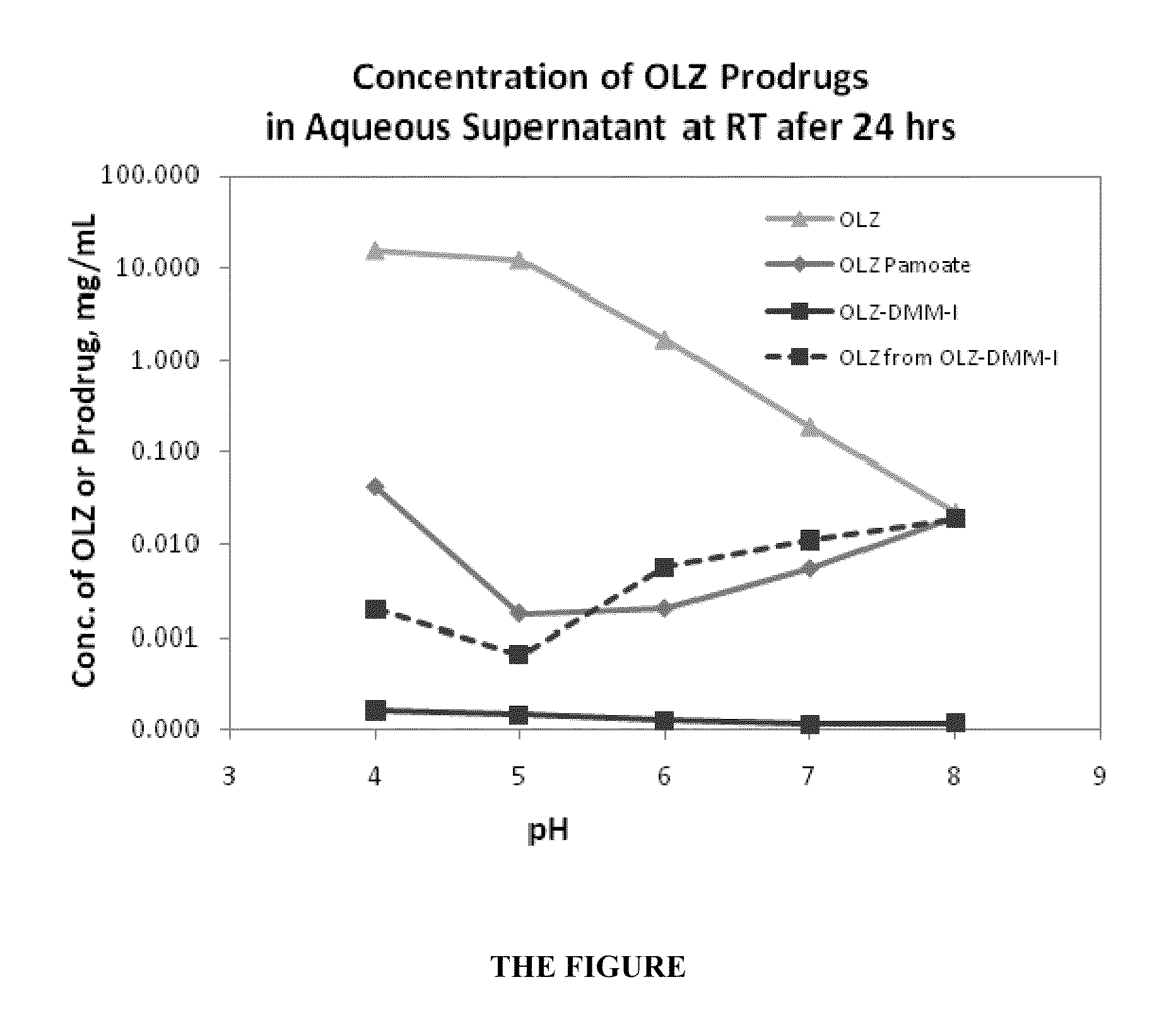
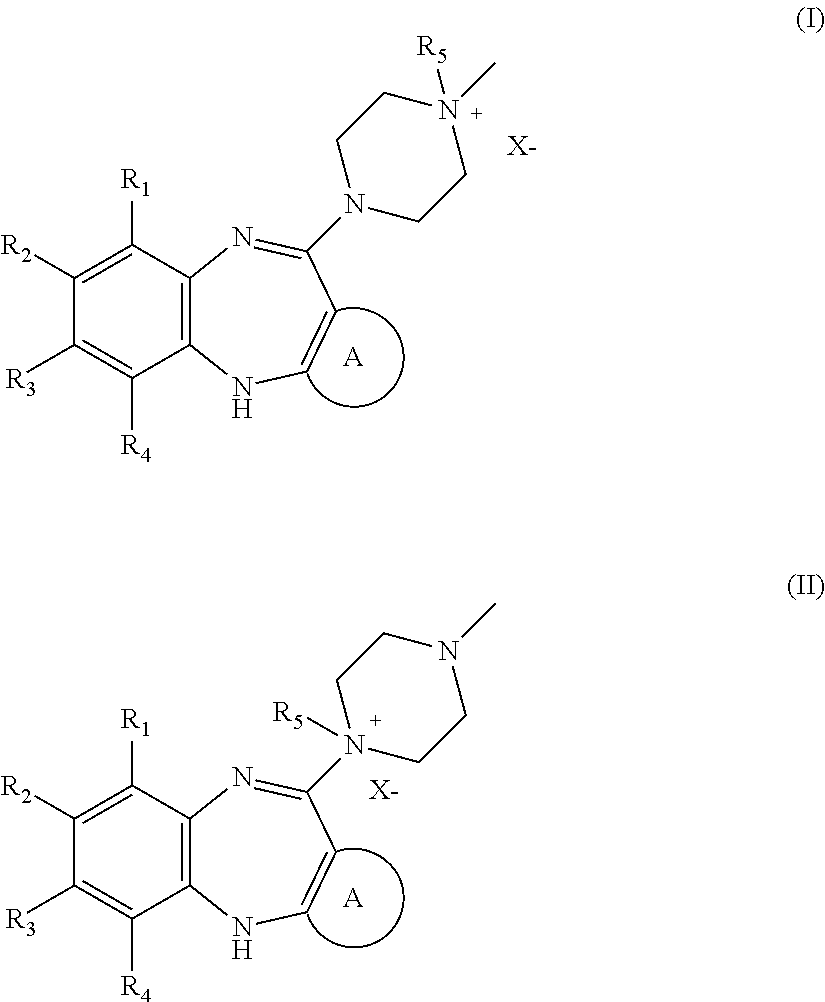
Diaryldiazepine Prodrugs for the Treatment of Neurological and Psychological Disorders
a diaryldiazepine and drug technology, applied in the field of diaryldiazepine drug prodrugs, can solve the problems of unreliable control of the degradation process required for the release of active agents, not all of these drugs have pharmacokinetic properties, and complicate dosage reproducibility, so as to reduce the solubility and polarity of the prodrug compound. , the effect of prolonging the action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1-methyl-4-(2-methyl-10H-benzo[b]thieno[2,3-e][1,4]diazepin-4-yl)-1-((stearoyloxy)methyl)piperazin-1-ium iodide (Compound 1)
A. Formation of Acid Chloride
[0133]To a stirred suspension of stearic acid (20 g, 70.3 mmol) in dichloromethane (100 mL) was added oxalyl chloride (8.92 mL, 105.5 mmol). 1 drop dimethylformamide was added and the reaction stirred at room temperature for 3 hours. The solvent was removed in vacuo and the resulting product used in the next step without further purification.
[0134]1H-NMR (CDCl3) δ 2.87 (t, 2H), 1.65-1.70 (m, 2H), 1.20-1.40 (m, 28H), 0.87 (3H, t).
B. Formation of Chloromethyl Alkyl Ester
[0135]Paraformaldehyde (2.11 g, 70.3 mmol) and zinc chloride (258 mg) were added to the acid chloride prepared above and the reaction mixture was heated at 65° C. for 16 hours and then allowed to cool to room temperature. Dichloromethane (200 mL) and saturated aqueous NaHCO3 (70 mL) were added. The aqueous emulsion was extracted with dichloromethane (2×50 mL) and the comb
example 2
1-methyl-4-(2-methyl-10H-benzo[b]thieno[2,3-e][1,4]diazepin-4-yl)-1-((palmitoyloxy)methyl)piperazin-1-ium iodide (Compound 2)
[0142]This compound was synthesized employing palmitoyl chloride. The product precipitated from the reaction mixture to give Compound 2 (1.23 g, endotherm peak in the DSC at 164° C.).
[0143]1H-NMR (CDCl3) δ 7.02-6.89 (3H, m), 6.67 (1H, dd), 6.35 (1H, s), 5.83 (2H, s), 5.32 (1H, s), 4.03-3.96 (2H, m), 3.79-3.71 (6H, m), 3.56 (3H, s), 2.52 (2H, t), 2.31 (3H, s), 1.64 (2H, t), 1.39-1.21 (24H, m), 0.87 (3H, t).
example 3
1-((butyryloxy)methyl)-1-methyl-4-(2-methyl-10H-benzo[b]thieno[2,3-e][1,4]diazepin-4-yl)piperazin-1-ium iodide (Compound 3)
[0144]This compound was synthesized employing butyryl chloride instead of stearyl chloride. The final precipitated from the reaction mixture to give Compound 3 (1.8 g, endotherm peak in the DSC at 203.2° C.).
[0145]1H-NMR (d6-DMSO) δ 6.77-6.88 (m, 3H), 6.65-6.69 (m, 1H), 6.37 (s, 1H), 5.42 (s, 2H), 3.78-3.89 (m, 2H), 3.45-3.60 (m, 6H), 3.16 (s, 3H), 2.51 (t, 2H), 2.25 (s, 3H), 1.57 (st, 2H), 0.89 (t, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap