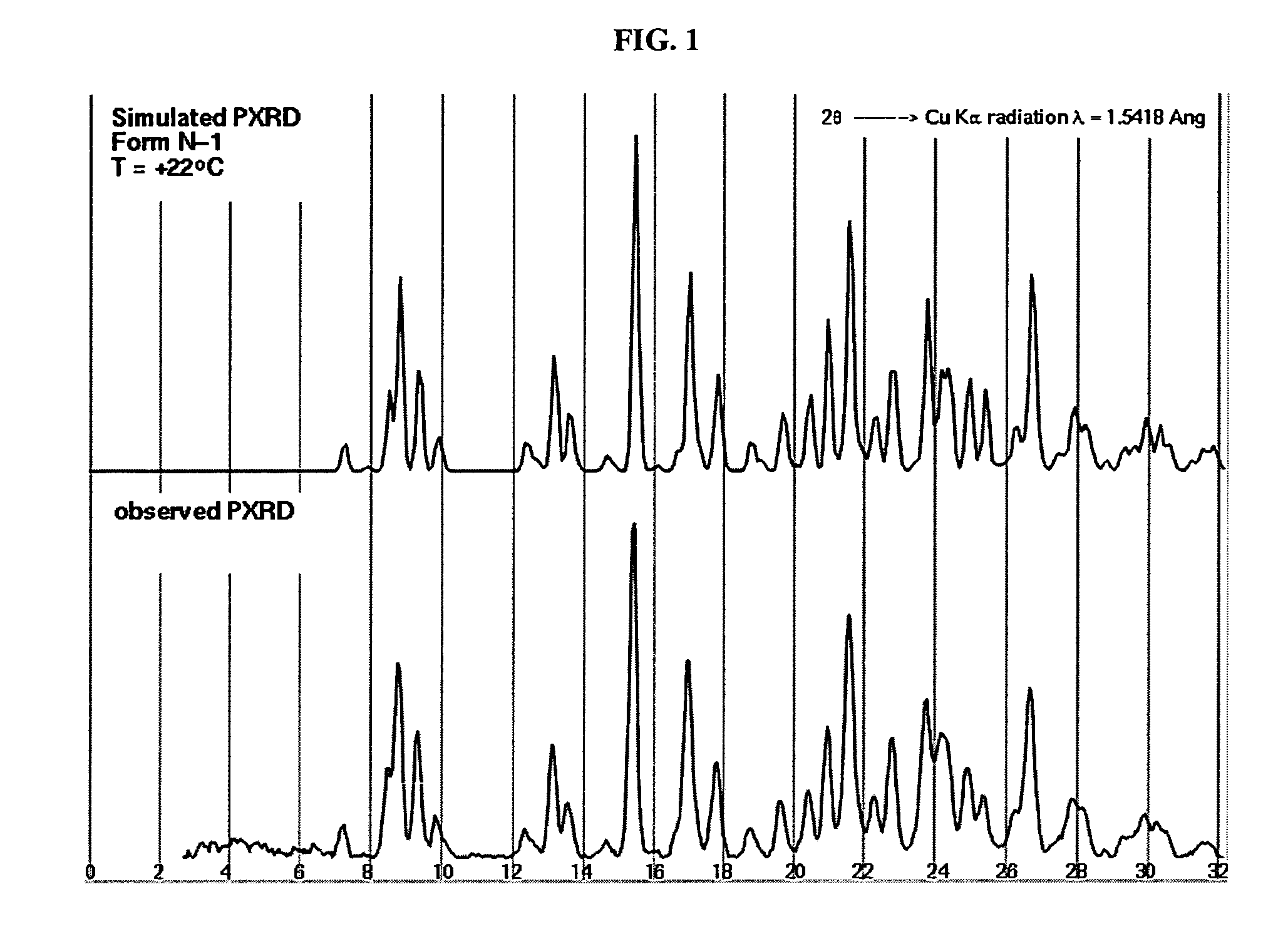
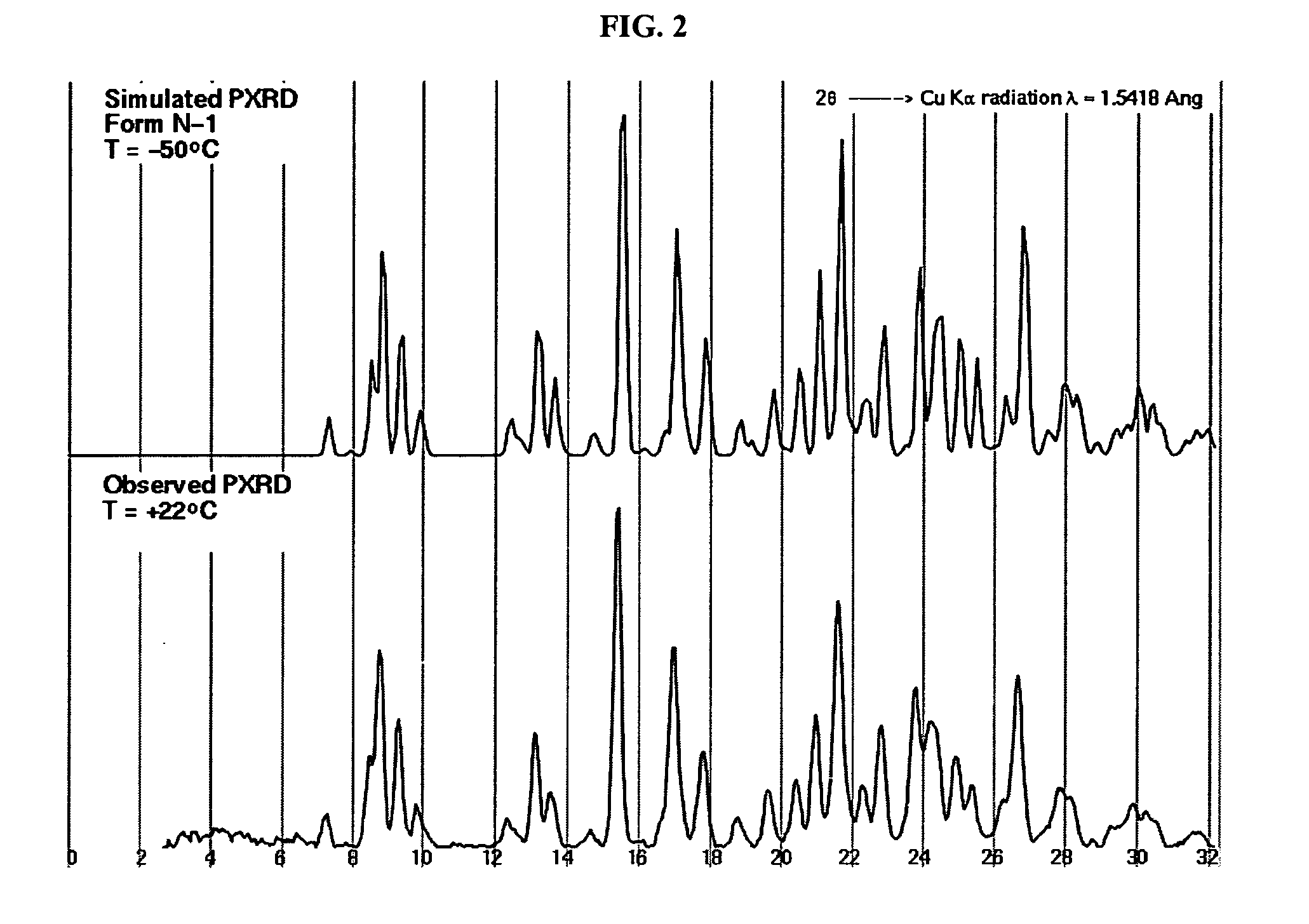
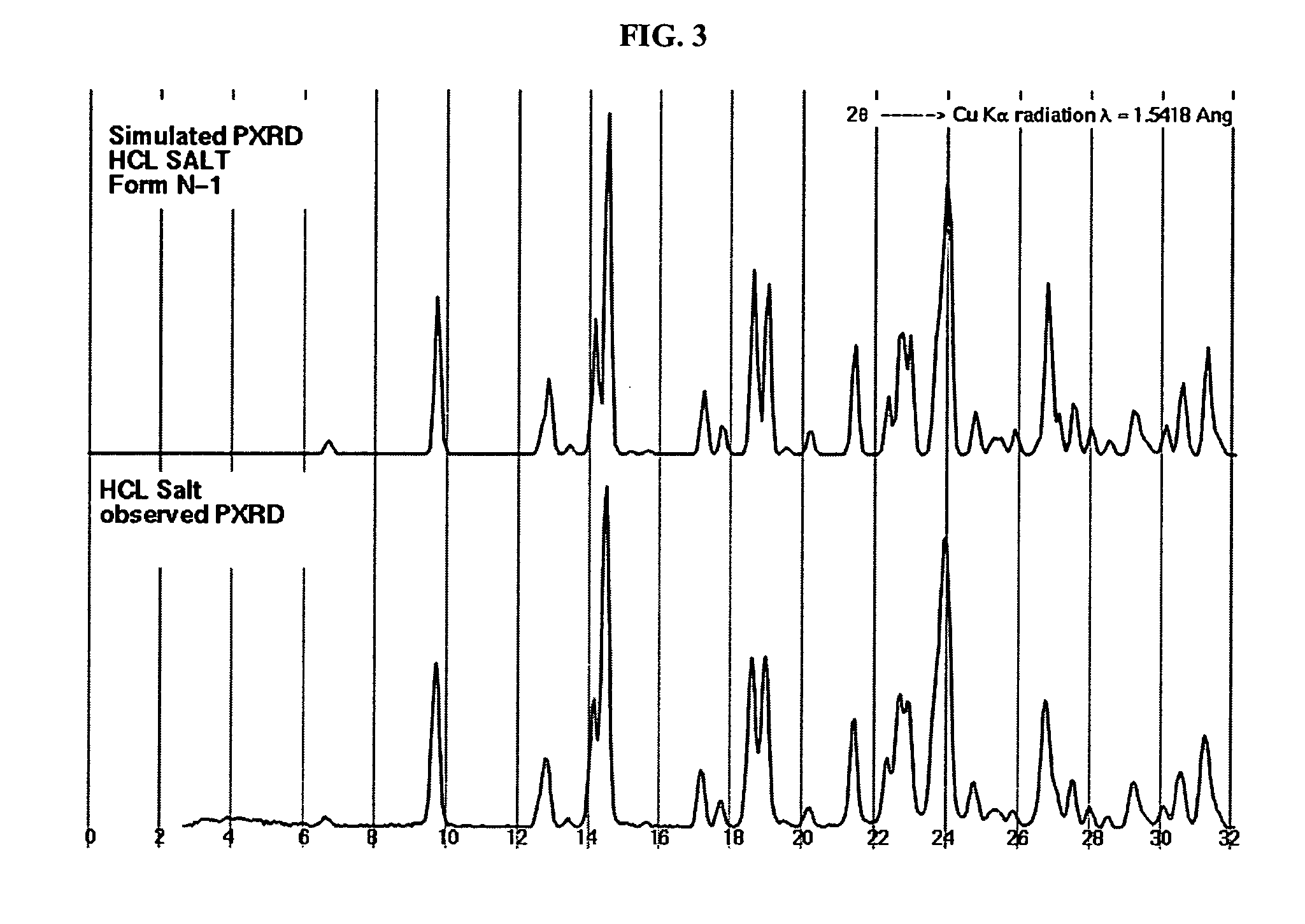
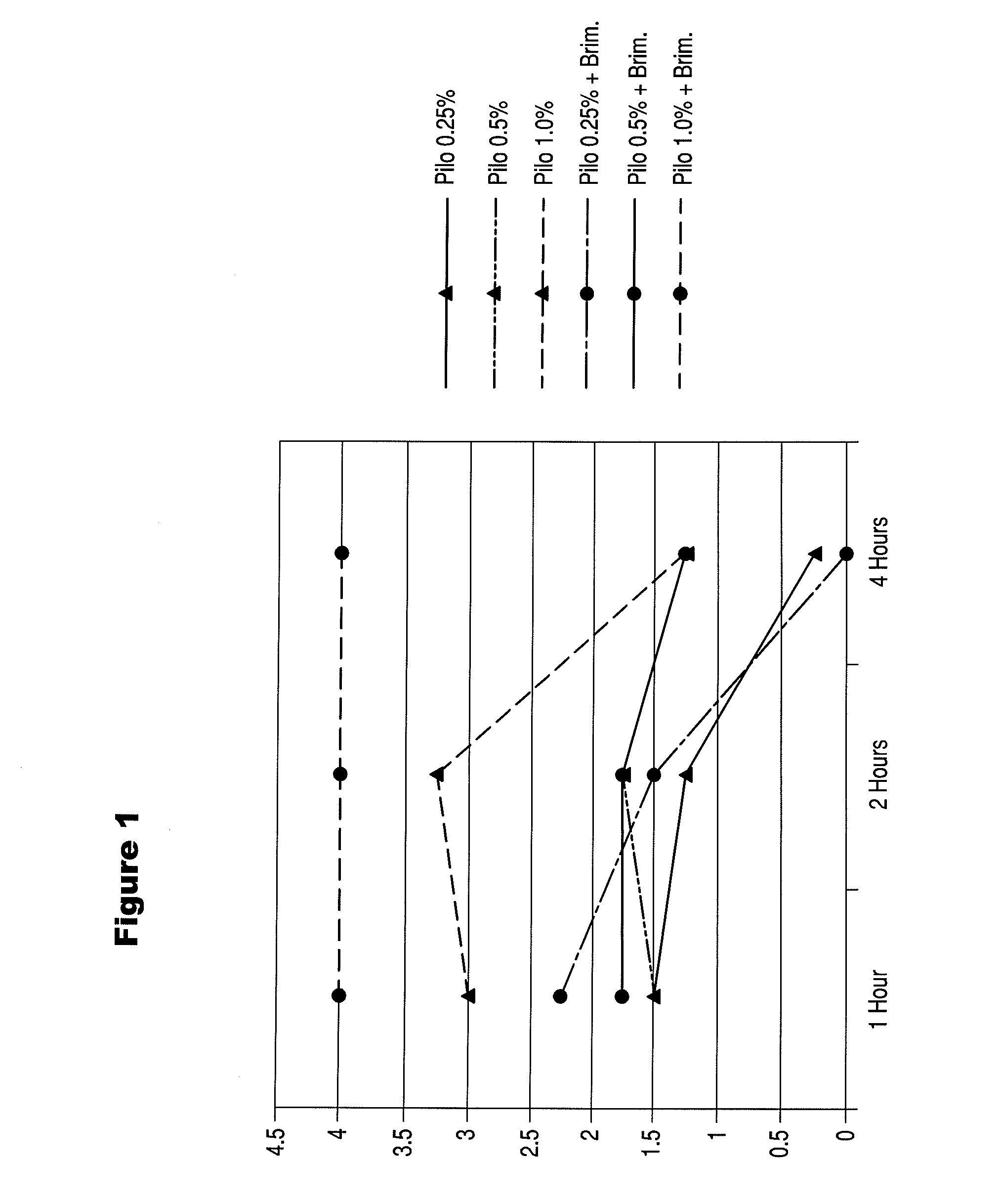
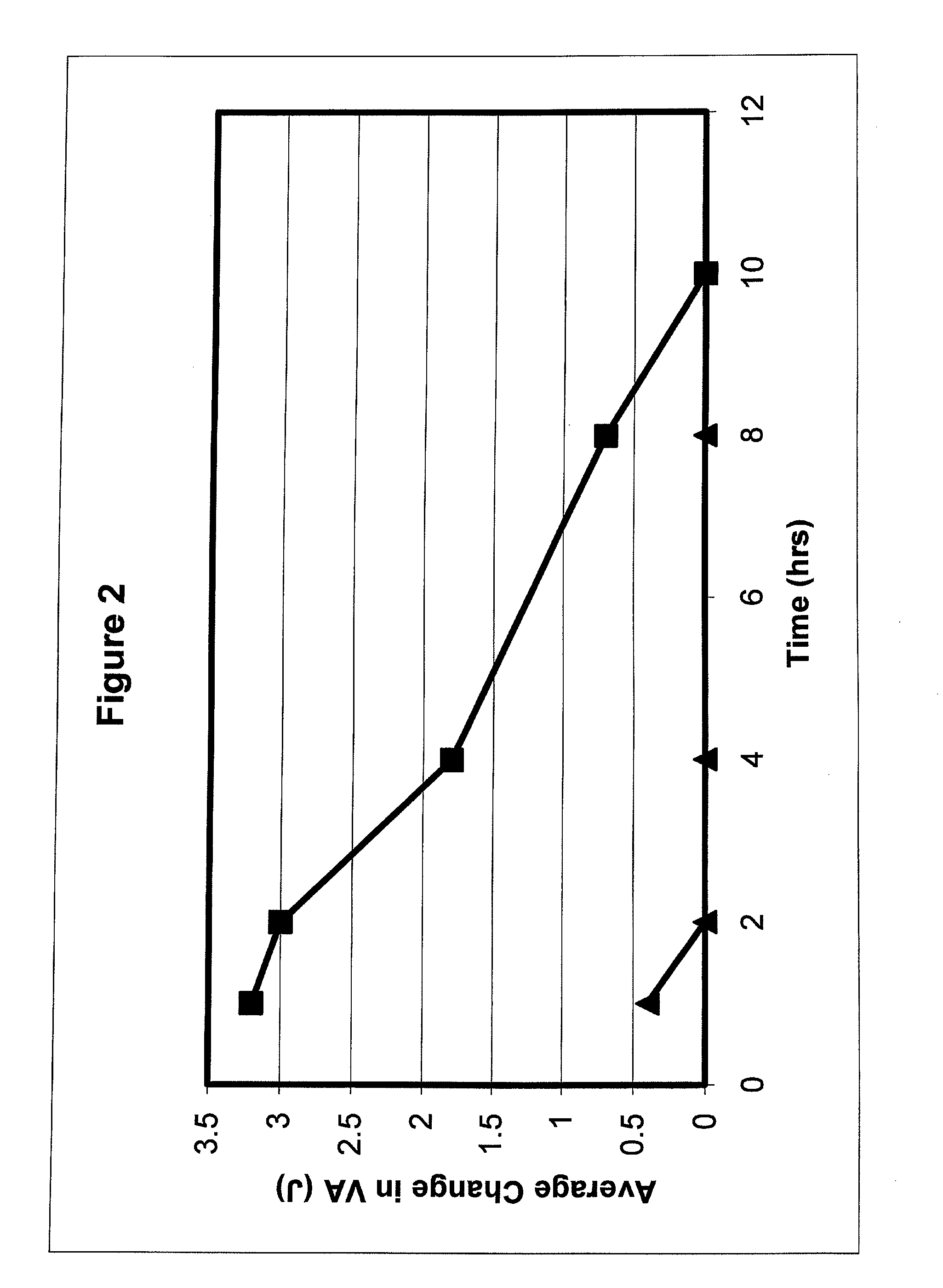
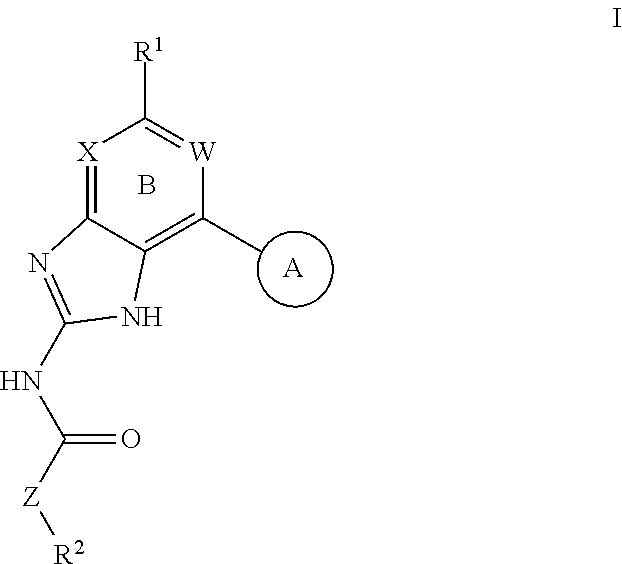
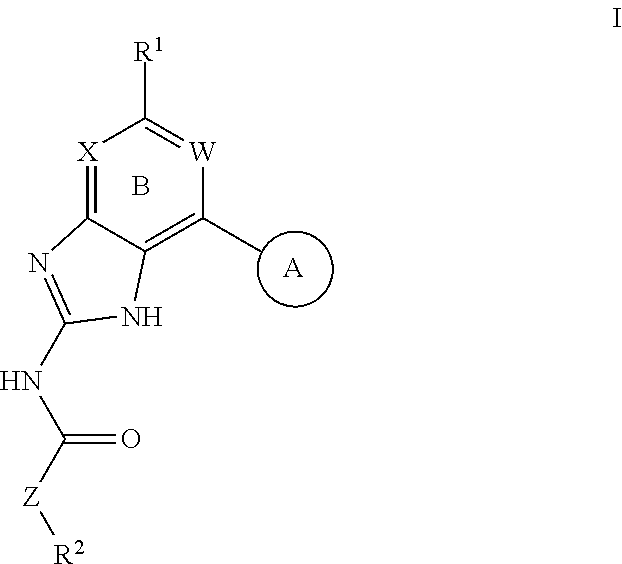
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
289results about "Plant growth regulators" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Beneficial effects of increasing local blood flow
InactiveUS20110028548A1Increase oxygenationImprove tissue nutritionBiocidePeptide/protein ingredientsArginineNitric oxide
Owner:STRATEGIC SCI & TECH
Child's cleaning implement comprising a biological extract
Owner:THE PROCTER & GAMBLE COMPANY
Compositions and methods for weight loss
InactiveUS20050238654A1Normalize fatNormalize carbohydrate metabolismBiocideMetabolism disorderDietary supplementWeight decreasing
Owner:TAKEDA YOSHINORI
Compositions for sustrained release of nitric oxide, methods of preparing same and uses thereof
The invention provides compositions for releasing nitric oxide (NO) comprising a matrix that encapsulates nitric oxide. Nitric oxide is released when the composition is exposed to an aqueous environment. The invention further provides methods of preparing the compositions and uses of the compositions for treating infections and disorders.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Injectable microdispersions for medical applications
The present invention is directed to microdispersions and pharmaceutical compositions containing a synthetic, bioabsorbable, biocompatible liquid polymer that is the reaction product of a polybasic acid or derivative thereof, a polyol and a fatty acid, the liquid polymer having a melting point less than about 40° C., as determined by differential scanning calorimetry, and a synthetic, bioabsorbable, biocompatible polymeric wax comprising the reaction product of a polybasic acid or derivative thereof, a fatty acid and a polyol, the polymeric wax having a melting point less than about 70° C., as determined by differential scanning calorimetry.
Owner:ETHICON INC
Wound healing polymeric networks
Owner:UNIVERSITY OF PITTSBURGH
Diaryldiazepine Prodrugs for the Treatment of Neurological and Psychological Disorders
InactiveUS20110166128A1Long duration of actionReduce solubilityBiocideNervous disorderDrug compoundProdrug
Owner:ALKERMES INC
Materials and methods for treating neuropathies and related disorders including those involving a keystone nerve
InactiveUS20160030408A1Increase blood flowAlter perception of painBiocideElectrotherapyDiseaseMedicine
Methods, apparatus, compositions and kits for inhibiting a disorder in a human patient, including non-cerebral neurovascular disorder or muscular headache pain, or loss of motor or sensory function, sympathetic tone or range or fluidity of motion that affect a nerve pathway at more than one locus associated with the disorder to inhibit the disorder. Alternatively or in addition, neuropathy associated with a disorder is treatable by palpating to determine a Keystone nerve essential to the neuropathy, applying pressure to determine a point of maximum discomfort or trigger of increased symptoms to identify a Levin Sign as a locus of initial intervention, and intervening to treat the neuropathy at the location of the Levin Sign by administering a pharmaceutically active agent, internal implanted or external neuro stimulation affecting the nerve pathway to inhibit the neuropathy.
Owner:BHL PATENT HLDG
Modified nucleosides for the treatment of viral infections and abnormal cellullar proliferation
The disclosed invention is a composition for and a method of treating a Flaviviridae (including BVDV and HCV), Orthomyxoviridae (including Influenza A and B) or Paramyxoviridae (including RSV) infection, or conditions related to abnormal cellular proliferation, in a host, including animals, and especially humans, using a nucleoside of general formula (I)-(XXIII) or its pharmaceutically acceptable salt or prodrug.This invention also provides an effective process to quantify the viral load, and in particular BVDV, HCV or West Nile Virus load, in a host, using real-time polymerase chain reaction (“RT-PCR”). Additionally, the invention discloses probe molecules that can fluoresce proportionally to the amount of virus present in a sample.
Owner:GILEAD PHARMASSET LLC
Use of macrolide compounds for treating glaucoma
Owner:ASTELLAS PHARMA INC
Genes differentially expressed in cancer cells to design cancer vaccines
Owner:GENZYME CORP
Plant antifreezer and its preparation method
Owner:DAQIAN ECOLOGY & LANDSCAPE
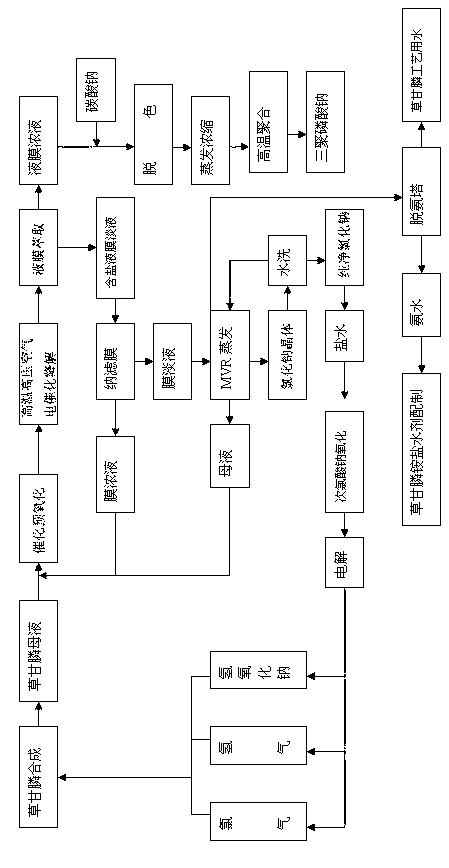
Cyclic production method of glyphosate
ActiveCN103012474AAvoid influenceReduce pollutionBiocideGroup 5/15 element organic compoundsEvaporationHigh pressure
Owner:HANGZHOU JINFADA CHEM IND
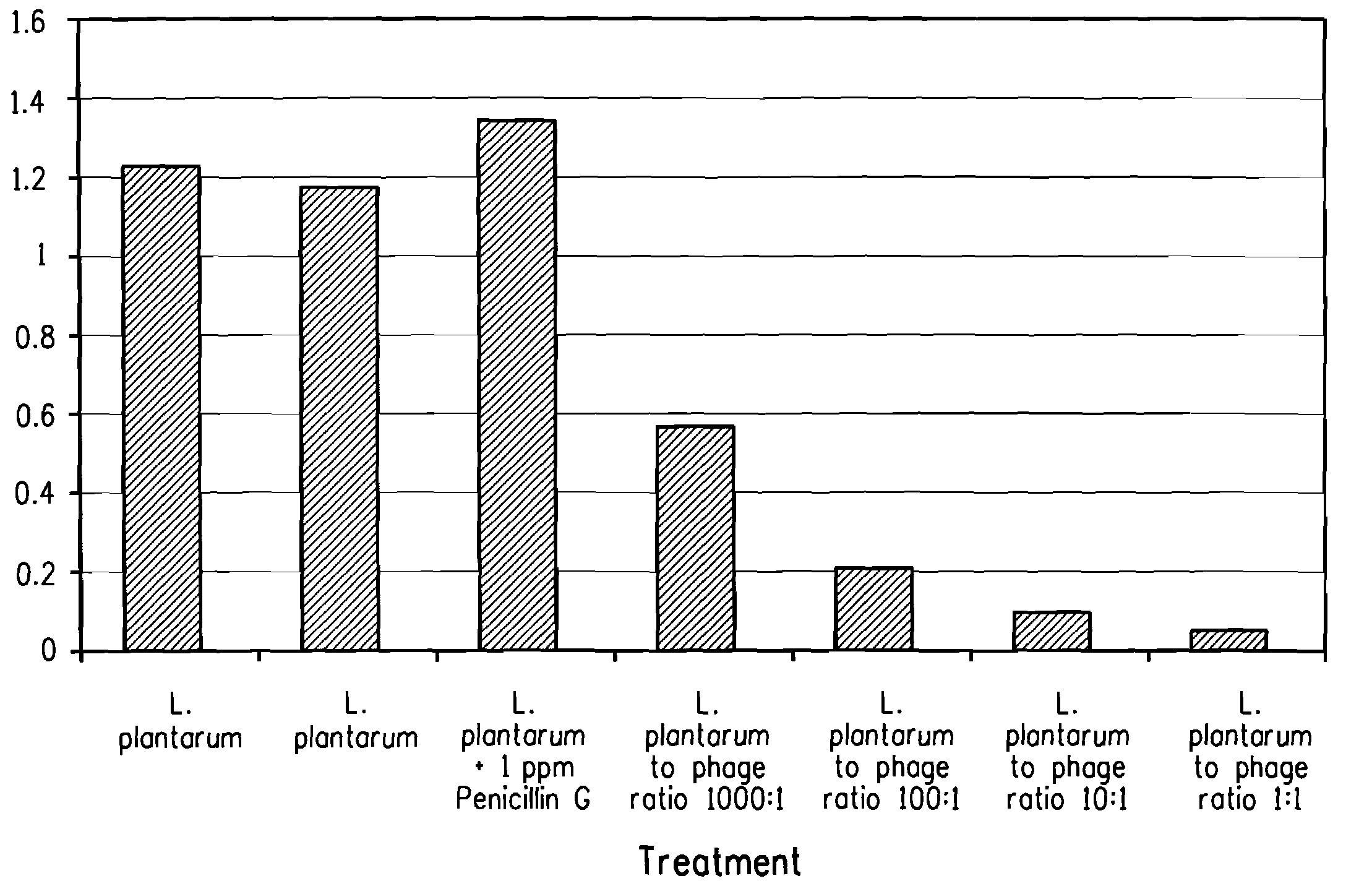
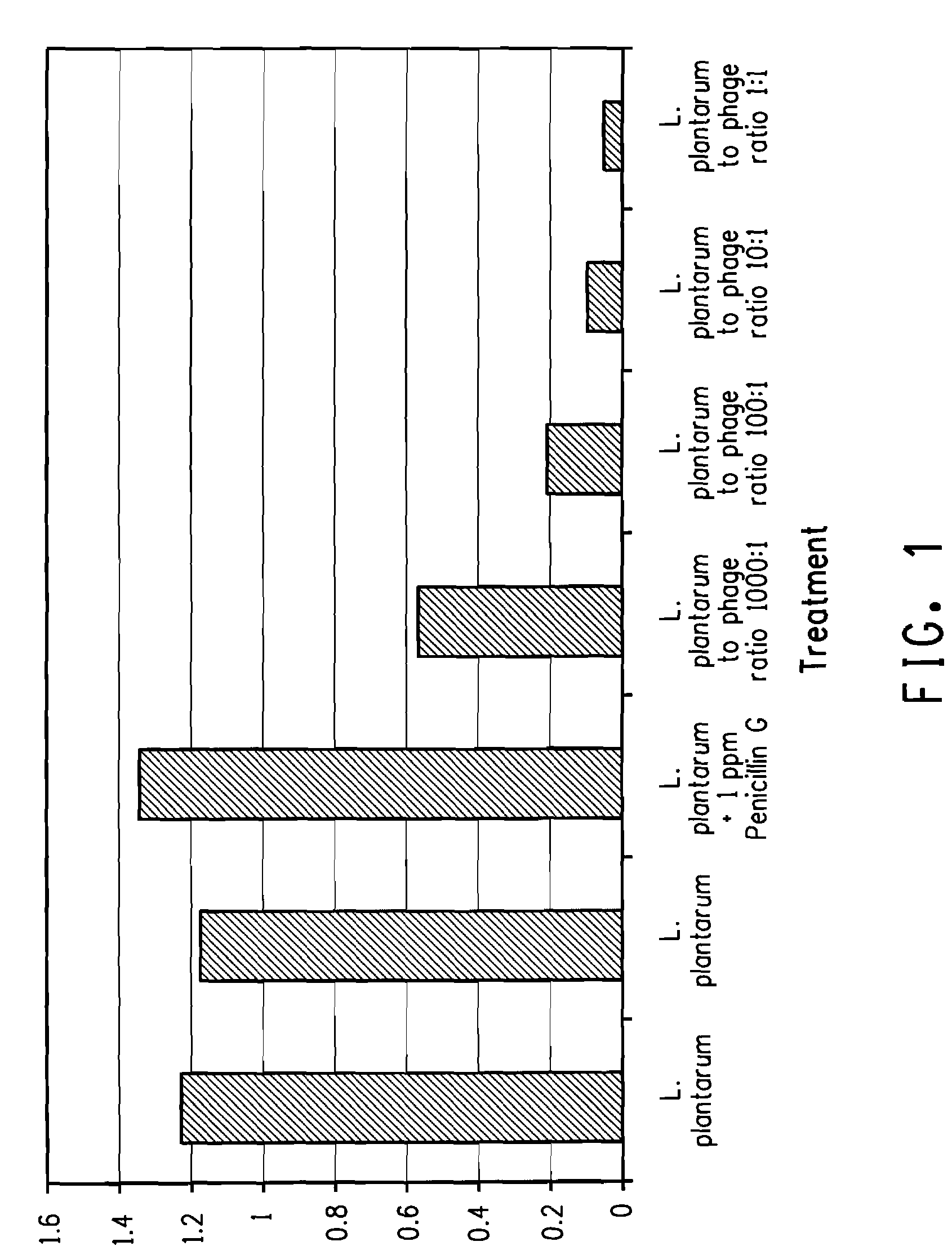
Utilization of bacteriophage to control bacterial contamination in fermentation processes
Owner:EI DU PONT DE NEMOURS & CO
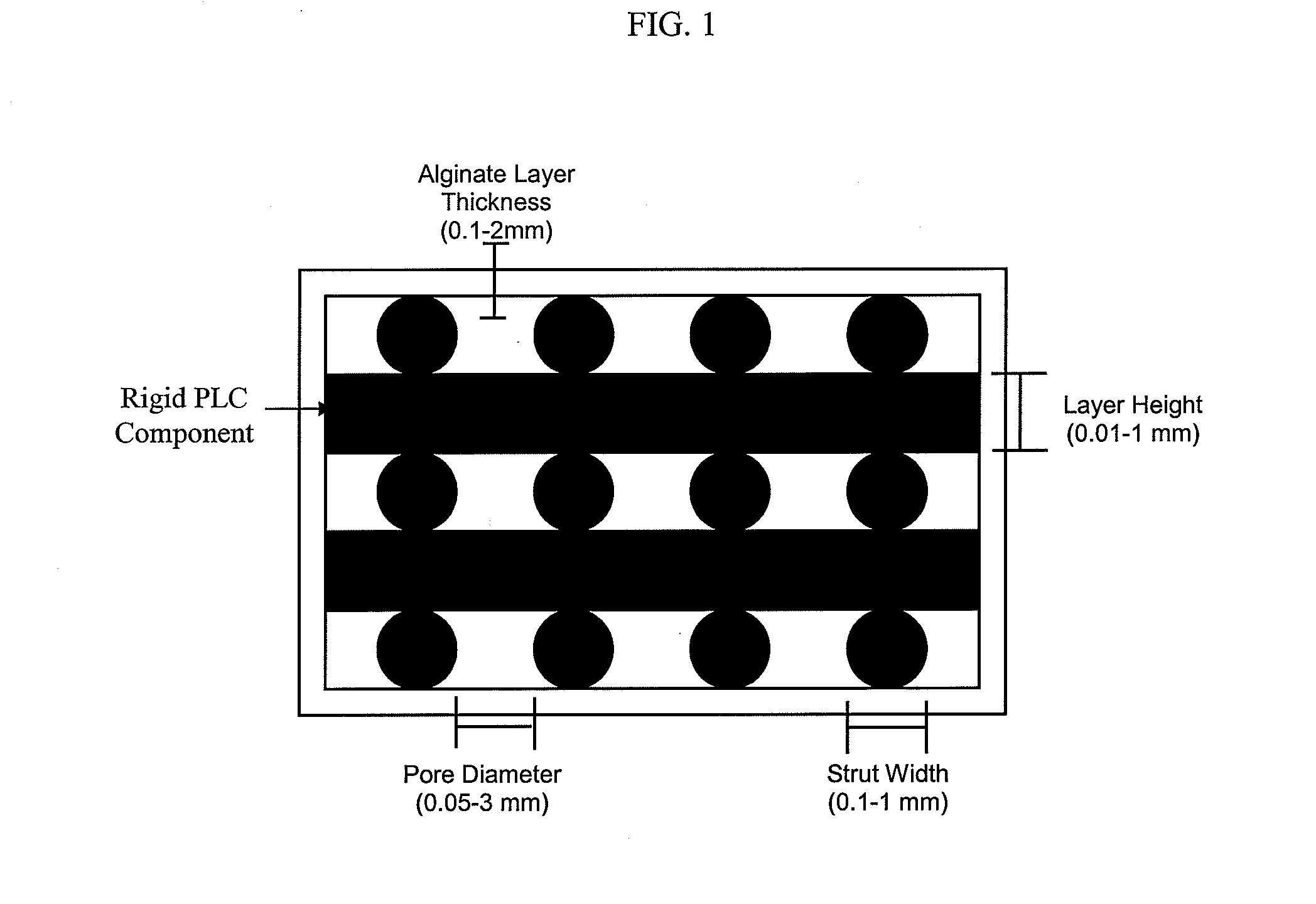
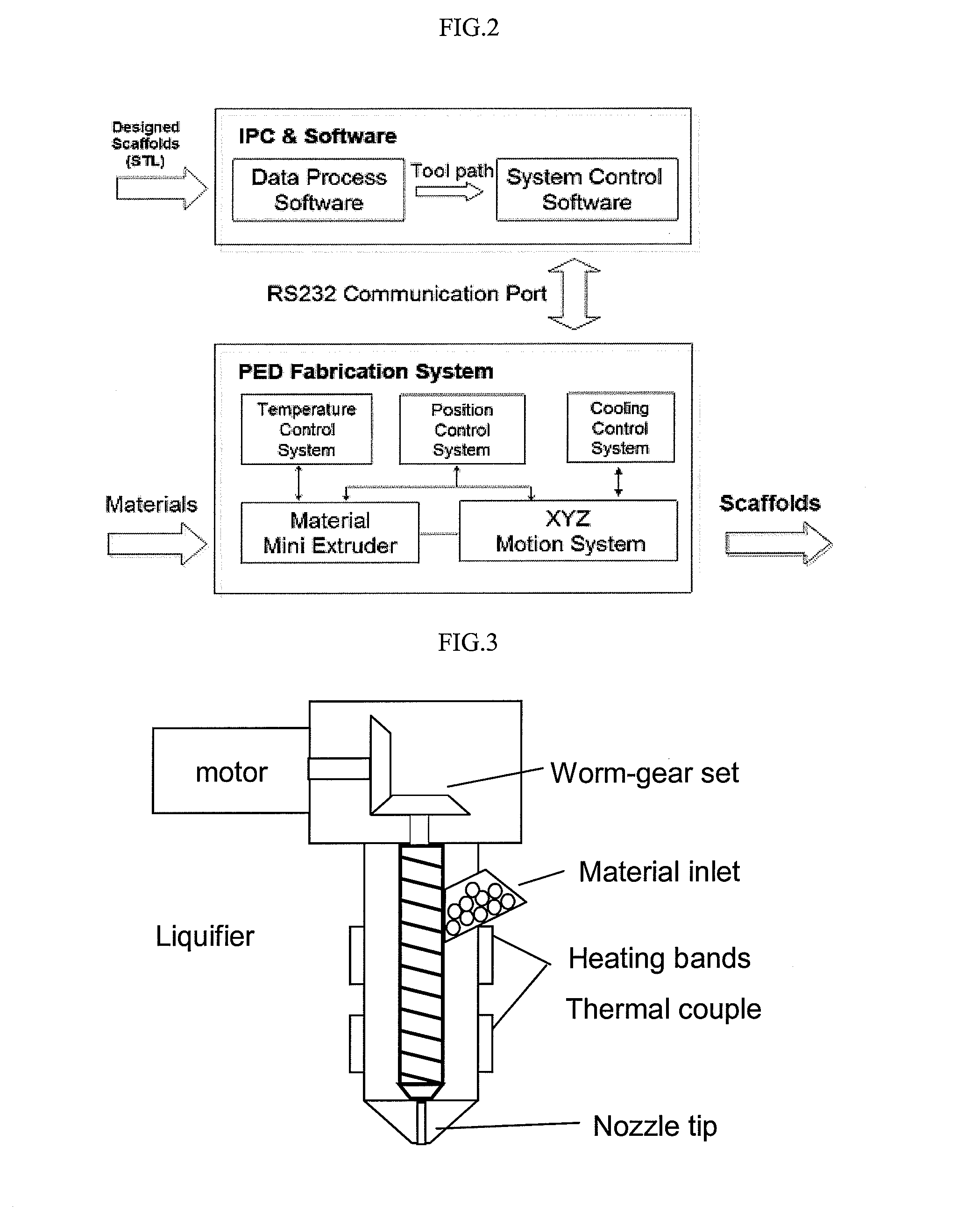
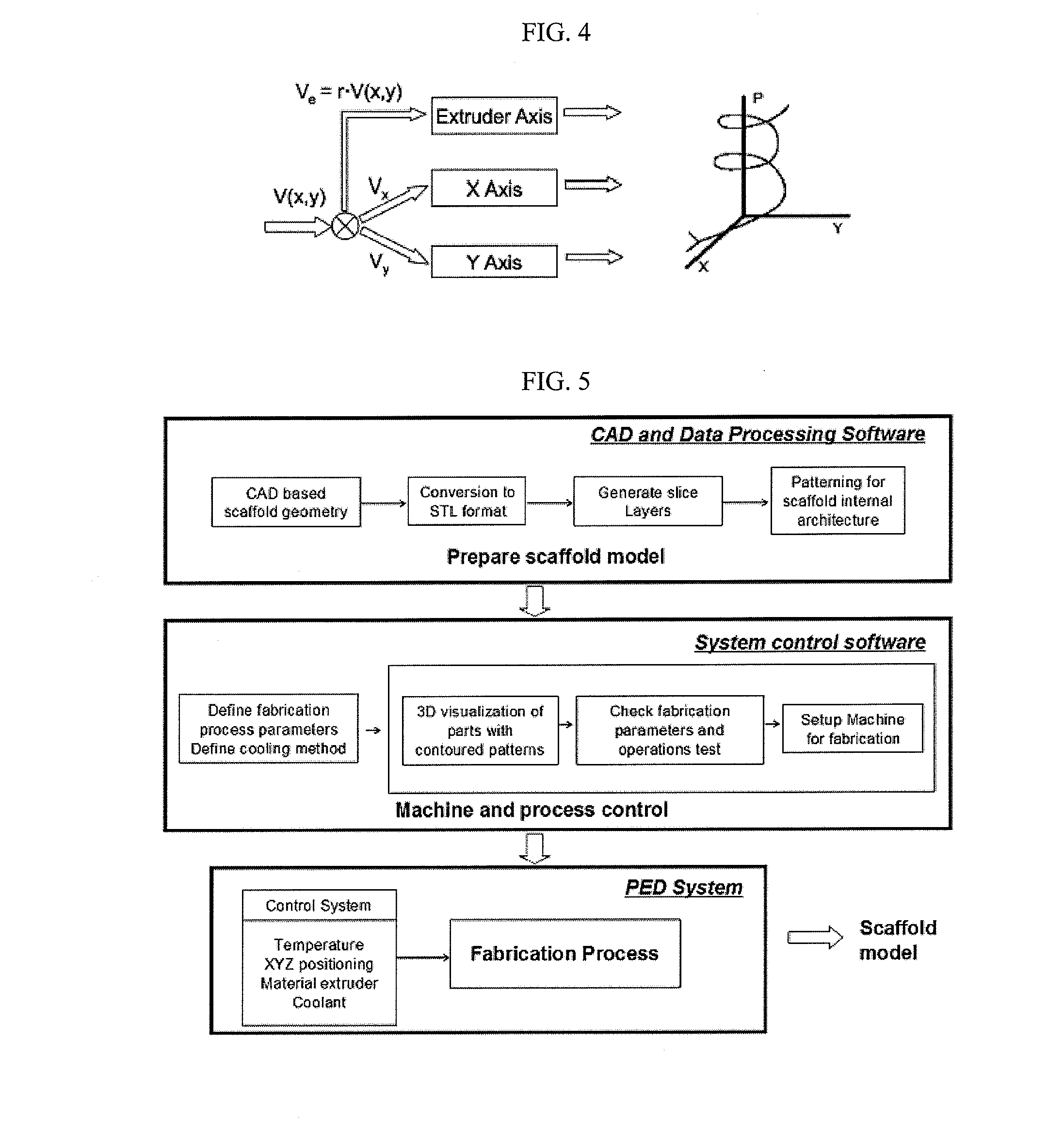
Super-sparger microcarrier beads and precision extrusion deposited poly-epsilon-caprolactone structures for biological applications
Owner:DARLING ANDREW +3
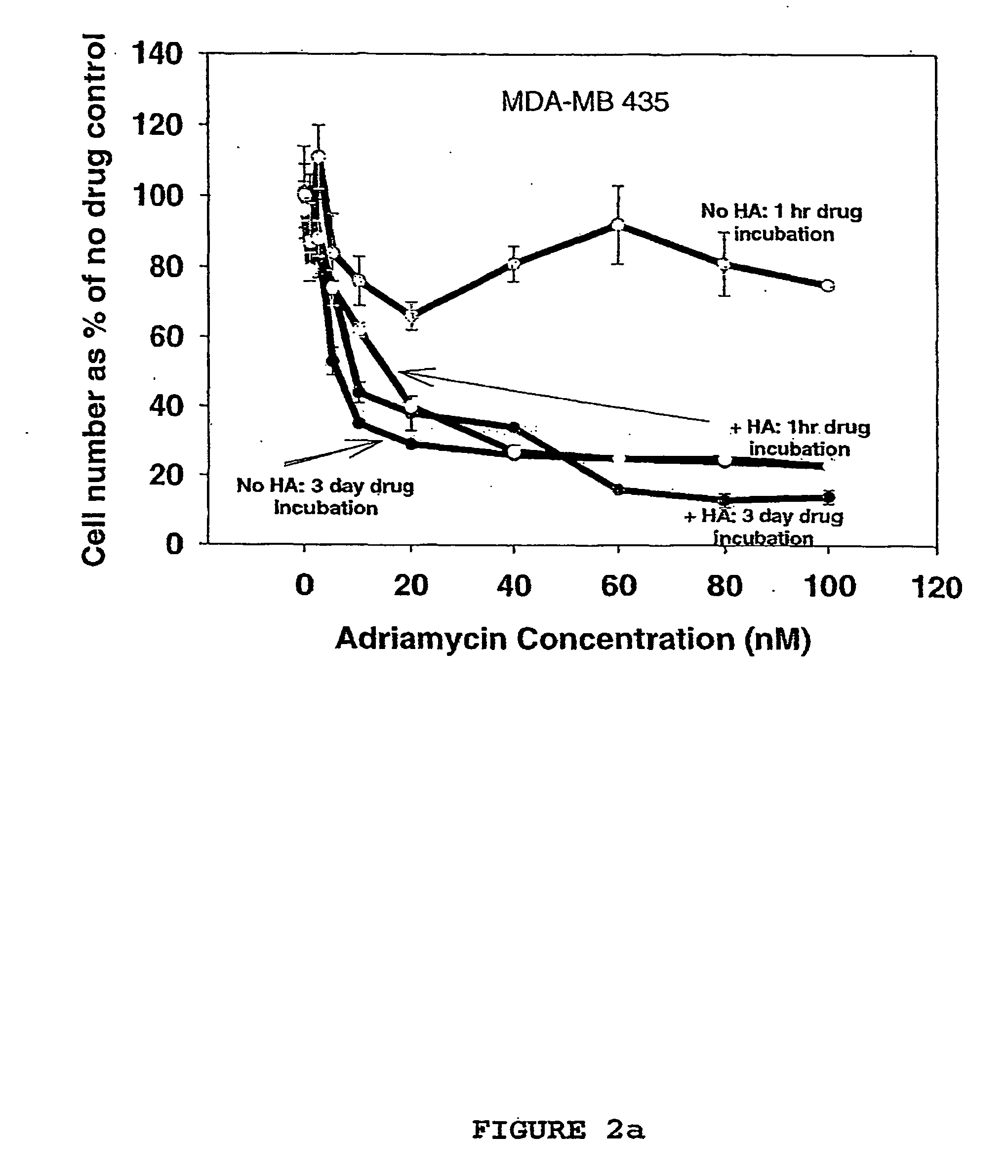
Hyaluronan as a cytotoxic agent, drug pre-sensitizer and chemo-sensitizer in the treatment of disease
InactiveUS20060178342A1Eliminate side effectsHeavy metal active ingredientsBiocideIrinotecanHyaluronic acid
Owner:ALCHEMIA ONCOLOGY PTY LTD
Bioadhesive compositions and their use in medical electrodes
InactiveUS20070196320A1Poor adhesionImprove skinBiocideNon-fibrous pulp additionBioadhesiveCopolymer
Owner:MANTRA INT
Hard capsule
InactiveUS20060153909A1Impair stability of drugImpair propertyOrganic active ingredientsBiocideWater activityHard Capsule
Owner:WAKUNAGA PHARMA CO LTD
Compositions and methods for treating conditions of the nail unit
InactiveUS20060275230A1Improve blood supplyPromote circulationBiocideCosmetic preparationsMicroparticlePhase change
Owner:TALIMA THERAPEUTICS INC
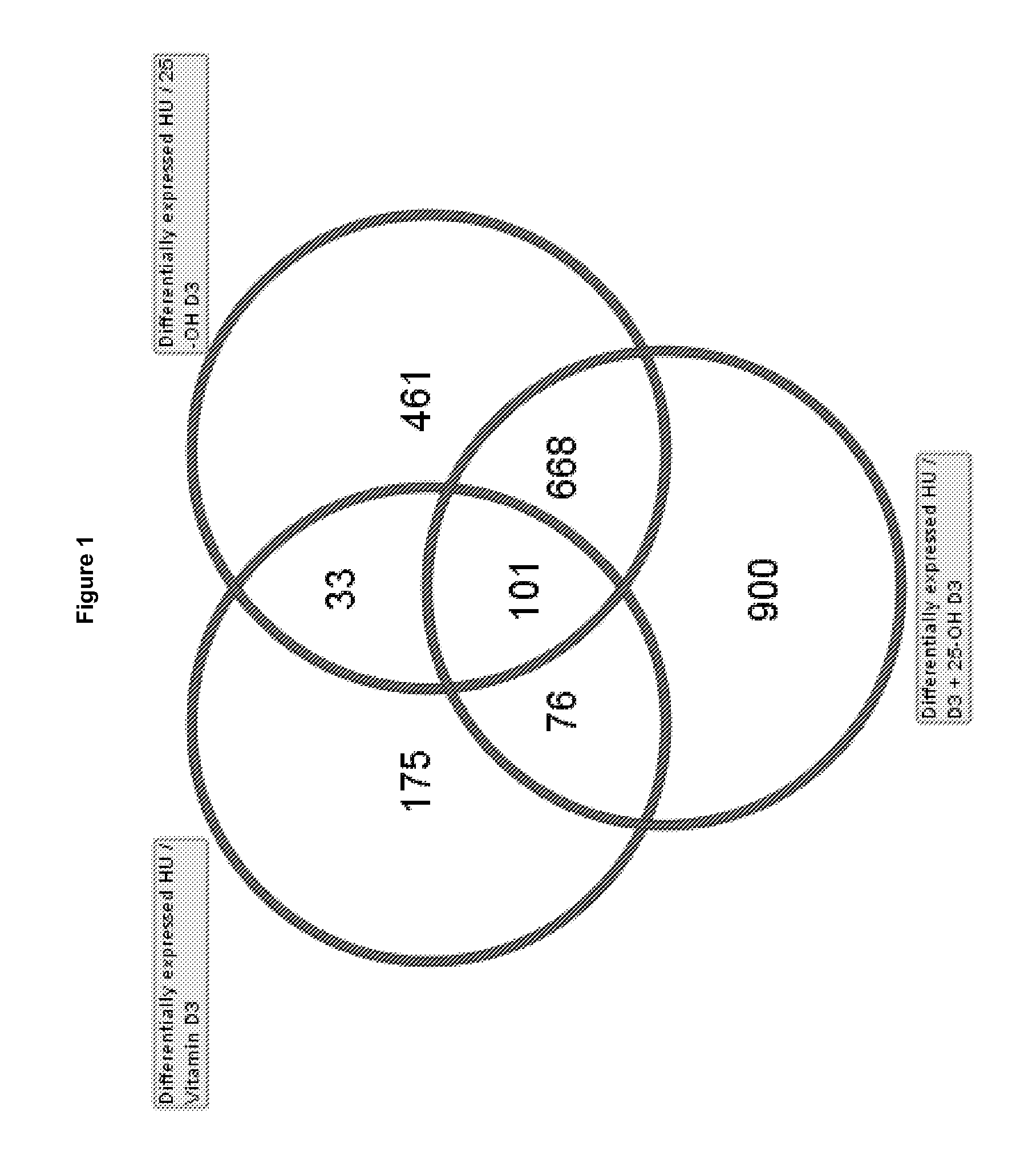
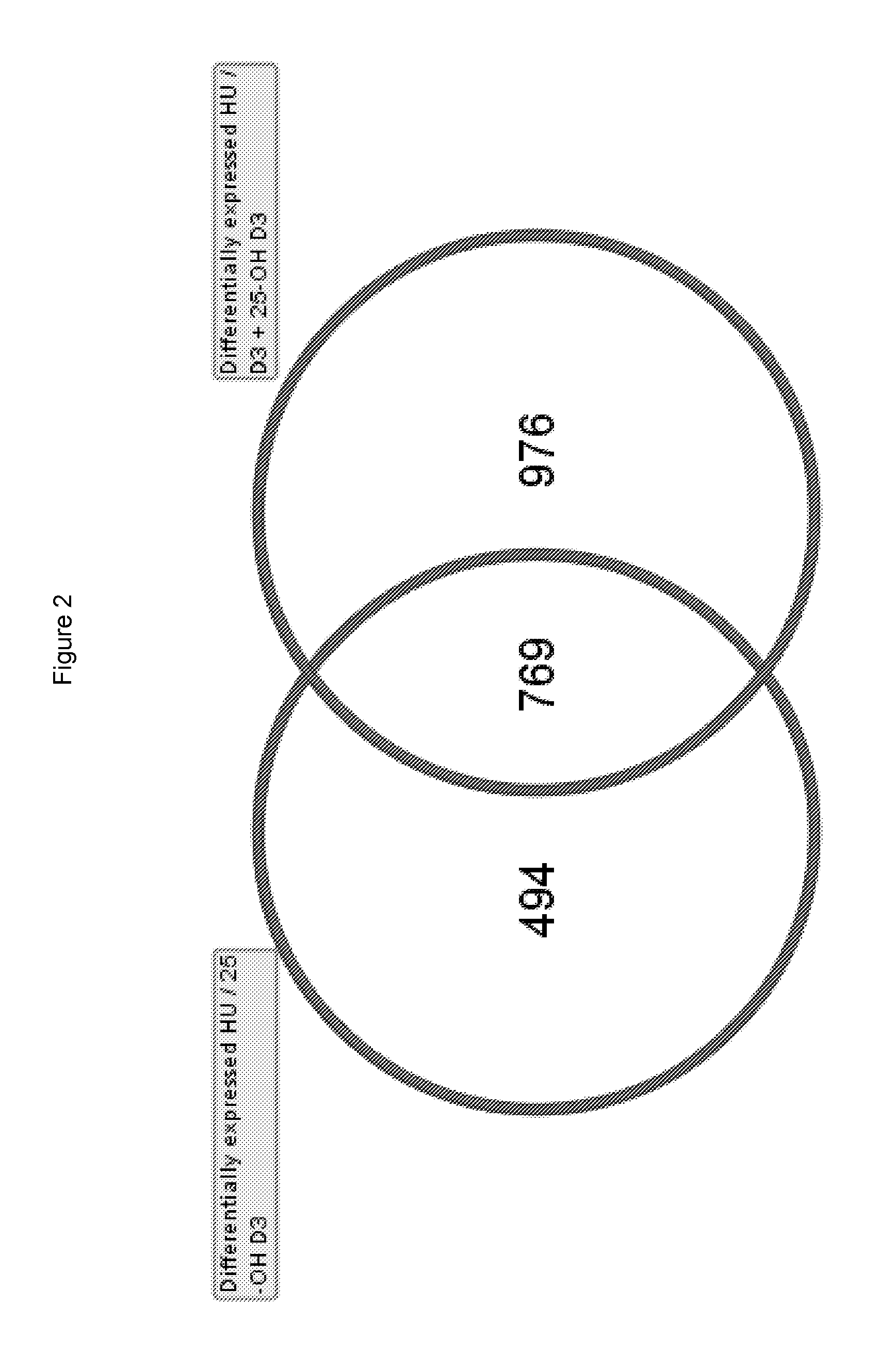
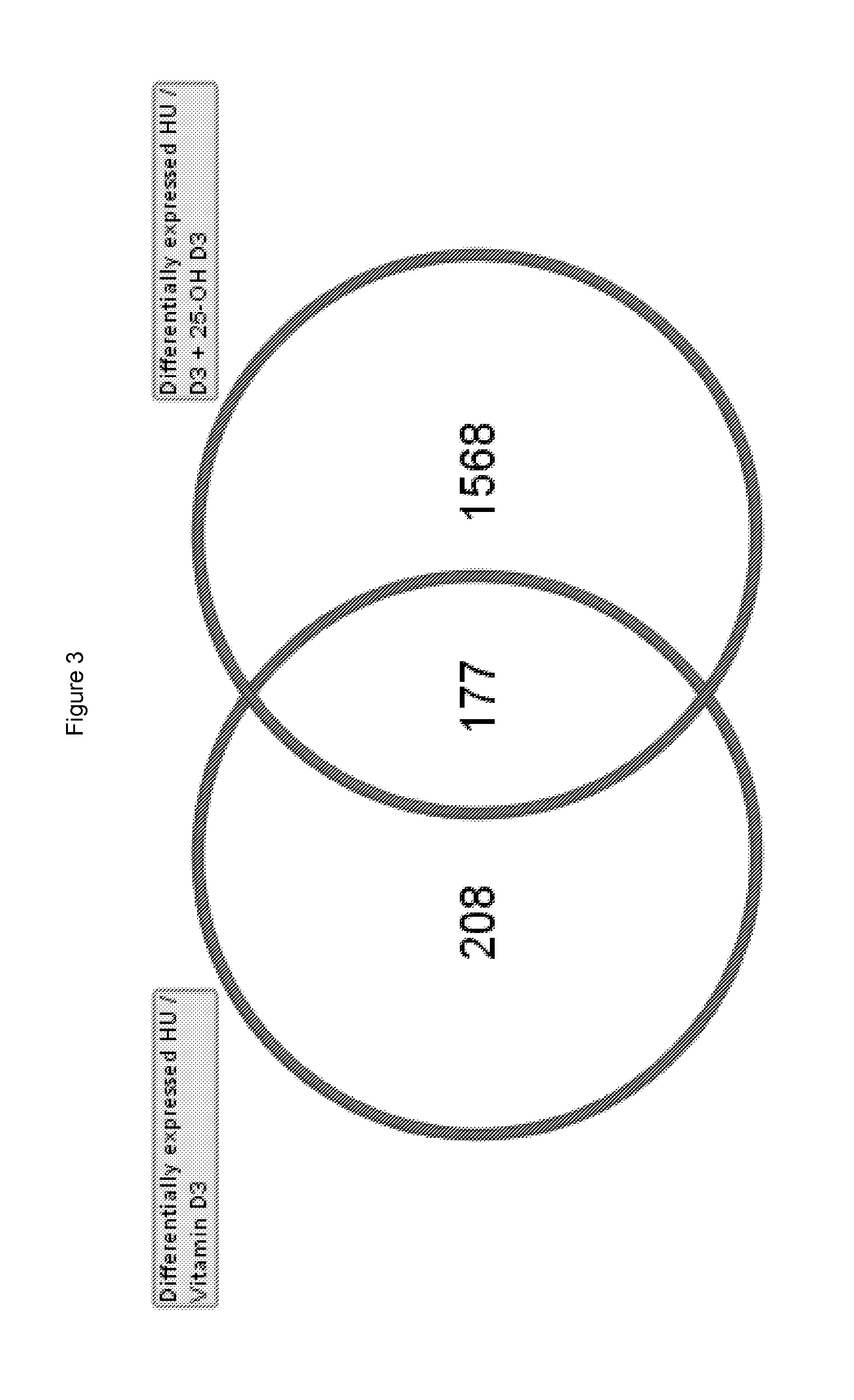
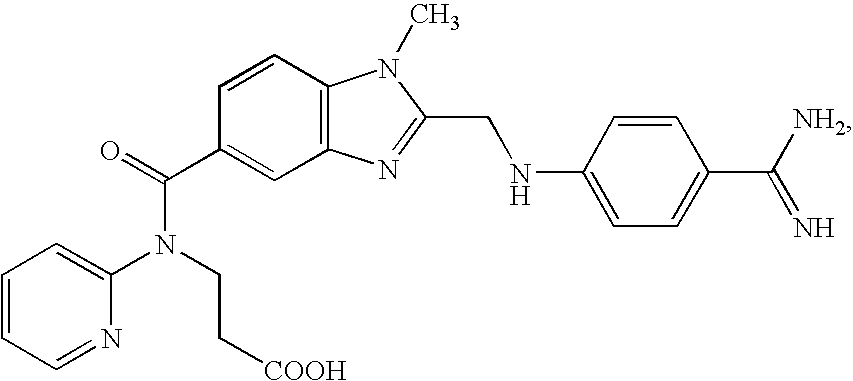
Use of 25-hydroxy-vitamin d3 to affect human muscle physiology
InactiveUS20110039810A1Function increaseIncrease muscle strengthBiocideOrganic active ingredientsMuscle strengthPhysiology
Owner:DSM IP ASSETS BV
Use Of Dipyridamole For Treatment Of Resistance To Platelet Inhibitors
InactiveUS20090048173A1Reduce decreaseBiocidePeptide/protein ingredientsDipyridamolePlatelet inhibitor
Owner:EISERT WOLFGANG +1
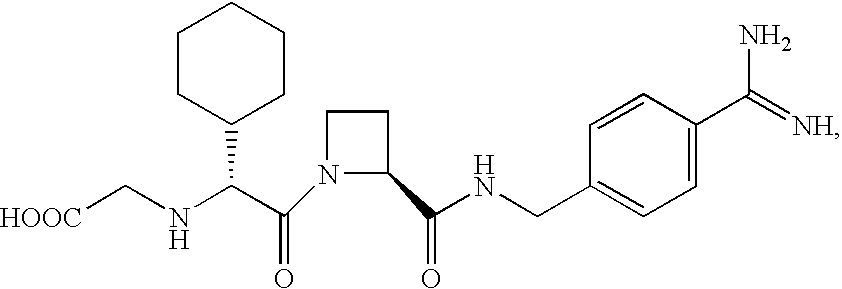
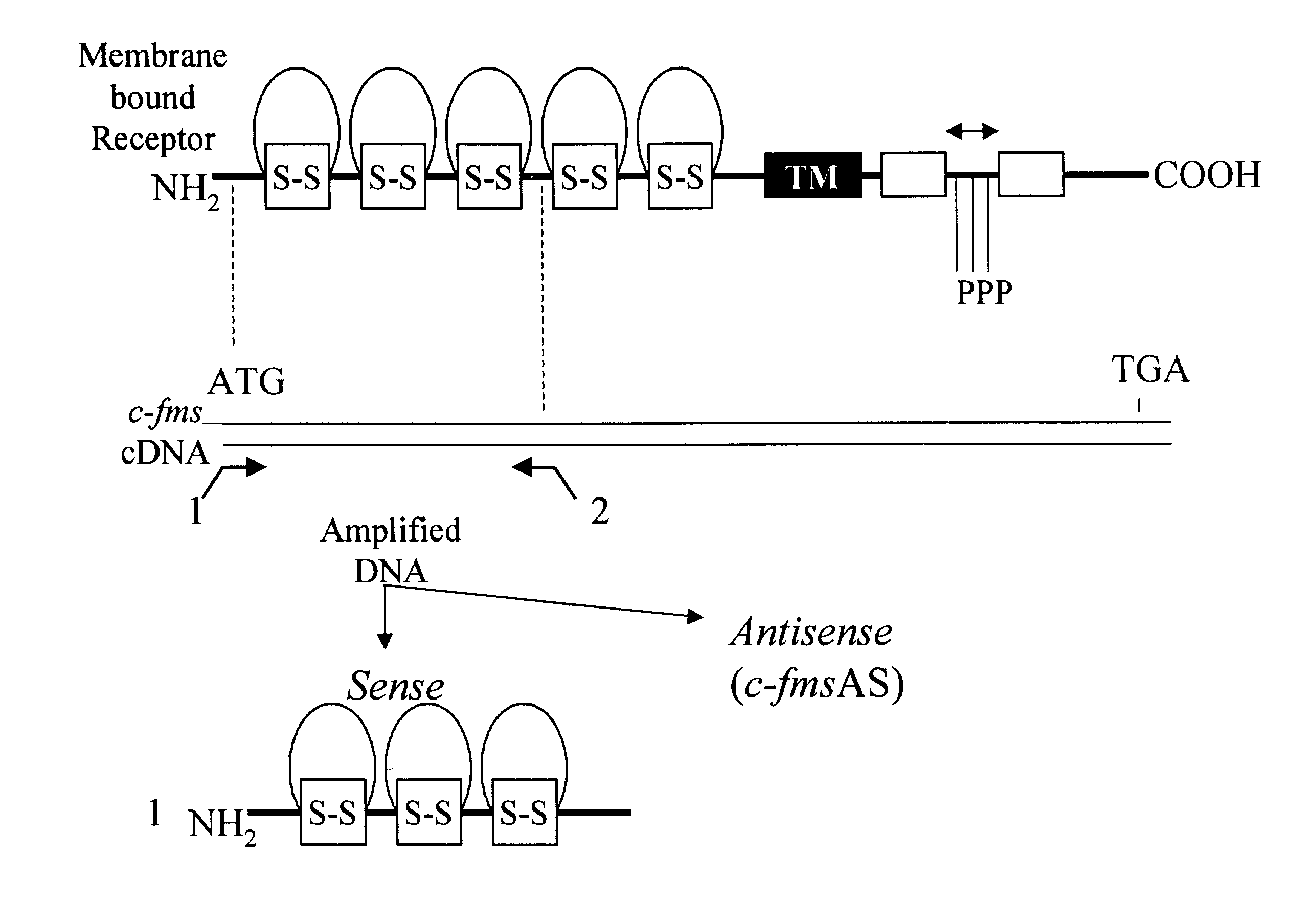
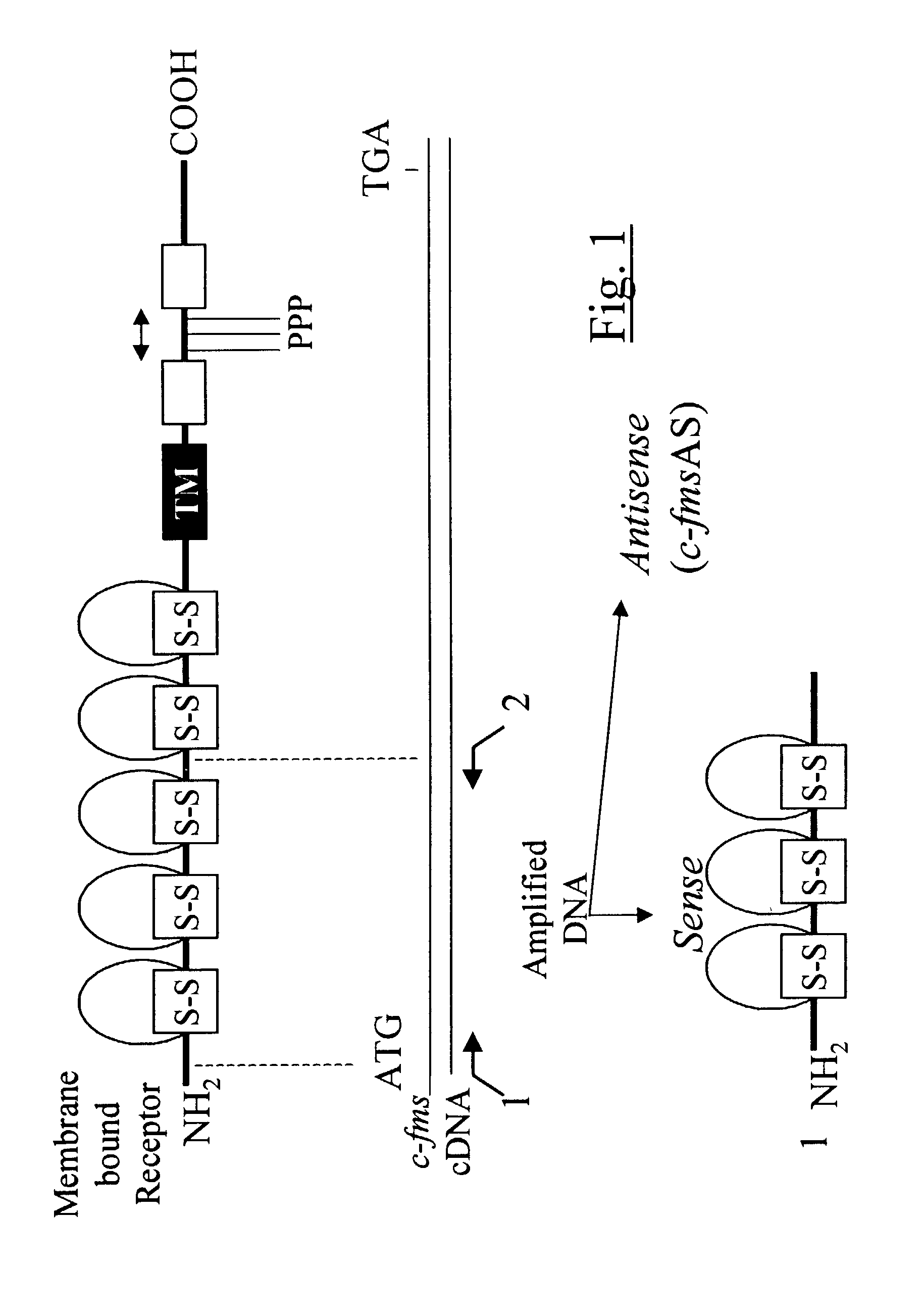
Methods for inhibiting macrophage colony stimulating factor and c-FMS-dependent cell signaling
Owner:RAJAVASHISTH TRIPATHI
Crystalline forms and process for preparing spiro-hydantoin compounds
Owner:BRISTOL MYERS SQUIBB CO
Novel compounds as cannabinoid receptor ligands
Disclosed herein are cannabinoid receptor ligands of formula (I)wherein Y, X1, X2, X3, R1, and R2 are as defined in the specification. Compositions comprising such compounds, and methods for treating conditions and disorders using such compounds and compositions are also disclosed.
Owner:ABBVIE INC
Preparations and Methods for Ameliorating or Reducing Presbyopia
InactiveUS20100298335A1Ameliorating and reducing presbyopiaEfficient productionBiocideSenses disorderCholinesteraseAgonist
Owner:KAUFMAN HERBERT E
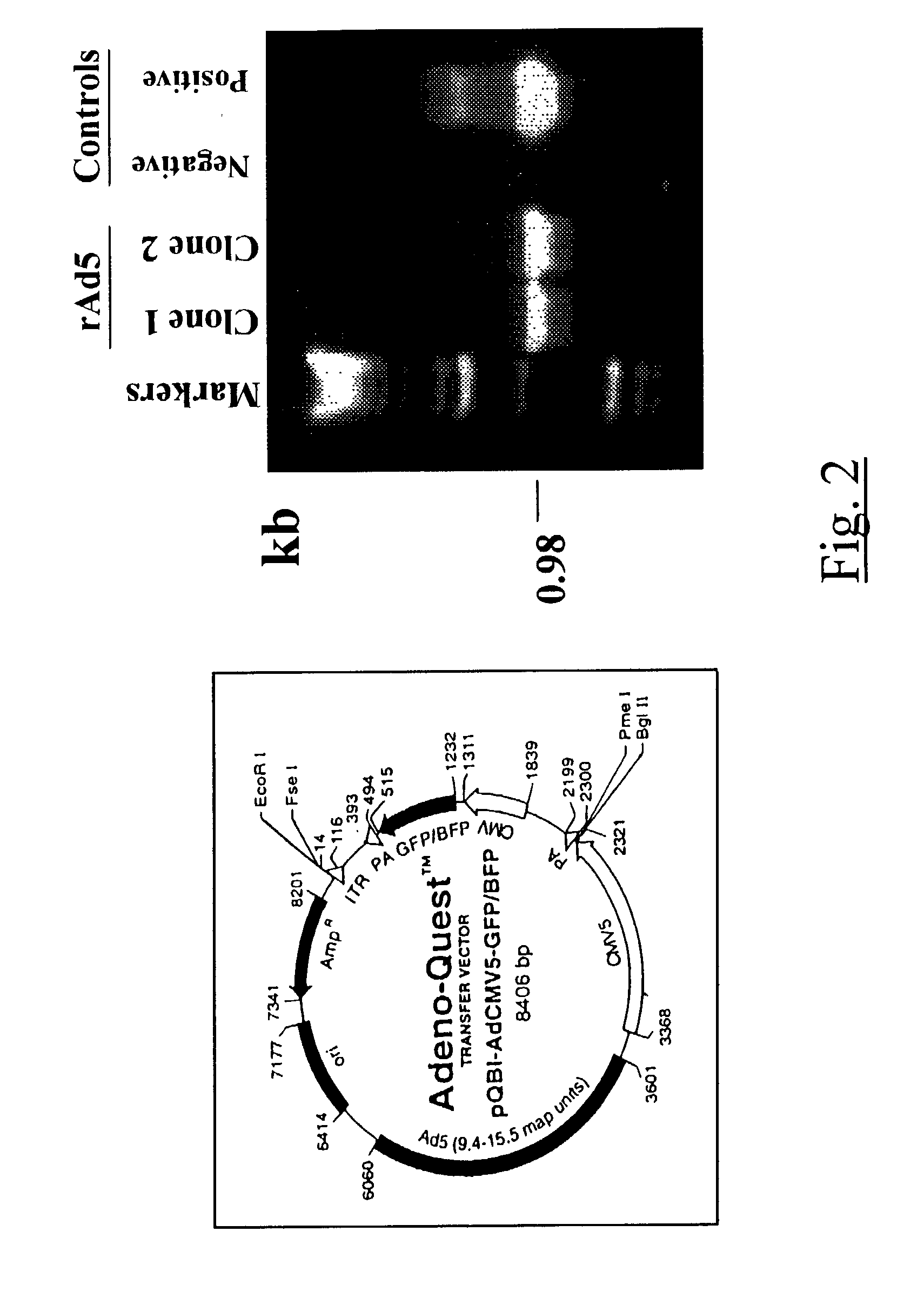
Adenovirus vector containing a heterologous peptide epitope in the hi loop of the fiber knob
InactiveUS7297542B2Efficient transductionRaise transfer toBiocideAntibody mimetics/scaffoldsHeterologousEpitope
The present invention provides means to modify the tropism of recombinant adenoviral vectors using genetic methods to alter the adenoviral fiber cell-binding protein. The present invention generates an adenovirus with modified fiber gene such that novel tropism is achieved. This recombinant adenovirus has a fiber gene modified in the HI loop domain.
Owner:UAB RES FOUND
Biological control strain capable of preventing and curing root knot nematode disease for greenhouse vegetable
Owner:NANJING AGRICULTURAL UNIVERSITY
Amorphous and a crystalline form of genz 112638 hemitartrate as inhibitor of glucosylceramide synthase
The hemitartrate salt of a compound represented by the following structural formula: (Formula I Hemitartrate), which may be used in pharmaceutical applications, are disclosed. Particular single crystalline forms of the Formula (I) Hemitartrate are characterized by a variety of properties and physical measurements. As well, methods of producing crystalline Formula (I) Hemitartrate, and using it to inhibit glucosylceramide synthase or lowering glycosphingolipid concentrations in subjects to treat a number of diseases, are also discussed. Pharmaceutical compositions are also described.
Owner:GENZYME CORP
Clinic compliant method for banking human placental mesenchymal cells
Owner:AFFILIATED HOSPITAL OF NINGXIA MEDICAL UNIV
Gyrase Inhibitors and Uses Thereof
Owner:VERTEX PHARMA INC
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap