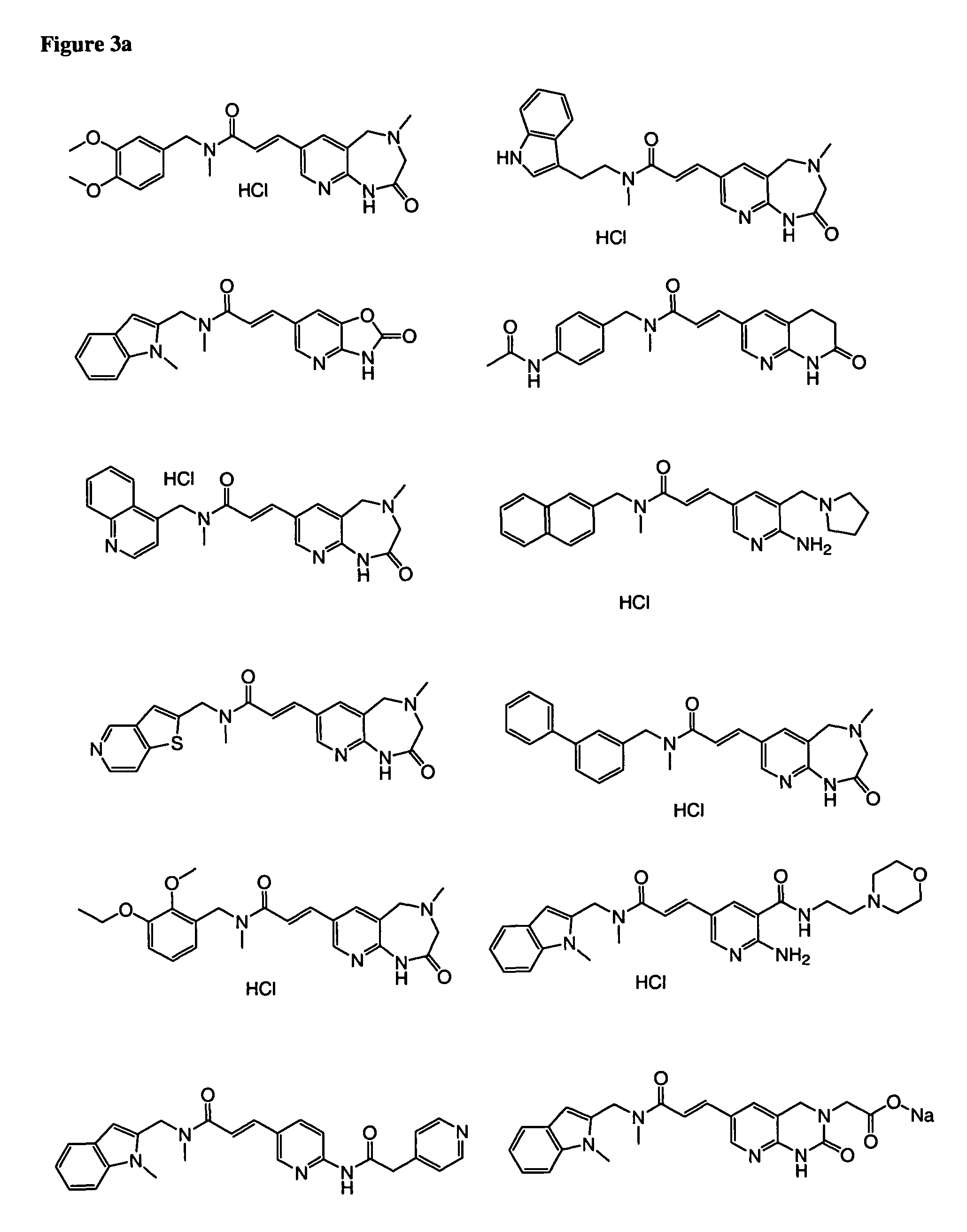
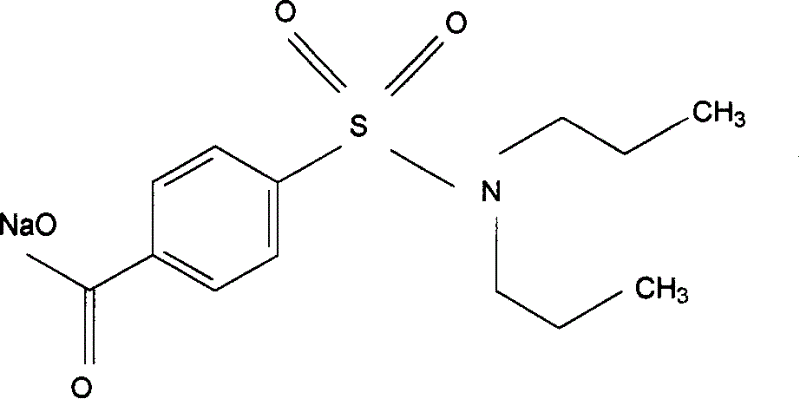
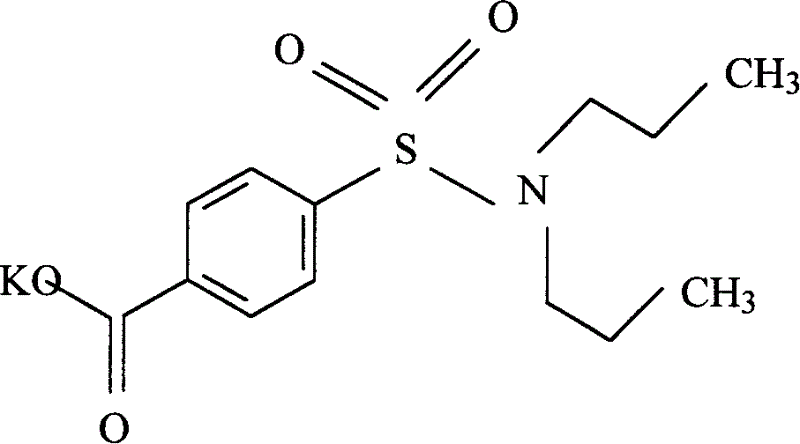
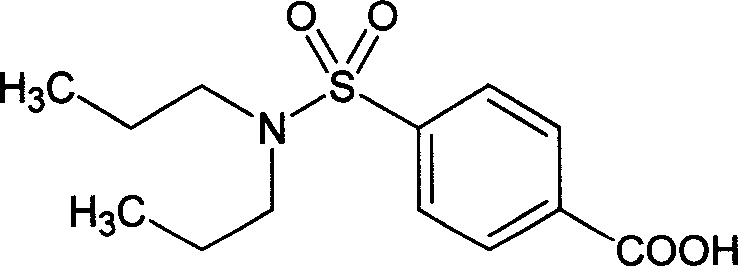
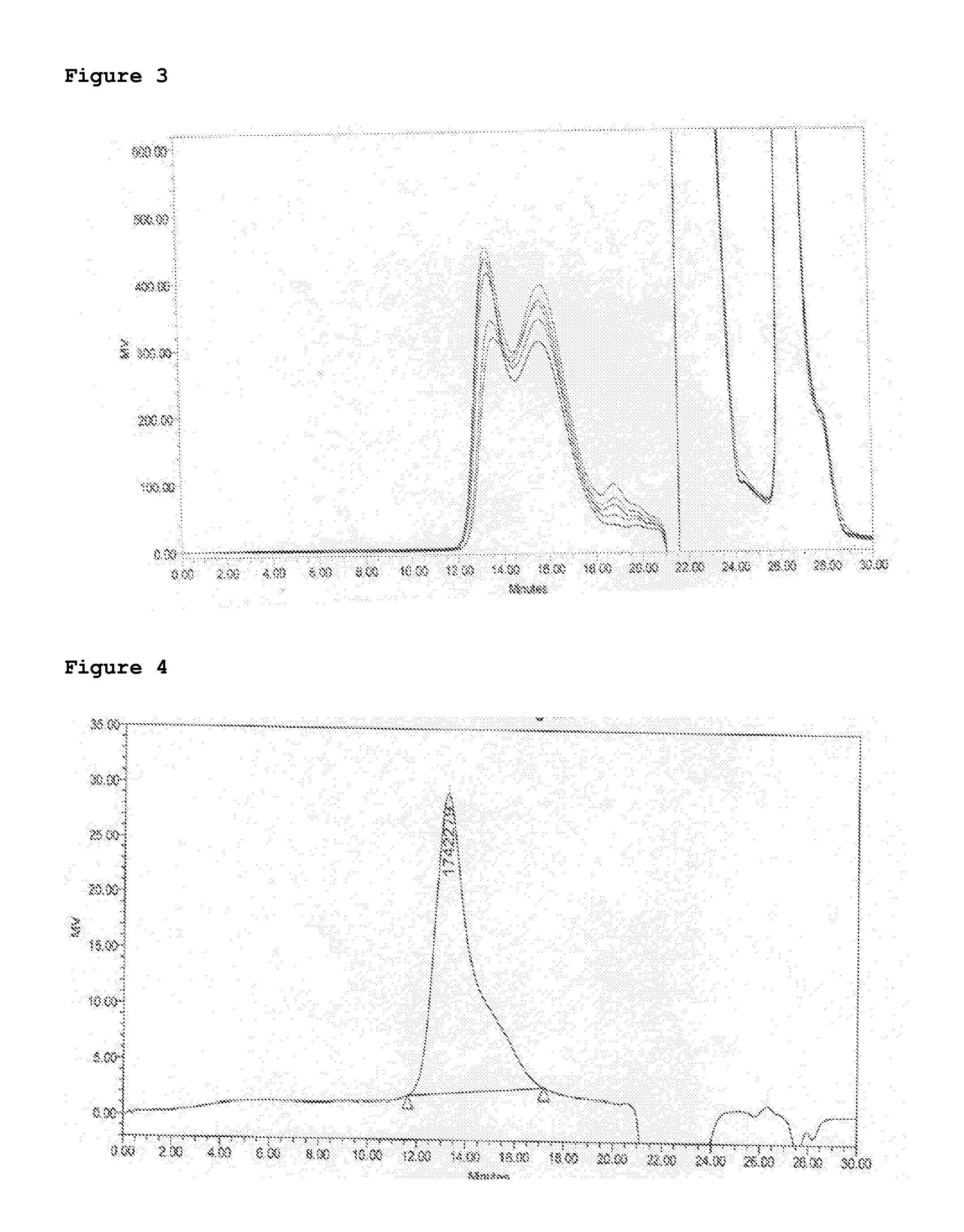
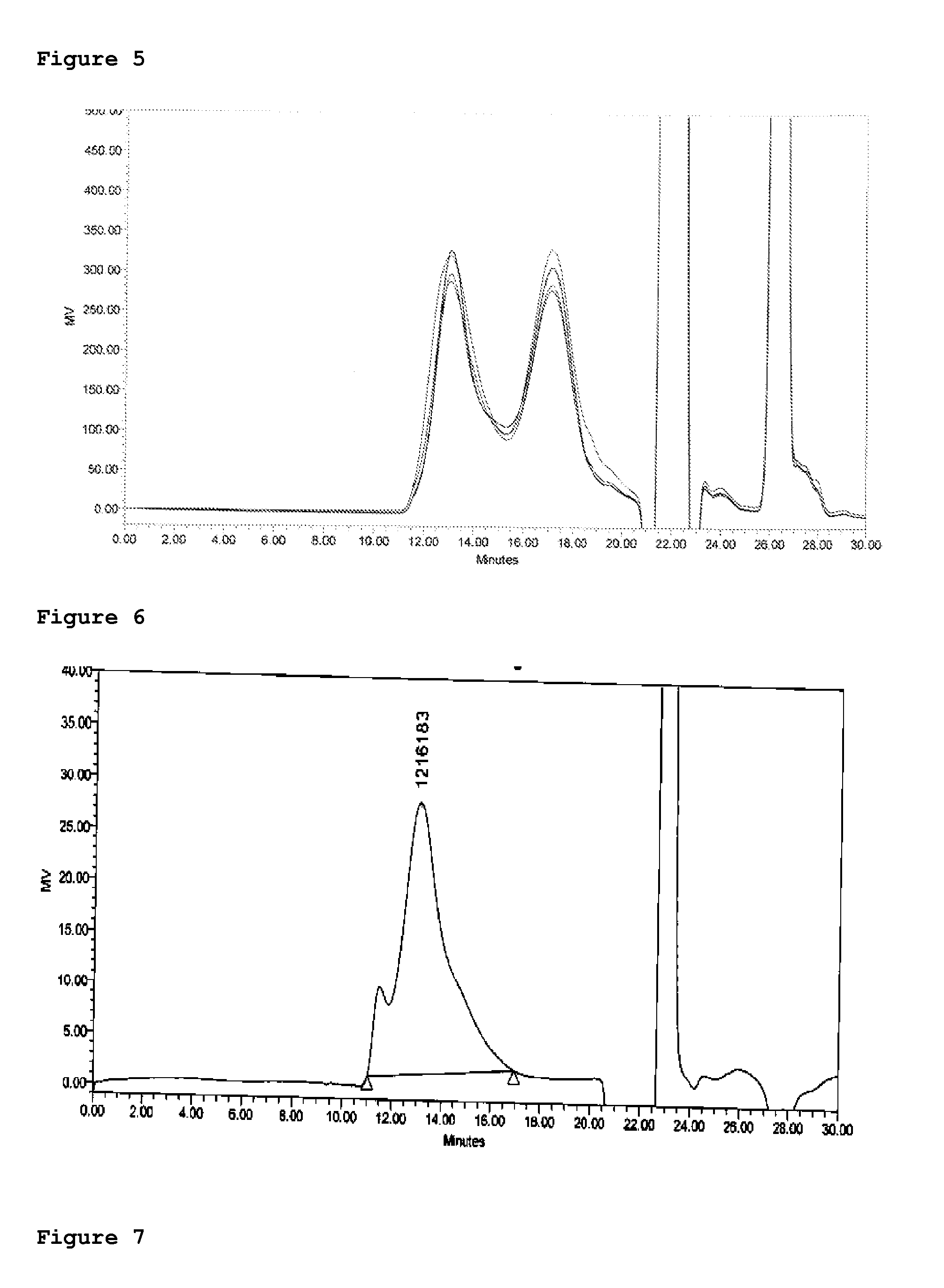
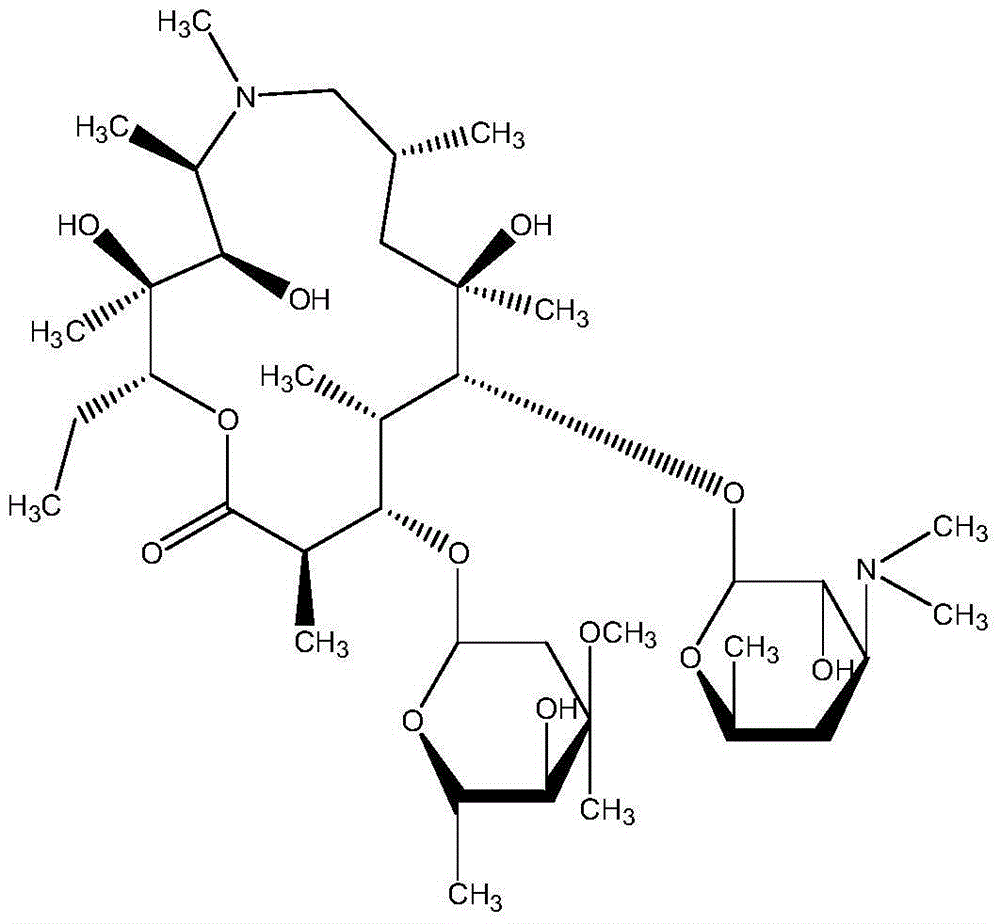
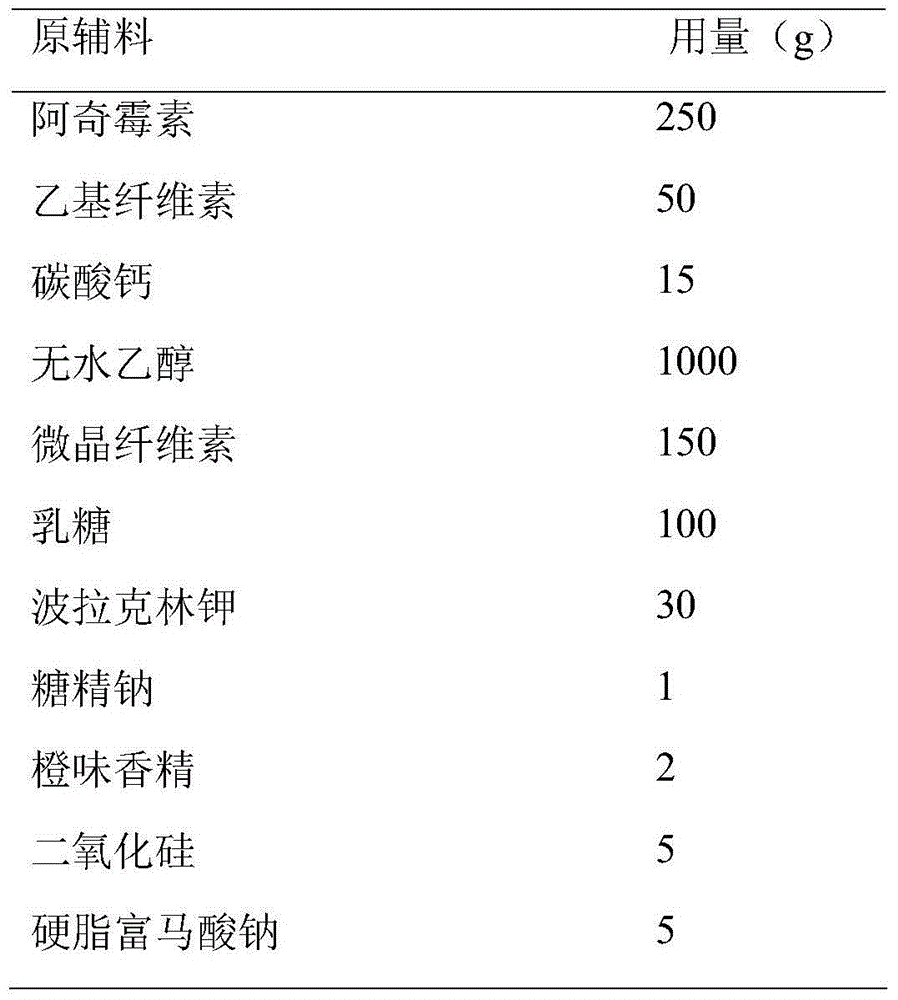
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
124results about "Antibacterial agents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
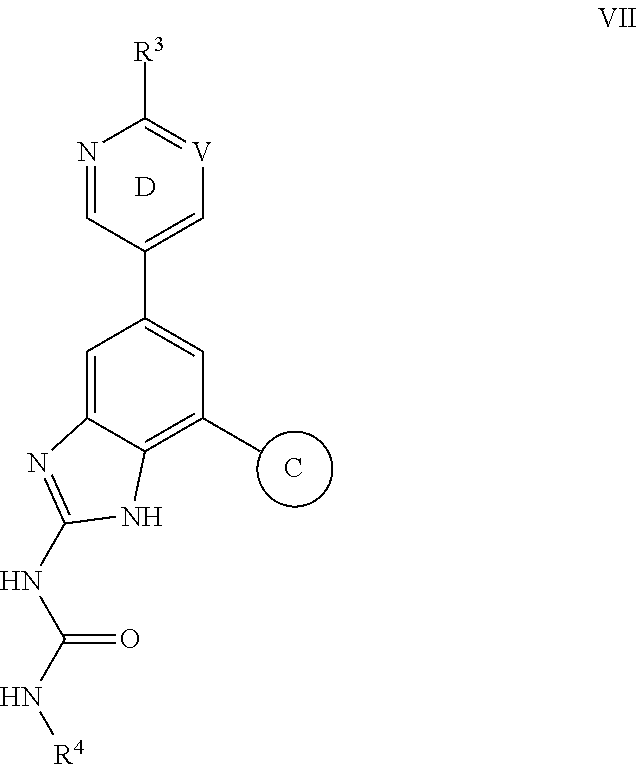
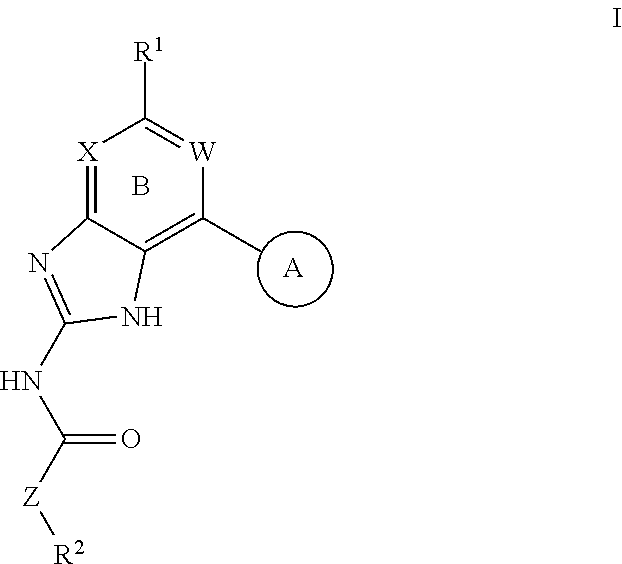
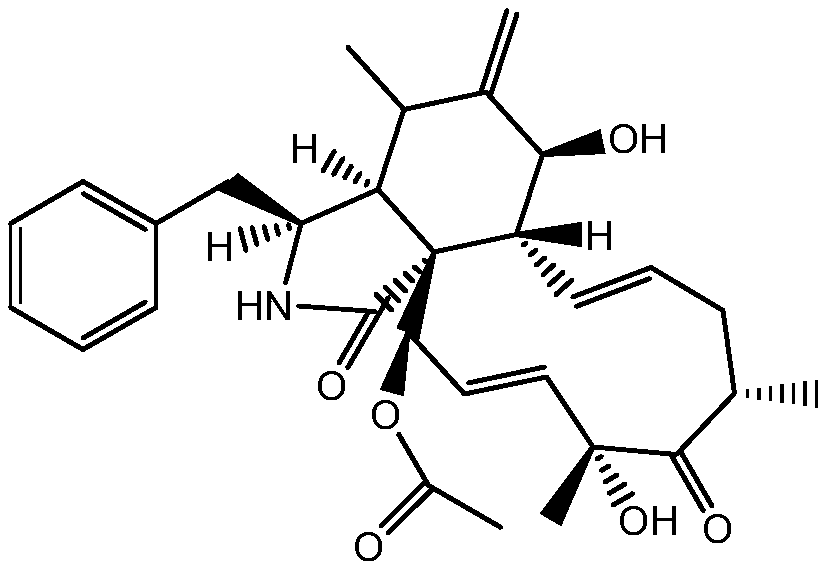
Compositions for sustrained release of nitric oxide, methods of preparing same and uses thereof
The invention provides compositions for releasing nitric oxide (NO) comprising a matrix that encapsulates nitric oxide. Nitric oxide is released when the composition is exposed to an aqueous environment. The invention further provides methods of preparing the compositions and uses of the compositions for treating infections and disorders.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Method of Delivering Rna Interference and Uses Thereof
InactiveUS20080153737A1Limiting potential side effectQuantity minimizationFusion with RNA-binding domainAntibacterial agentsGeneticsDouble strand
Owner:CHILDRENS MEDICAL CENT CORP
Gyrase Inhibitors and Uses Thereof
Owner:VERTEX PHARMA INC
Antifungal compound process
Owner:MYCOVIA PHARMA INC +1
Health-care and health-promotion pillow
InactiveCN102396950AEnhance body functionNo side effectsAntibacterial agentsPillowsCistancheCommon cold
The invention relates to a health-care and health-promotion pillow. The health-care and health-promotion pillow comprises a pillow inner, wherein the pillow inner is filled with the following Chinese herbal medicines: platycodon root, simpleleaf shrub chastetree fruit, platycladi seed, turmeric, evodia rutaecarpa, rhizoma atractylodis macrocephalae, mint, cinnamon, ligusticum wallichii, incised notopterygium rhizome, angelica sinensis, common monkshood mother root, sharpleaf galangal fruit, cistanche deserticola, manyprickle acathopanax root, divaricate saposhnikovia root, magnolia flower, ulmus macrocarpa hance, liquoric root, chrysanthemum, eucommia, combined spicebush root, pinellia ternate, angelica dahurica, prepared common monkshood daughter root, white paeony root, Chinese ligusticum rhizome, Chinese wild ginger, Chinese honeylocust fruit, bitter orange, Paris polyphylla, root of red-rooted salvia, cherokee rose fruit and hawthorn. When people sleep on the health-care and health-promotion pillow normally, the medicinal powder inside the pillow inner gives off slowly, and physical diseases (such as common cold, insomnia, hyper tension, scapulohumeral periarthritis, cervical spondylosis and the like) can be relieved or cured unknowingly. The health-care and health-promotion pillow integrates the effects of treatment, health protection and health promotion, is convenient to use, does not have stimulus, toxic or side effects on human bodies, and is suitable for home use.
Owner:王丰田
Mucosal Bioadhesive SLow Release Carrier for Delivering Active Principles
A mucosal bioadhesive slow release carrier comprising an active principle and devoid of starch, lactose, which can release the active principal for a duration of longer than 20 hours. This bioadhesive carrier contains a diluent, an alkali metal alkylsulfate, a binding agent, at least one bioadhesive polymer and at least one sustained release polymer, as well as a method for its preparation.
Owner:ONXEO SA
Fibrin-Binding Peptides and Conjugates Thereof
ActiveUS20100158814A1High degreeSuperior fibrin specific bindingUltrasonic/sonic/infrasonic diagnosticsCompound screeningBinding peptideCompanion animal
Owner:BRACCO IMAGINIG SPA
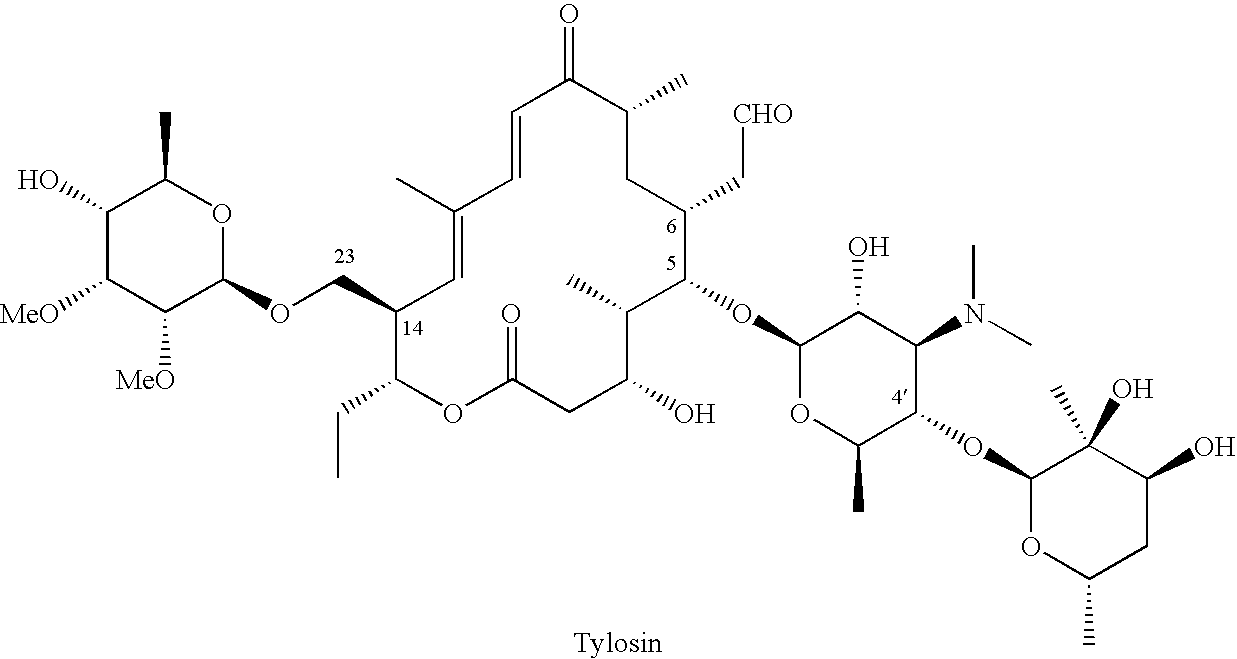
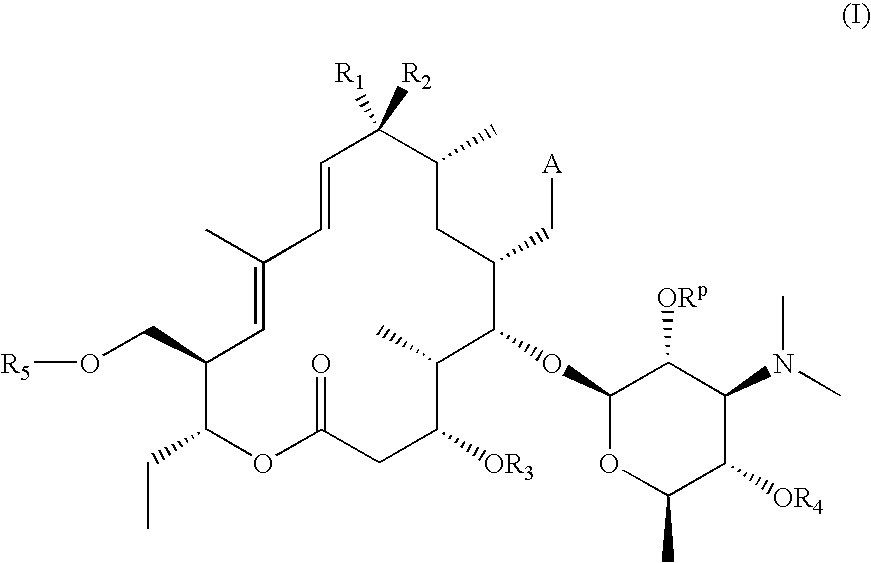
23-O-substituted 5-O-mycaminosyltylonide derivatives
InactiveUS20050020823A1High antibacterial activityOvercome bacterial resistanceAntibacterial agentsSugar derivativesGramAntibacterial activity
Owner:PHAN LY TAM +6
Tilmicosin soluble powder and preparation method thereof
ActiveCN104473876AImprove performanceCompliancePowder deliveryOrganic active ingredientsAdditive ingredientCombinatorial chemistry
Owner:SHANGHAI TONGREN PHARM CO LTD
Preparation methods of sulbenicillin sodium and injection thereof
InactiveCN101805356AReduce lossesEasy to operate the machineAntibacterial agentsOrganic chemistrySulbenicillinIon exchange
The invention discloses a preparation method of sulbenicillin sodium, which comprises the following steps of: sulfonating, hydrolyzing, crystallizing, ion-exchanging, acidylating, condensing, extracting and salifying to obtain sulbenicillin sodium. The method has the advantages of simple technological steps, fewer reaction impurities and high product purity, and the yield is higher than 50%. Meanwhile, the invention also relates to a preparation method of a sulbenicillin sodium injection.
Owner:HUNAN ERKANG XIANGYAO PHARMA
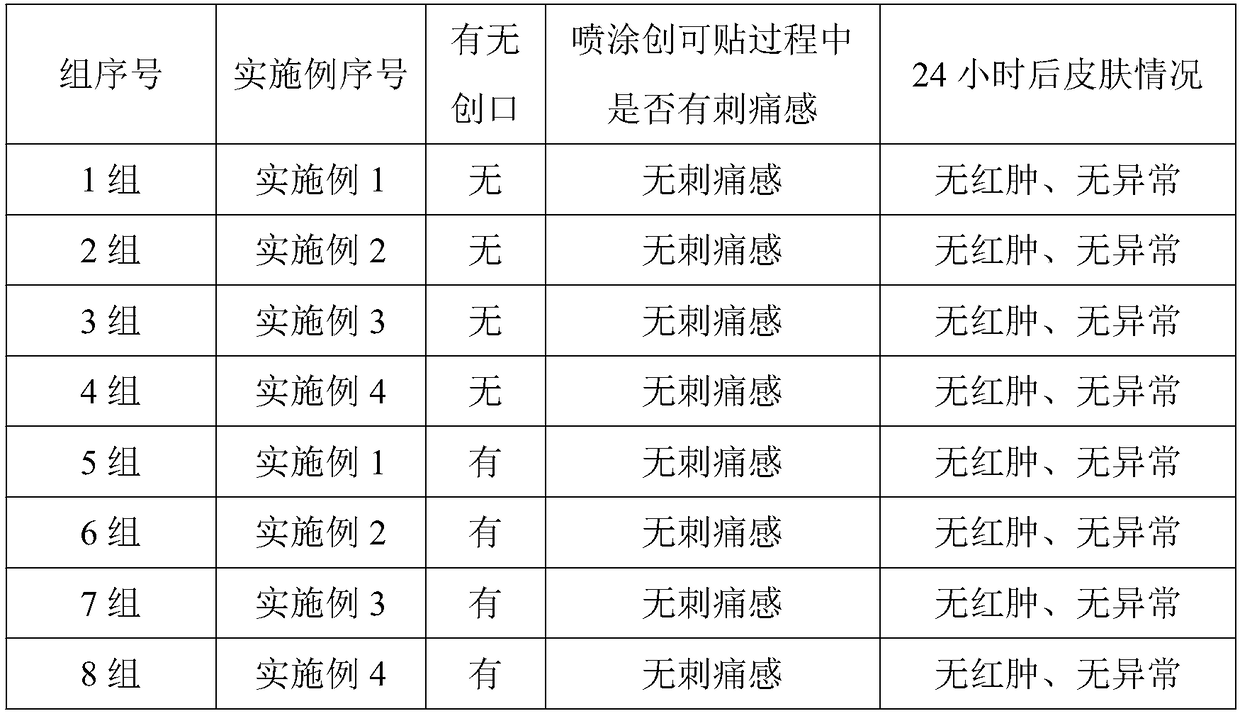
Wound cleaning liquid
ActiveCN103505490APromote healingNot easy to stayAntibacterial agentsAntipyreticMentha spicata extractMint extract
The invention provides a wound cleaning liquid, which comprises an active component, an osmotic pressure regulator and deionized water; the active component is one or more from a wormwood extract, an aloe extract and a mint extract. The wound cleaning liquid provided by the invention has no stimulation to a wound, can clean the wound, also has bactericidal, anti-inflammatory, analgesic, and wound healing promoting effects, and scars are not easy to leave.
Owner:BYD CO LTD
Compositions comprising multiple bioactive agents, and methods of using the same
Owner:DEBIOPHARM INTERNATIONAL SA
Preparation of sodium probenecid and potassium probenecid, compound injection prepared by sodium probenecid, potassium probenecid and beta-lactam antibiotics, and use thereof
ActiveCN1631876AEliminate hemolyticExtended half-lifeAntibacterial agentsOrganic chemistryBeta lactam antibioticHalf-life
Owner:BEIJING KANGZHENG KANGREN BIOTECH
Cefmetazole sodium proliposome preparation
ActiveCN101623264AThe encapsulation efficiency has not decreasedImprove stabilityOrganic active ingredientsAntibacterial agentsCholesterolPhospholipid
The invention provides a cefmetazole sodium proliposome preparation which comprises the following components by weight part: 1 part of cefmetazole sodium, 3-15 parts of liposome carrier and 2-10 parts of proppant, wherein the liposome carrier comprises polyene phosphatidyl choline, cholesterol and oleinic acid according to a weight ratio of (4-20):(1-5):1. The cefmetazole sodium proliposome preparation has good preparation stability and cannot crack because of dewatering, fusion, ice crystal generation, and the like in a freeze-drying process; and after hydrated re-dissolution, the cefmetazole sodium proliposome preparation still can maintain good entrapment rate.
Owner:HAINAN LINGKANG PHARMA CO LTD
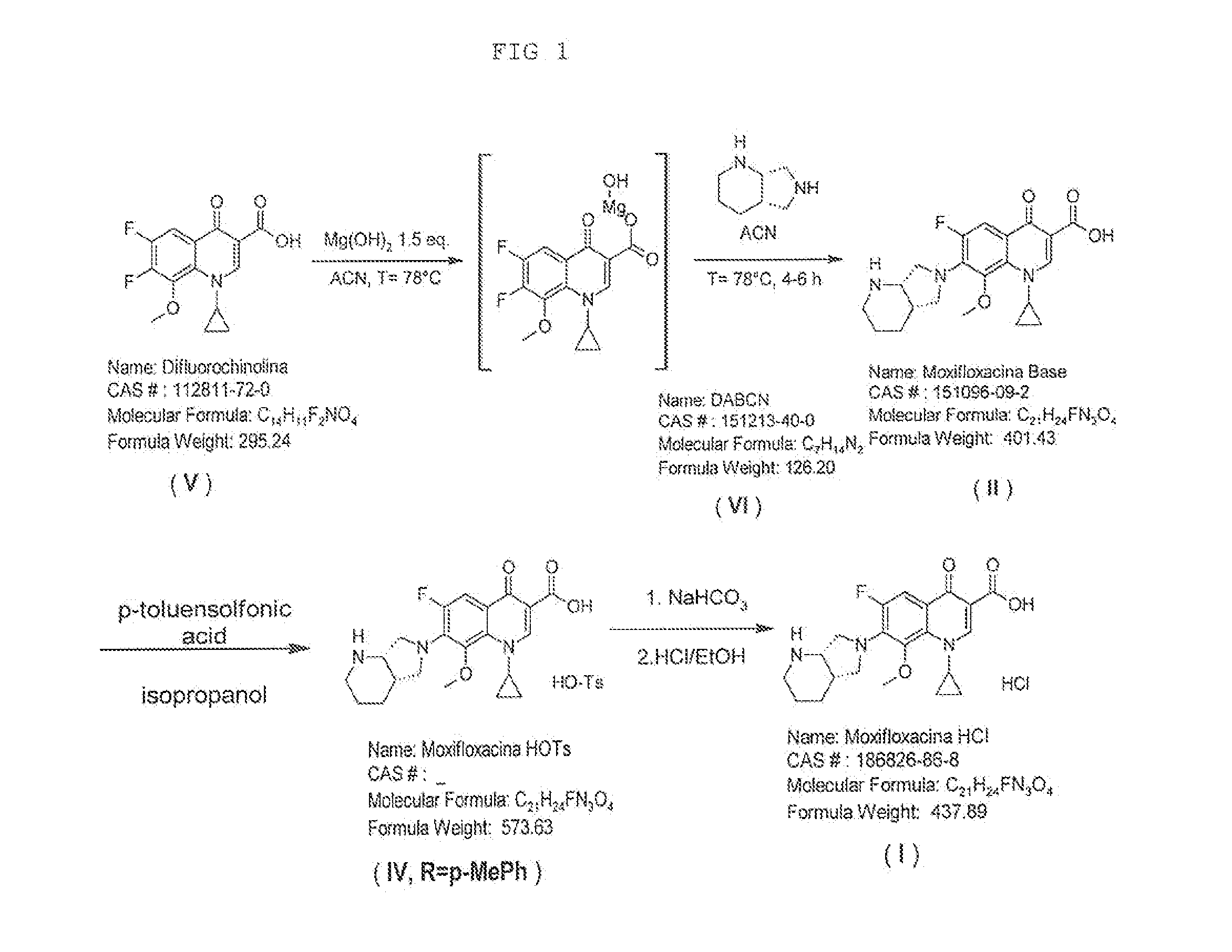
Moxifloxacin hydrochloride compounds and intermediates and methods for making same
Owner:F I S FAB ILTALIANA SINTETICI SPA
Compound ozone oil and preparation method
InactiveCN106728416AEfficient killingSignificantly sterilizedAntibacterial agentsInorganic active ingredientsTreatment effectCnidium monnieri
Owner:黄庆文 +1
Antibacterial wet wipe for skin care
ActiveUS10456437B1Good antibacterialExcellent disinfection propertyCosmetic preparationsAntibacterial agentsCeramideFungicide
Owner:JIANG SHULAN
A kind of traditional Chinese medicine composition for treating pulmonary tuberculosis
The invention discloses a traditional Chinese medicine composition for treating pulmonary tuberculosis. The components and ratios of raw materials used in the traditional Chinese medicine composition are as follows: 4-7 parts of aster, 4-7 parts of Anemarrhena, 3-6 parts of platycodon, batch 7-10 parts of leaves, 4-8 parts of Prunella vulgaris, 4-8 parts of Agrimony, 3-6 parts of Baibu, 3-6 parts of Chuanbei, 8-12 parts of Bletilla striata, 7-10 parts of L. 3-6 servings, 3-6 servings of Ophiopogon japonicus, 1.5-2.5 servings of Chonglou. The medicine of the invention has the functions of nourishing the lungs, relieving cough, reducing inflammation, sterilizing, dispelling stagnation and healing wounds, and can completely cure pulmonary tuberculosis in a short time.
Owner:白思蓉
A preparation method and application of cytochalasin compounds
Owner:GUANGXI NORMAL UNIV
Heat-stable, aqueous lactoferrin composition and its preparation and use
Owner:FRIESLANDCAMPINA NEDERLAND BV
Immunogenic composition
The invention provides immunogenic polysaccharide protein conjugates comprising capsular polysaccharides from N. Meningitidis serogroup X and methods for preparation thereof. The present invention relates to N. meningitidis X saccharide-carrier protein conjugates prepared by a conjugation reaction. Accordingly, the instant invention relates to multivalent meningococcal polysaccharide protein conjugate composition comprising capsular saccharide from serogroups X and at least one capsular saccharide from A, C, W135 and Y wherein, i) polysaccharides A C W135 X are sized mechanically whereas polysaccharide Y is sized chemically, ii) all saccharide are conjugated to carrier protein via a linker with a cyanylation conjugation chemistry iii) all saccharide to protein ratios in final conjugates are between 0.2-0.6 and iv) at least two different carrier proteins selected from the group consisting of TT, DT and CRM197 are utilized.
Owner:SERUM INST OF INDIA PTE LTD
Method for keeping quality stability of clindamycin phosphate injection
InactiveCN102018960APrevent oxidationNot easily oxidizedOrganic active ingredientsAntibacterial agentsSulfite saltAntioxidant
Owner:ANHUI WANBEI PHARMA
Sterilizing gel
InactiveCN1682703AAntibacterial agentsPharmaceutical delivery mechanismChlorhexidinePharmaceutical preservatives
Owner:GUANGZHOU PUIS PHARMA FACTORY
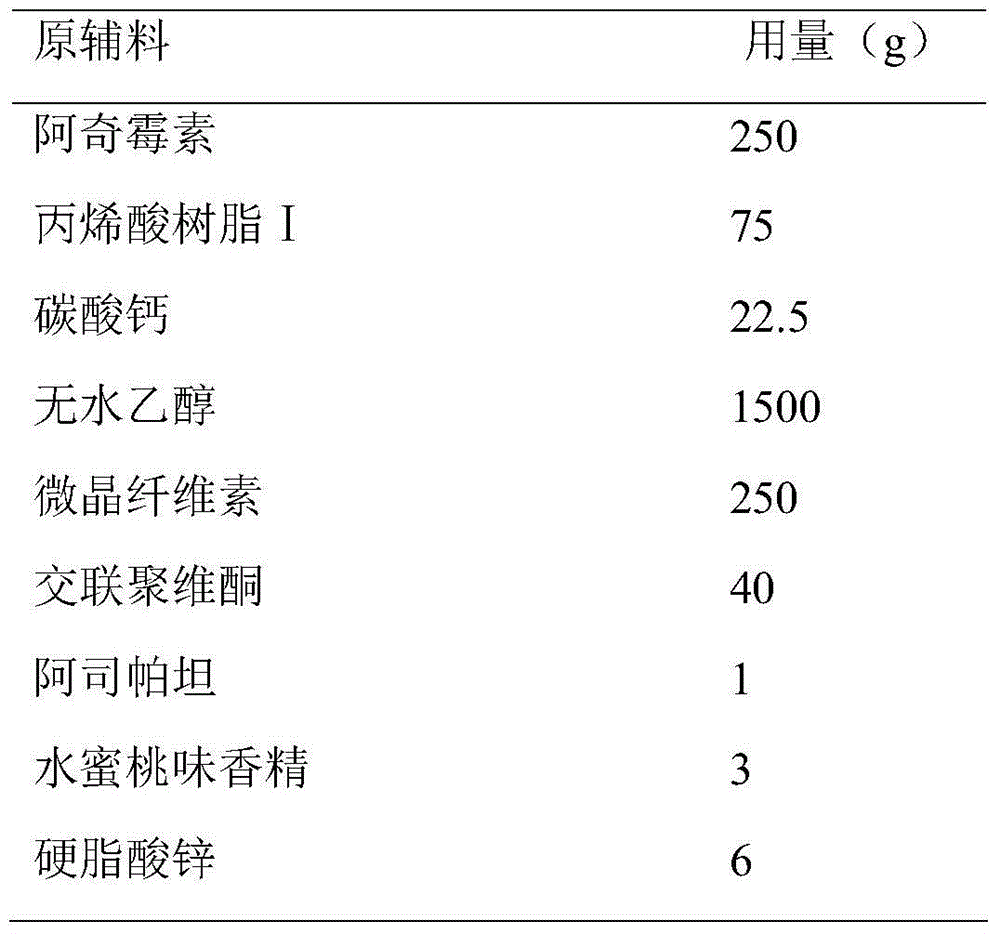
Azithromycin dispersible tablet
Owner:LUNAN BETTER PHARMA
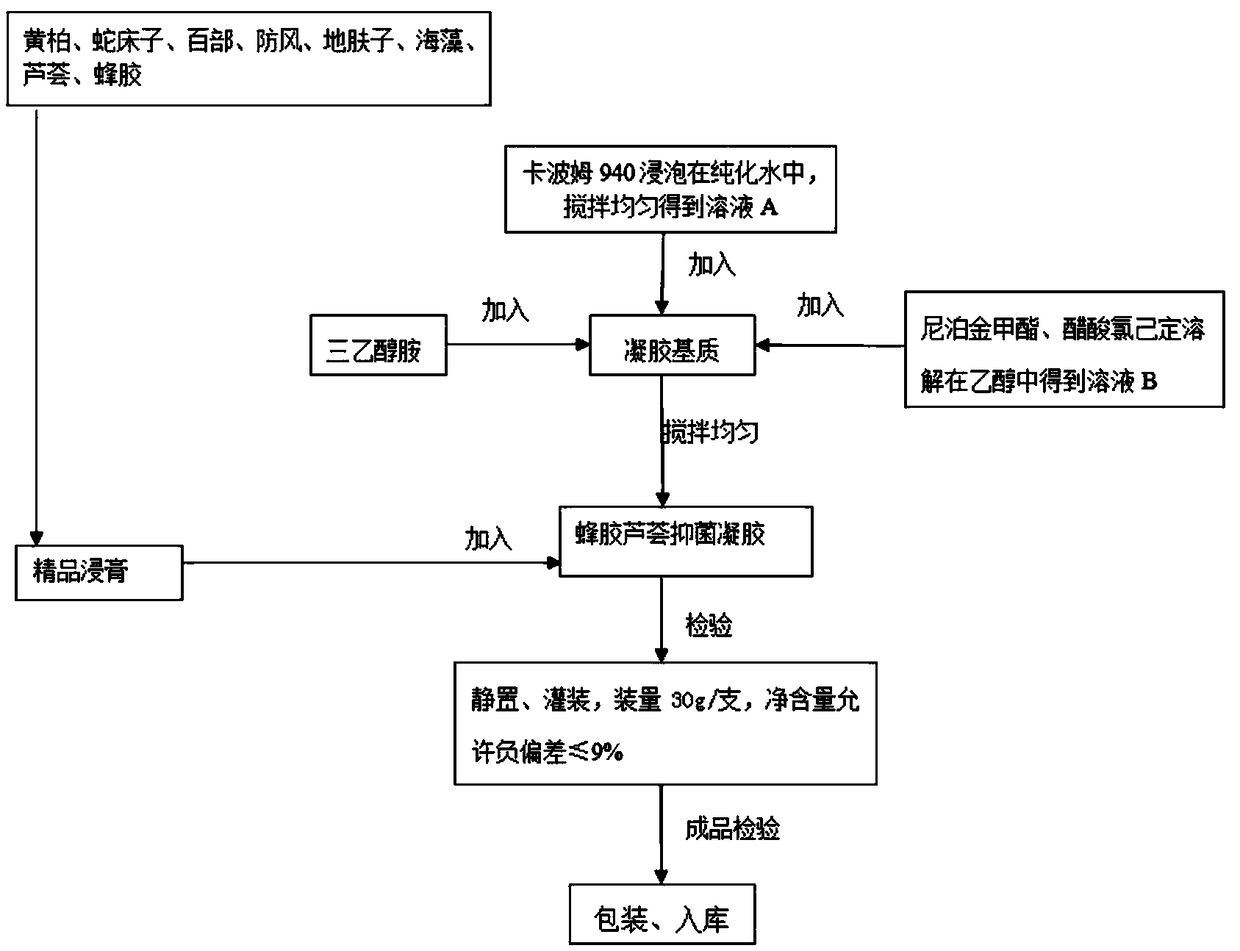
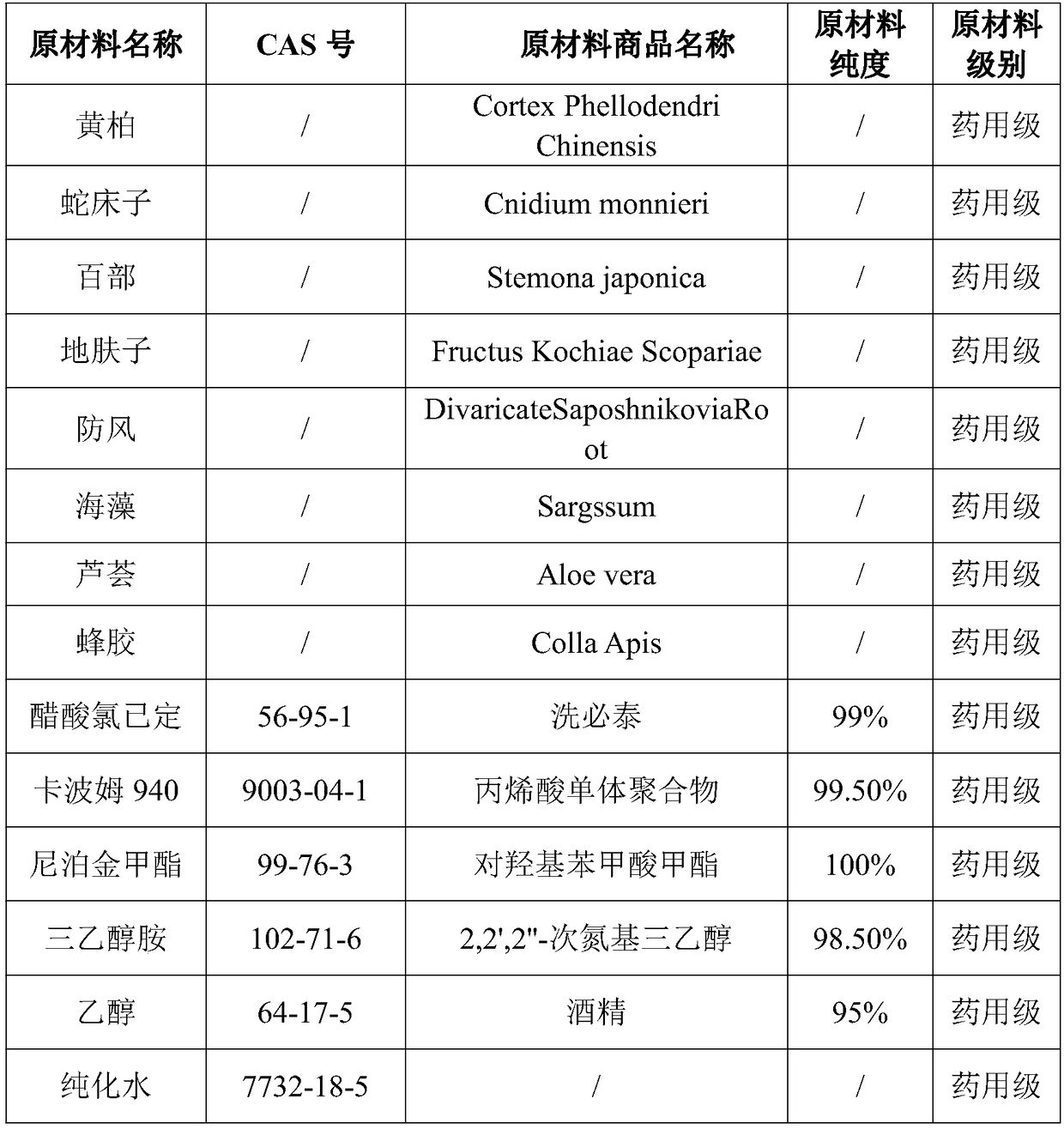
Propolis-aloe bacteriostatic gel and preparation method thereof
InactiveCN108853409AEasy to prepareEasy to useOrganic active ingredientsAntibacterial agentsEscherichia coliPersonal care
Owner:西安中天生物医药有限公司
Composition for oral cavity and application and preparation method of composition
ActiveCN108464963AReduce bad breathUniversalCosmetic preparationsAntibacterial agentsPropolisGum inflammation
Owner:HONGMEI PHARMA CHINA
Preparation method of high-purity gynostemma pentaphylla total saponin for veterinary drug
ActiveCN103520256AHigh purityMeet the protection requirementsAntibacterial agentsAntimycoticsReflux extractionMicrofiltration
Owner:江西嘉博生物工程有限公司 +1
Ephedra, almond gypsum and licorice granule pill
InactiveCN103623088AReduce manufacturing costAntibacterial agentsPharmaceutical delivery mechanismMonoglycerideAcrylic resin
Owner:JIANGSU HFQ BIO TECH CO LTD
Reinforced mosaic gene recombinant bacillus calmette-guerin and preparation method thereof
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap