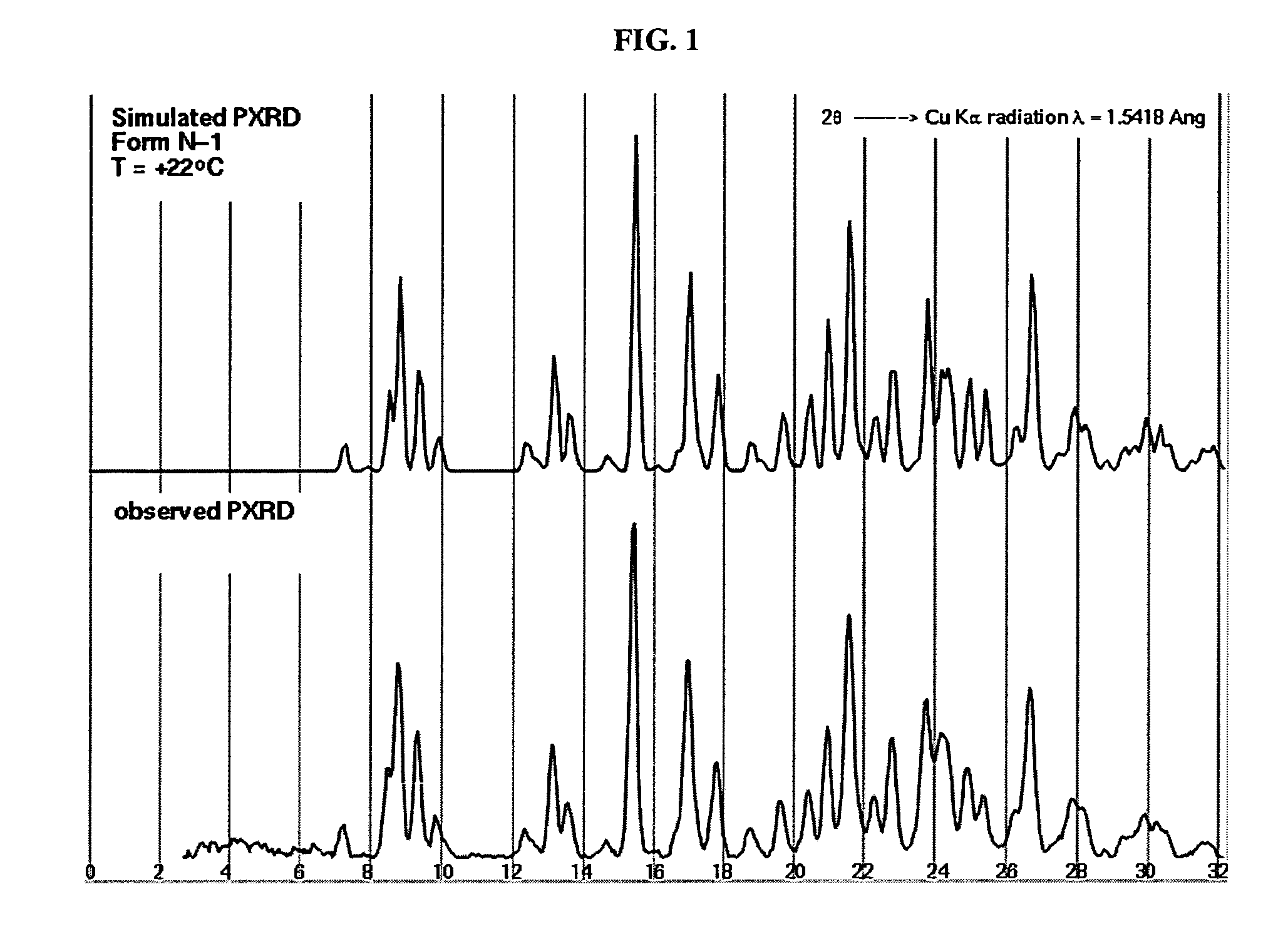
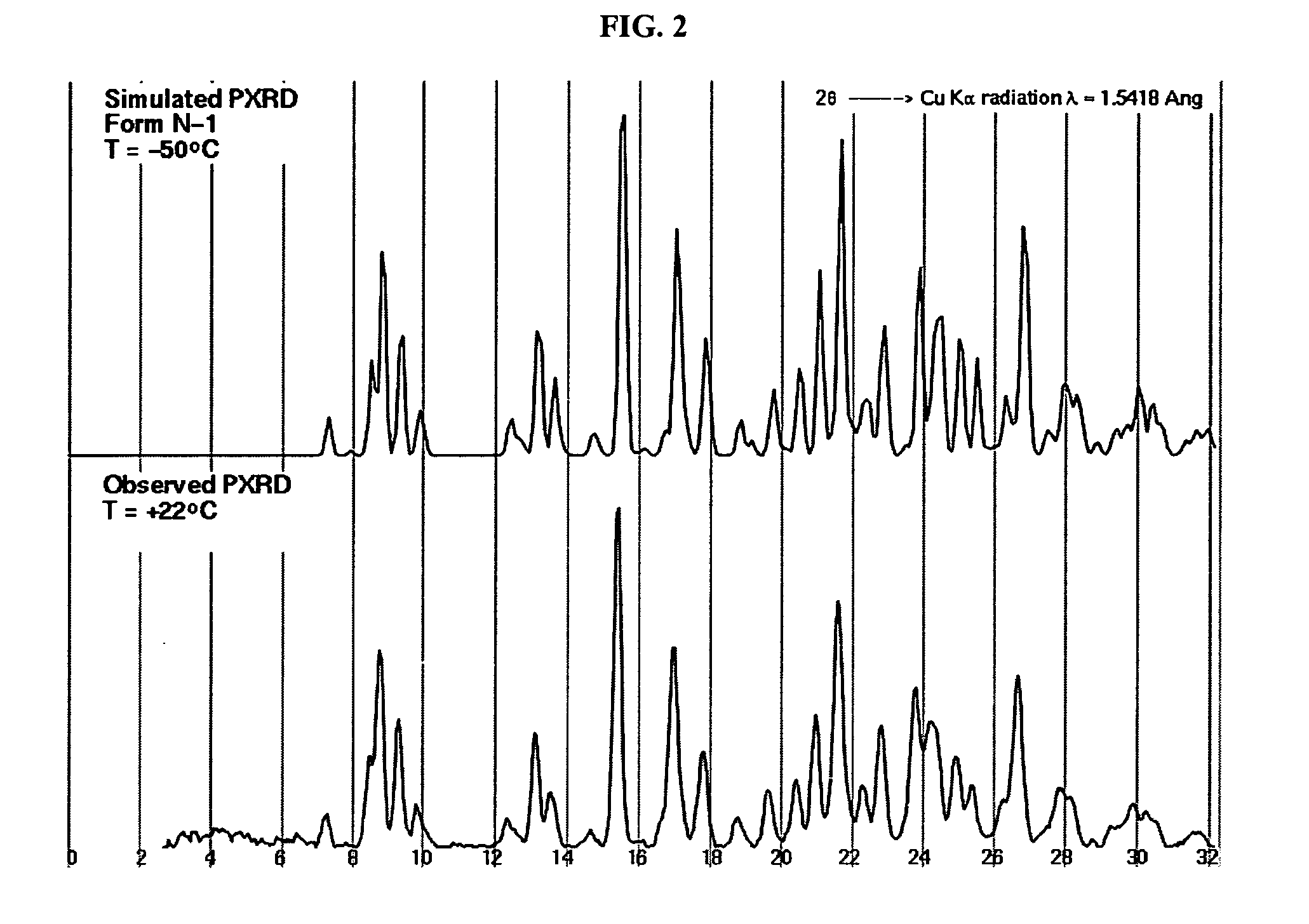
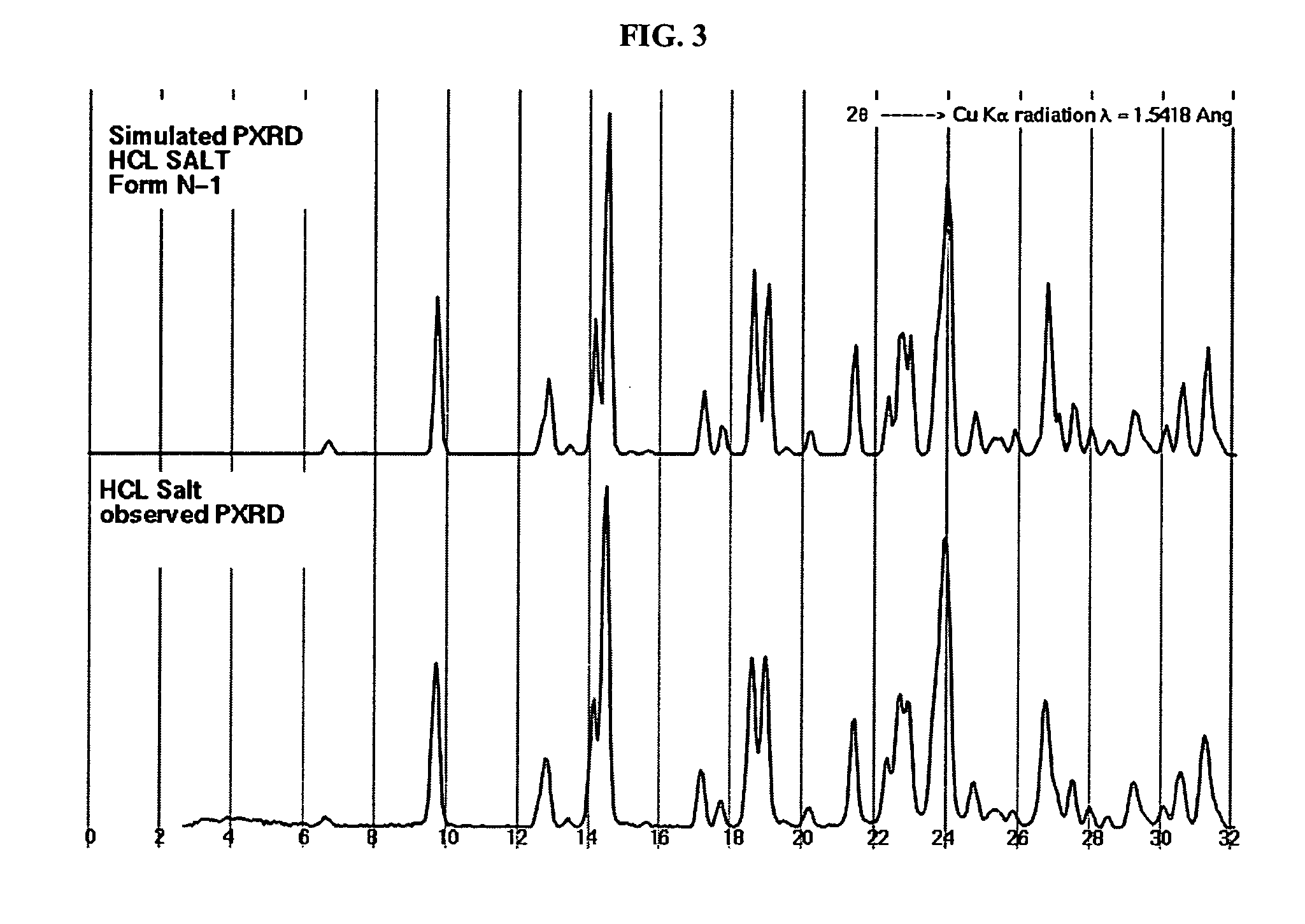
Crystalline forms and process for preparing spiro-hydantoin compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0227] The following examples illustrate embodiments of the inventive process, and are not intended to limit the scope of the claims. For ease of reference, the following abbreviations are used herein:
ABBREVIATIONS
[0228] DMSO=dimethyl sulfoxide [0229] DTTA=(+)-Di-p-toluoyl-D-tartaric acid [0230] EtOH=ethanol [0231] HCl=hydrochloric acid [0232] HPLC=high performance liquid chromatography [0233] kg=kilogram [0234] L=liter [0235] M=molar [0236] MTBE=methyl tertiary butyl ether [0237] MeOH=methanol [0238] mol=mole [0239] mp=melting point [0240] NMR=nuclear magnetic resonance [0241] TBME=t-butyl methyl ether [0242] THF=tetrahydrofuran
preparation 1
3-(3,5-dichlorophenyl)-1-methylimidazolidine-2,4-dione
[0243]
[0244] Triethylamine (0.78 kg, 7.75 mol) was added in 15-30 minutes with stirring to a thin suspension of sarcosine ethylene hydrochloride (1.00 kg, 6.51 mol) in dichloromethane (6.00 L). After stirring at room temperature for 1.5-2.0 hours, the mixture was filtered to remove the resulting triethylamine hydrochloride salt. The salt cake was washed with dichloromethane (2.00 L). The filtrate was cooled to 0-5° C.
[0245] A solution of 3,5-dichlorophenyl isocyanate (1.47 kg, 7.81 mol) in dichloromethane was prepared at 20-25° C. The solution was added to the above cooled filtrate slowly in 30-60 minutes. The temperature was maintained below 10° C. during the addition. After the addition, the mixture was stirred at 20-25° C. for 12-14 hours. The completeness of the reaction was followed by HPLC. Upon reaction completion, TBME (16.00 L) was added in one portion. The resulting suspension was stirred at 20-25° C. for 2-3 hours and w
preparation 2
(E)-4-((1-(3,5-dichlorophenyl)-3-methyl-2,5-dioxoimidazolidin-4-ylidene)methyl)benzonitrile
[0246]
[0247] A mixture of 3-(3,5-dichlorophenyl)-1-methylimidazolidine-2,4-dione (1.00 kg, 3.86 mol), 4-cyanobenzaldehyde (0.70 kg, 5.79 mol) and pyrrolidone (0.27 kg, 3.86 mmol) was refluxed in EtOH (13.00 L) for 20-24 hours at a temperature of 78° C. The completeness of the reaction was followed by HPLC. Upon reaction completion, the suspension was cooled to 65° C. and THF (4.33 L) was added in 5-10 minutes. The suspension was cooled to 20-25° C. in 3-4 hours and was then filtered. The filter cake was washed with EtOH (4×2.00 L) and dried at maximum 40° C. to a constant weight. (E)-4-((1-(3,5-dichlorophenyl)-3-methyl-2,5-dioxoimidazolidin-4-ylidene)methyl)benzonitrile (1.24 kg, 86%) was obtained as a fluffy, yellowish crystalline solid. mp=239-241° C. 1H NMR (DMSO-d6): 8.07 (2H, d, J=8.3 Hz), 7.86 (2H, d, J=8.4 Hz), 7.72 (1H, m), 7.59 (2H, m), 6.72 (1H, s), 3.35 (3H, s). 13C NMR (DMSO-d6): 14
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap