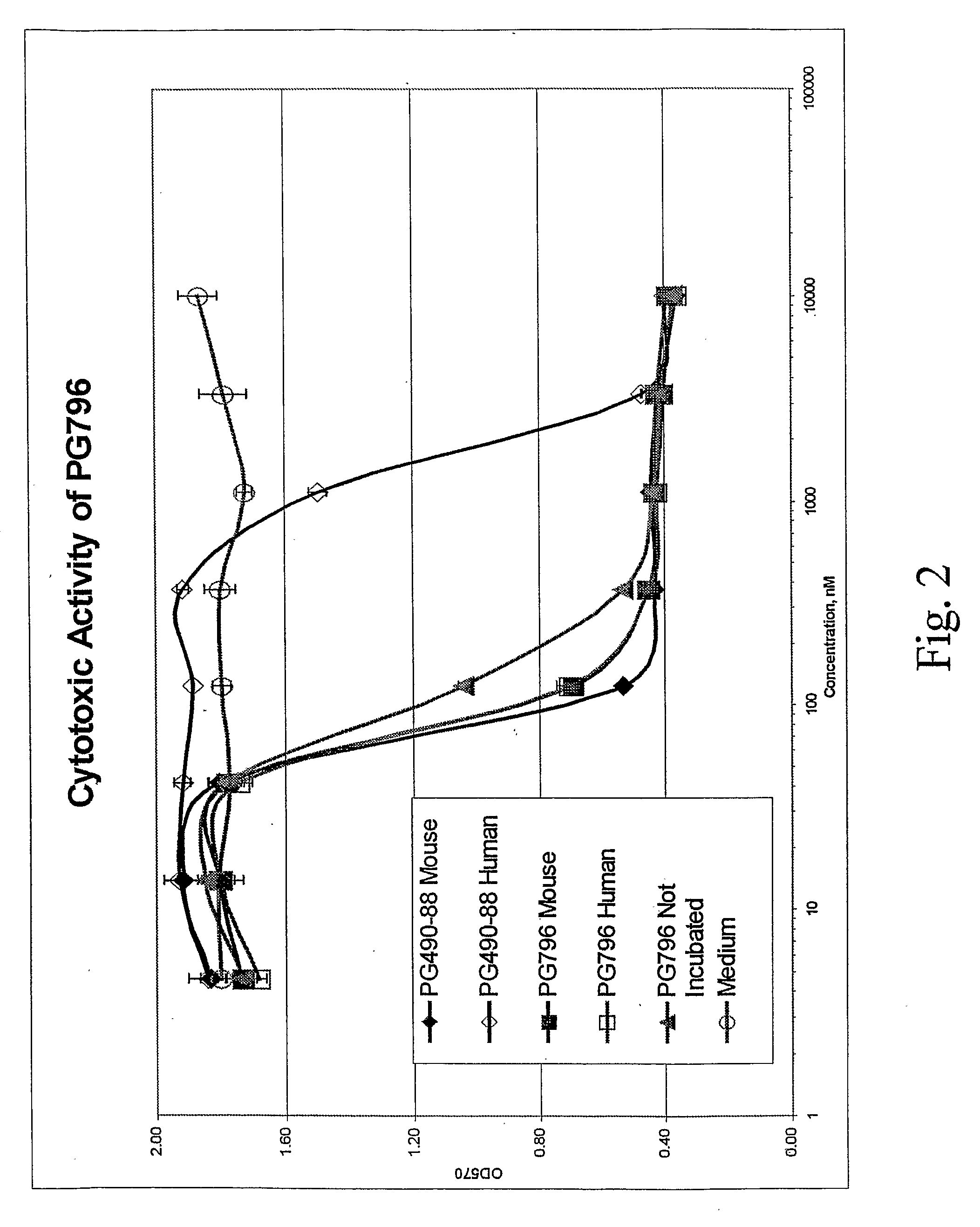
Tripolide Lactone Ring Derivatives as Immunomodulators and Anticancer Agents
Owner:PHARMAGENESIS
Analogs and prodrugs of buprenorphine
InactiveUS20040192714A1Improve lipophilicityImprove solubilityBiocideOrganic chemistryBuprenophineProdrug
Owner:PURDUE PHARMA LP
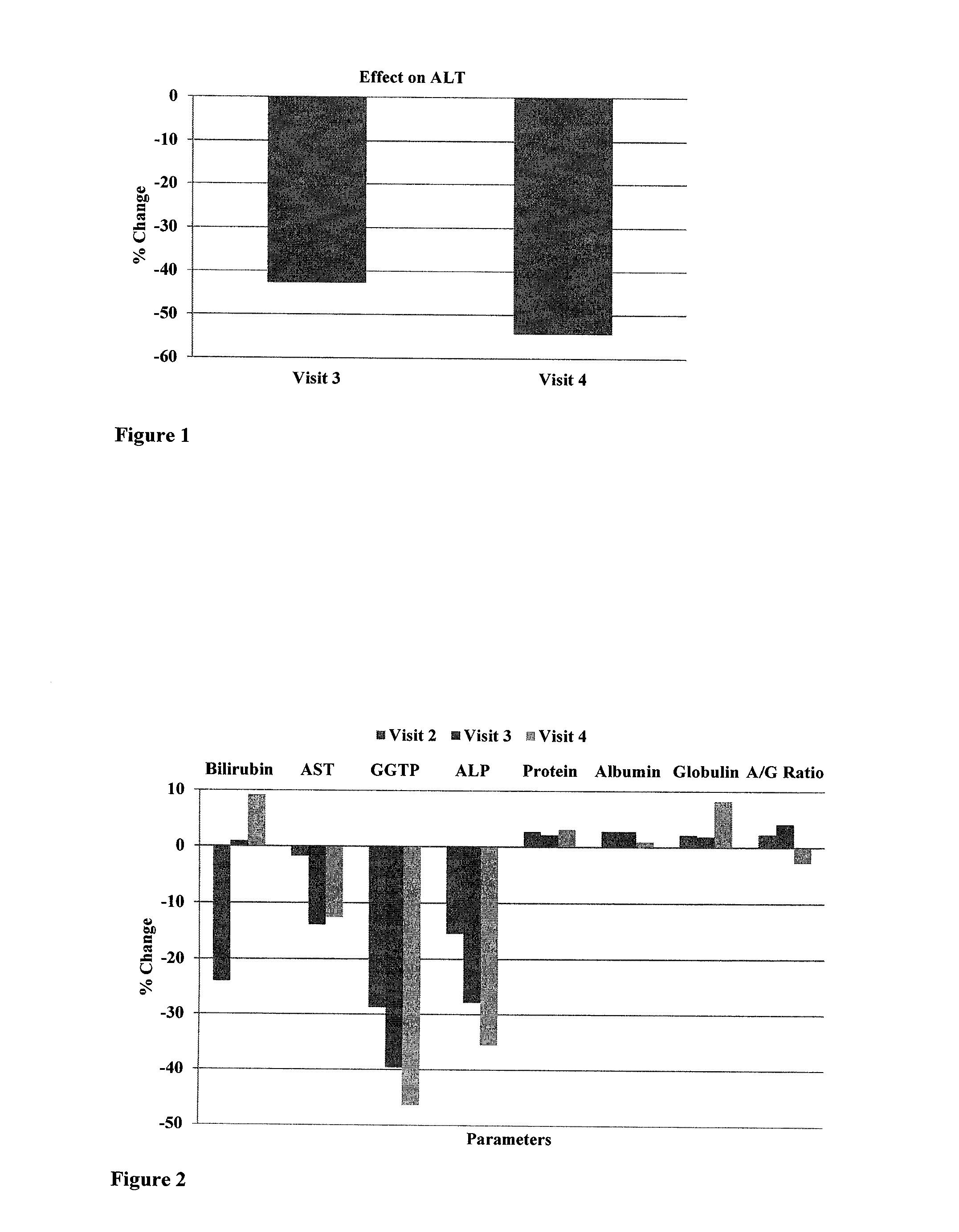
A novel composition for nonalcoholic fatty liver disease (NAFLD)
Owner:CADILA HEALTHCARE LTD
Novel imidazolidinedione derivatives and use thereof as drugs
Owner:SENJU PHARMA CO LTD
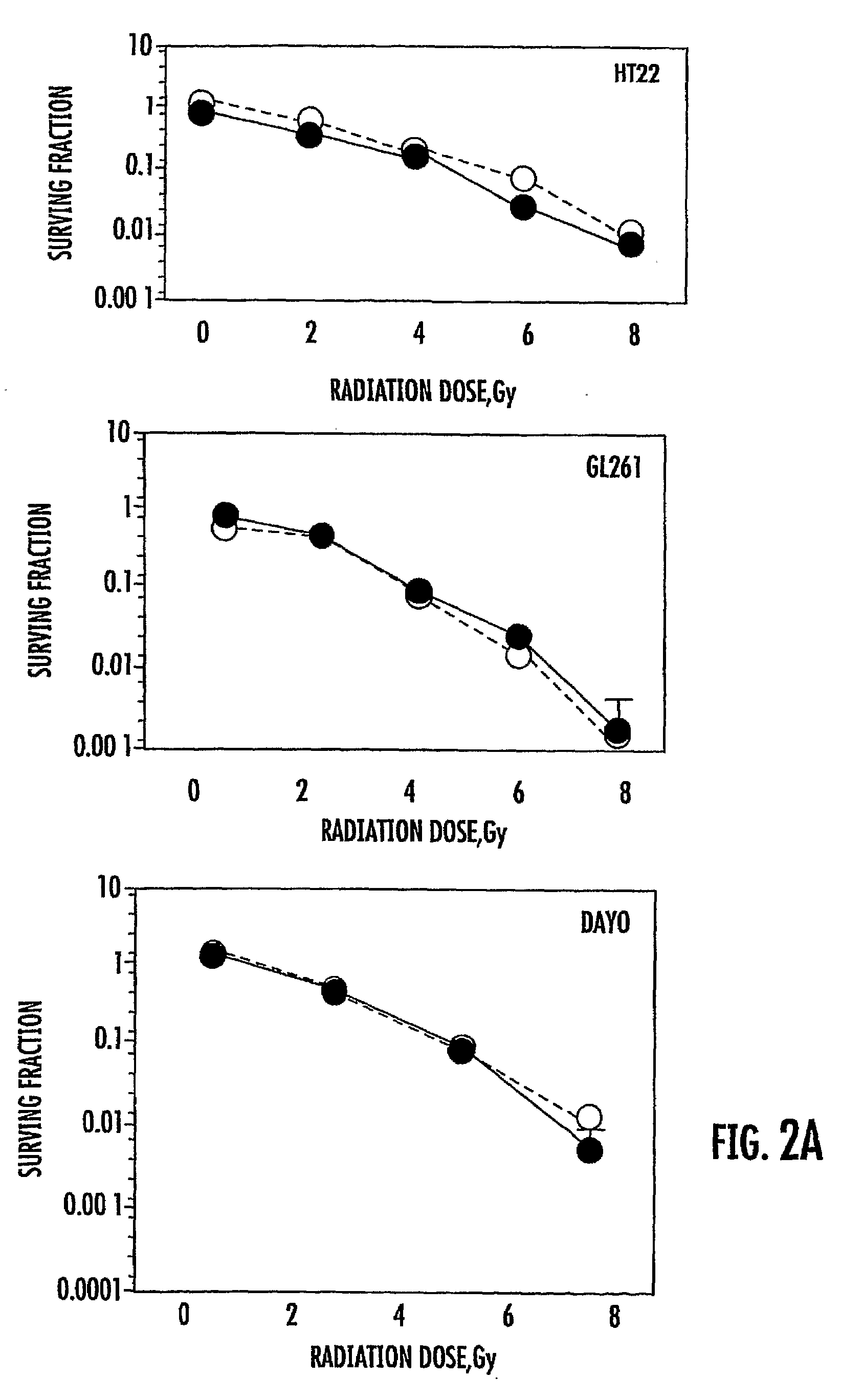
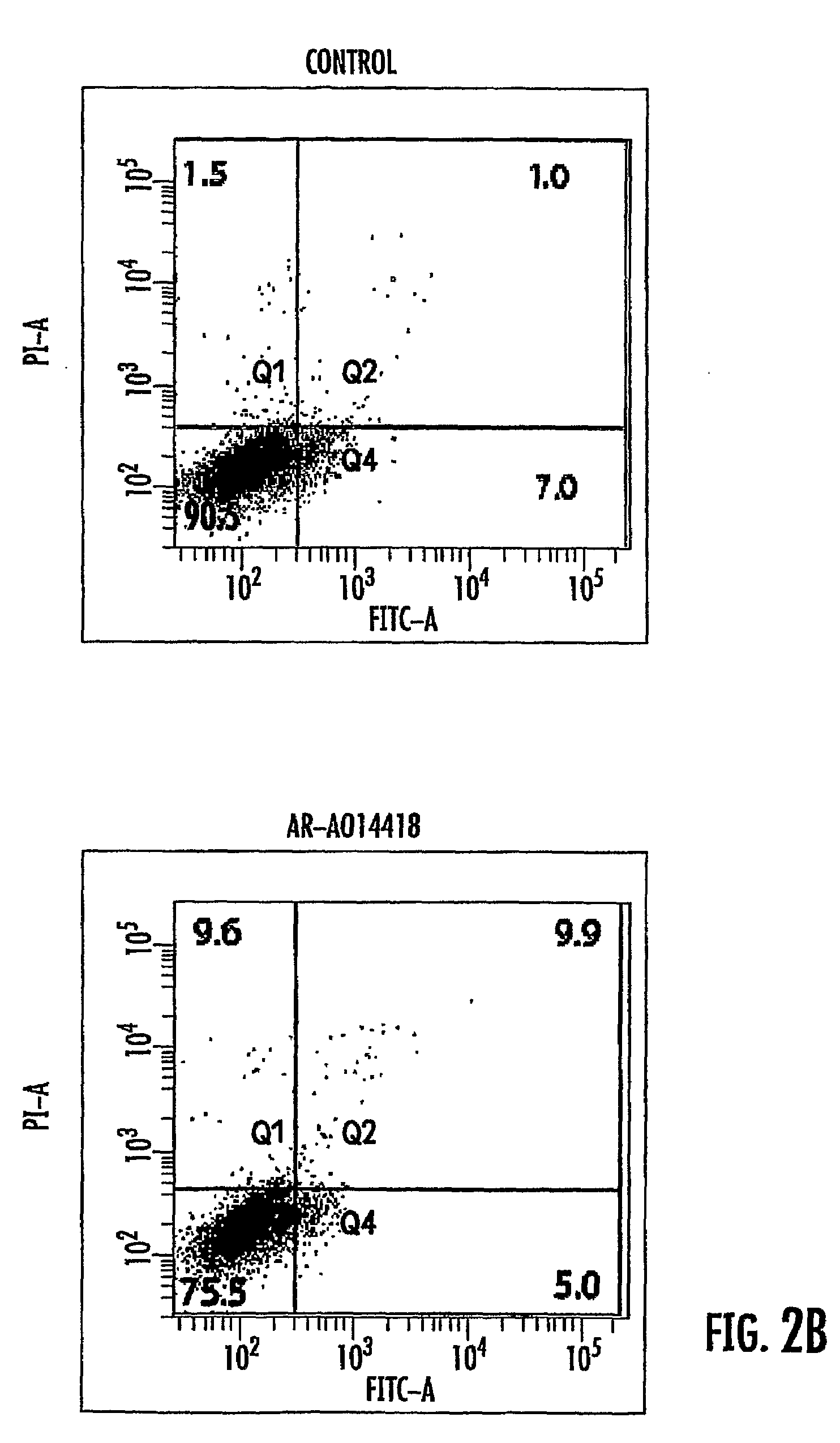
Use of GSK3 inhibitors in combination with radiation therapies
Owner:VANDERBILT UNIV
Fast dissolving pharmaceutical composition
ActiveUS20150045300A1High tensile strengthEasy to disassembleBiocidePeptide/protein ingredientsInulinMedicine
Owner:FERRING BV
Preparation methods of sulbenicillin sodium and injection thereof
InactiveCN101805356AReduce lossesEasy to operate the machineAntibacterial agentsOrganic chemistrySulbenicillinIon exchange
The invention discloses a preparation method of sulbenicillin sodium, which comprises the following steps of: sulfonating, hydrolyzing, crystallizing, ion-exchanging, acidylating, condensing, extracting and salifying to obtain sulbenicillin sodium. The method has the advantages of simple technological steps, fewer reaction impurities and high product purity, and the yield is higher than 50%. Meanwhile, the invention also relates to a preparation method of a sulbenicillin sodium injection.
Owner:HUNAN ERKANG XIANGYAO PHARMA
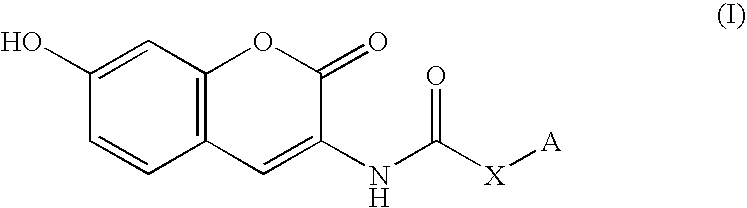
Coumarin derivative
InactiveUS20050054717A1Good effectEliminate side effectsBiocideHeavy metal active ingredientsArylSulfur
Owner:INST OF MEDICINAL MOLECULAR DESIGN
Compositions comprising multiple bioactive agents, and methods of using the same
Owner:DEBIOPHARM INTERNATIONAL SA
Nucleoside prodrug and application thereof
ActiveCN113999237AImprove oral bioavailabilityImprove performanceOrganic chemistryAntiviralsAnimal virusOral treatment
The invention relates to a nucleoside prodrug capable of being orally taken for treating mammalian virus infection, and especially relates to a compound shown as a formula (I) or pharmaceutically acceptable salt or stereoisomer thereof, or a pharmaceutical composition thereof, and application of the compound or the composition in preparation of drugs for treating, inhibiting or preventing diseases caused by virus infection.
Owner:RISEN SUZHOU PHARMA TECH CO LTD
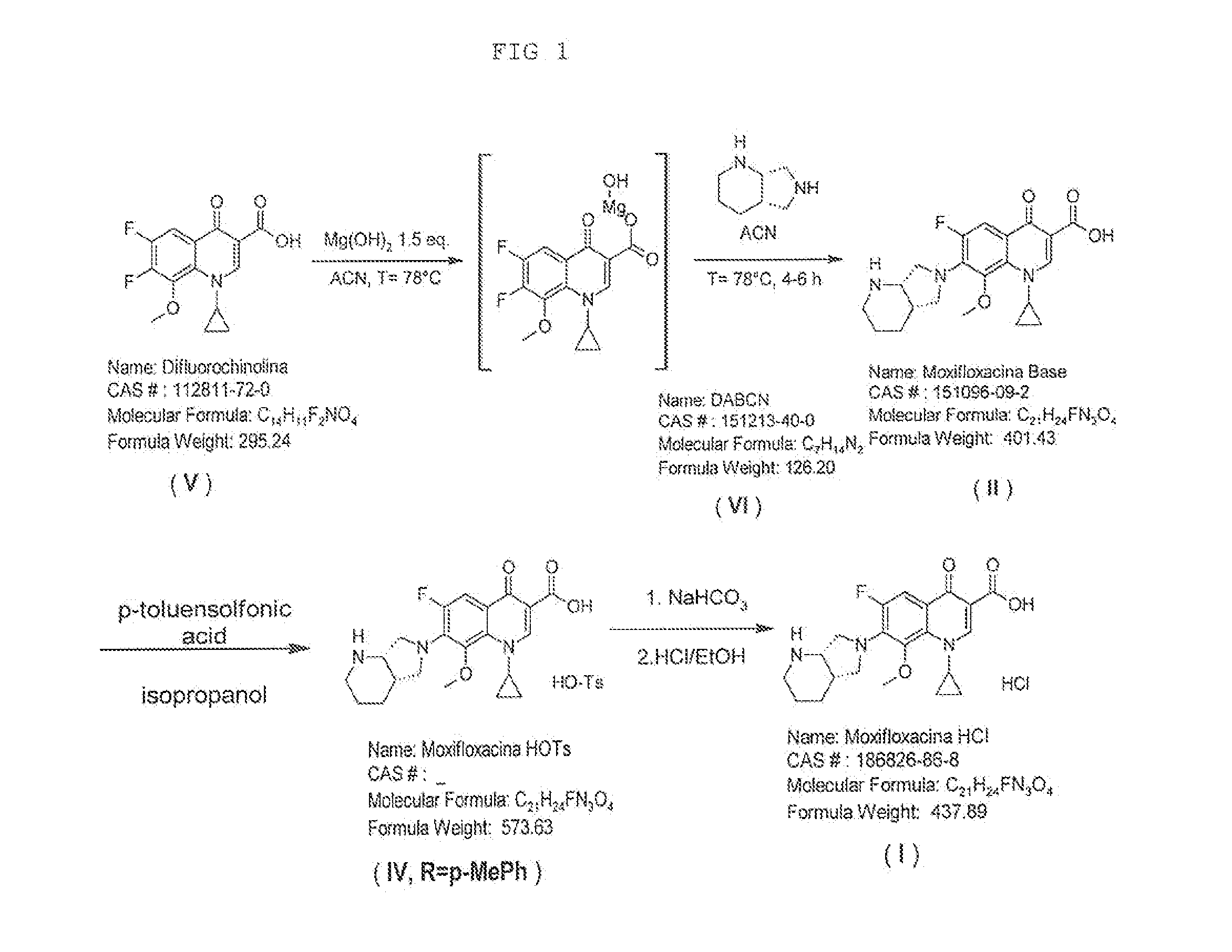
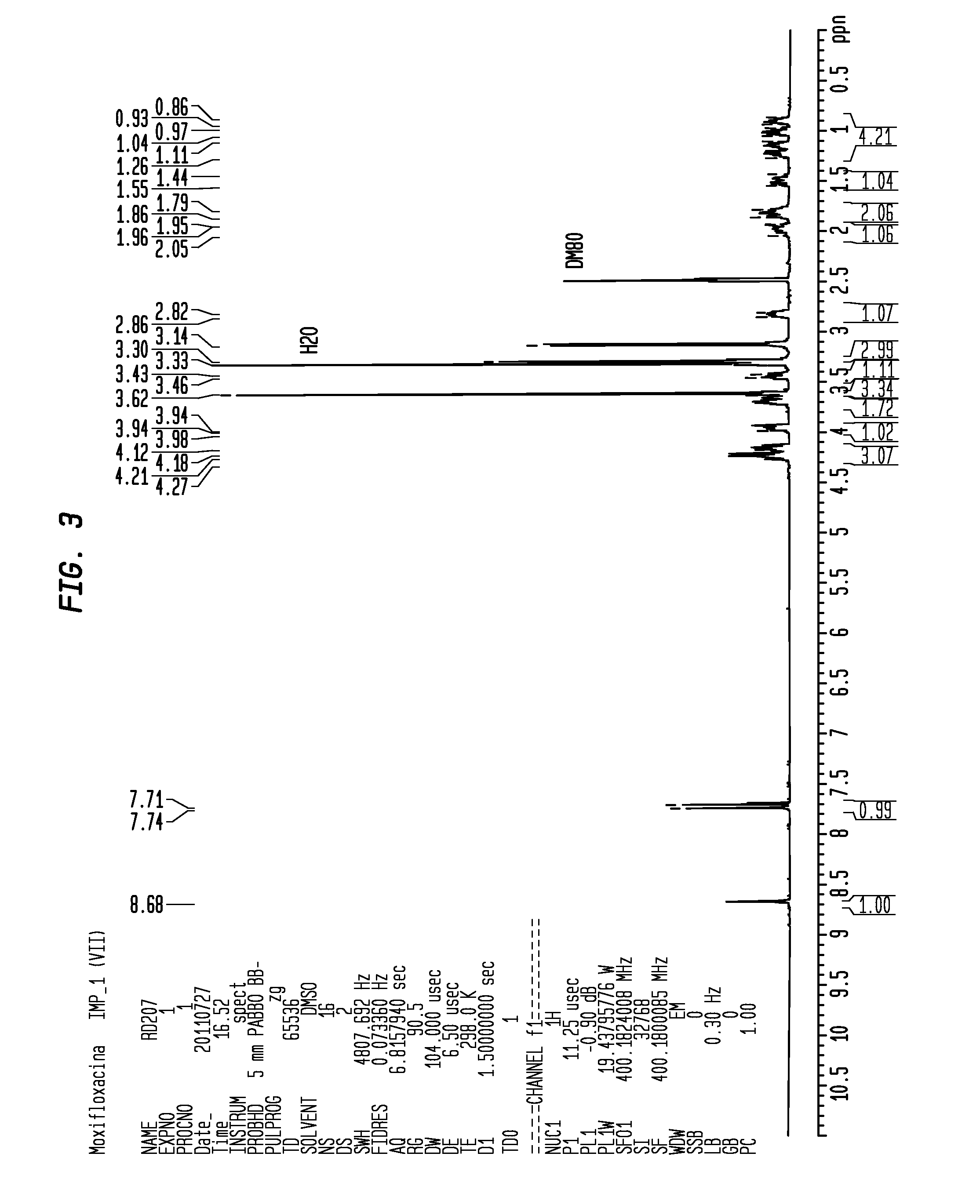
Moxifloxacin hydrochloride compounds and intermediates and methods for making same
Owner:F I S FAB ILTALIANA SINTETICI SPA
Acne-removing liquid containing tea tree flower extract
InactiveCN106726998ANo side effectsLong-term useSalicyclic acid active ingredientsCosmetic preparationsSide effectAdditive ingredient
Owner:NANJING INST FOR THE COMPREHENSIVE UTILIZATION OF WILD PLANTS CHINA COOP
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap