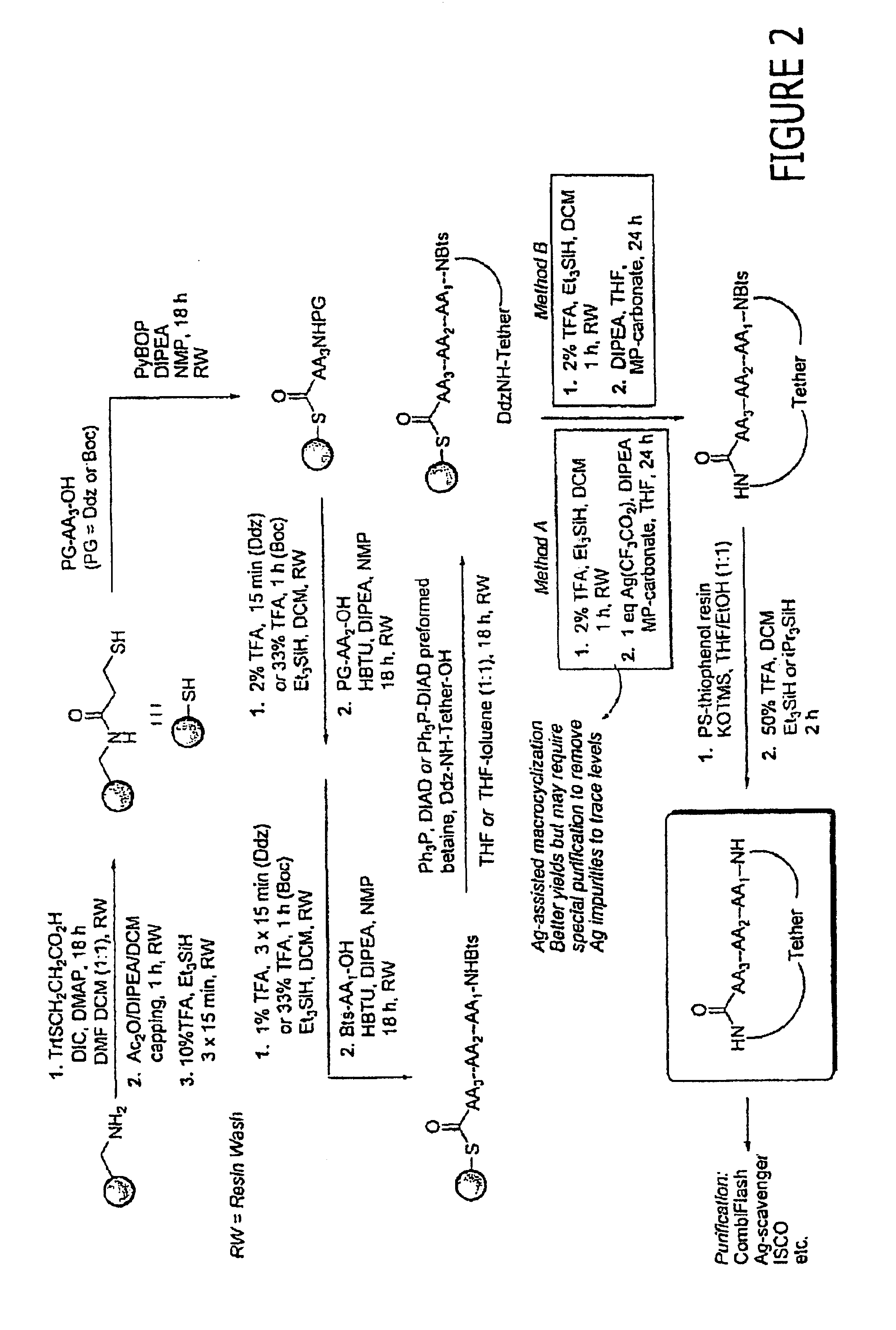
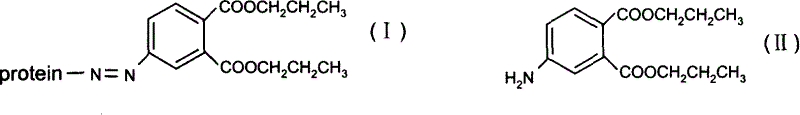Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44results about "Immunoglobulins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
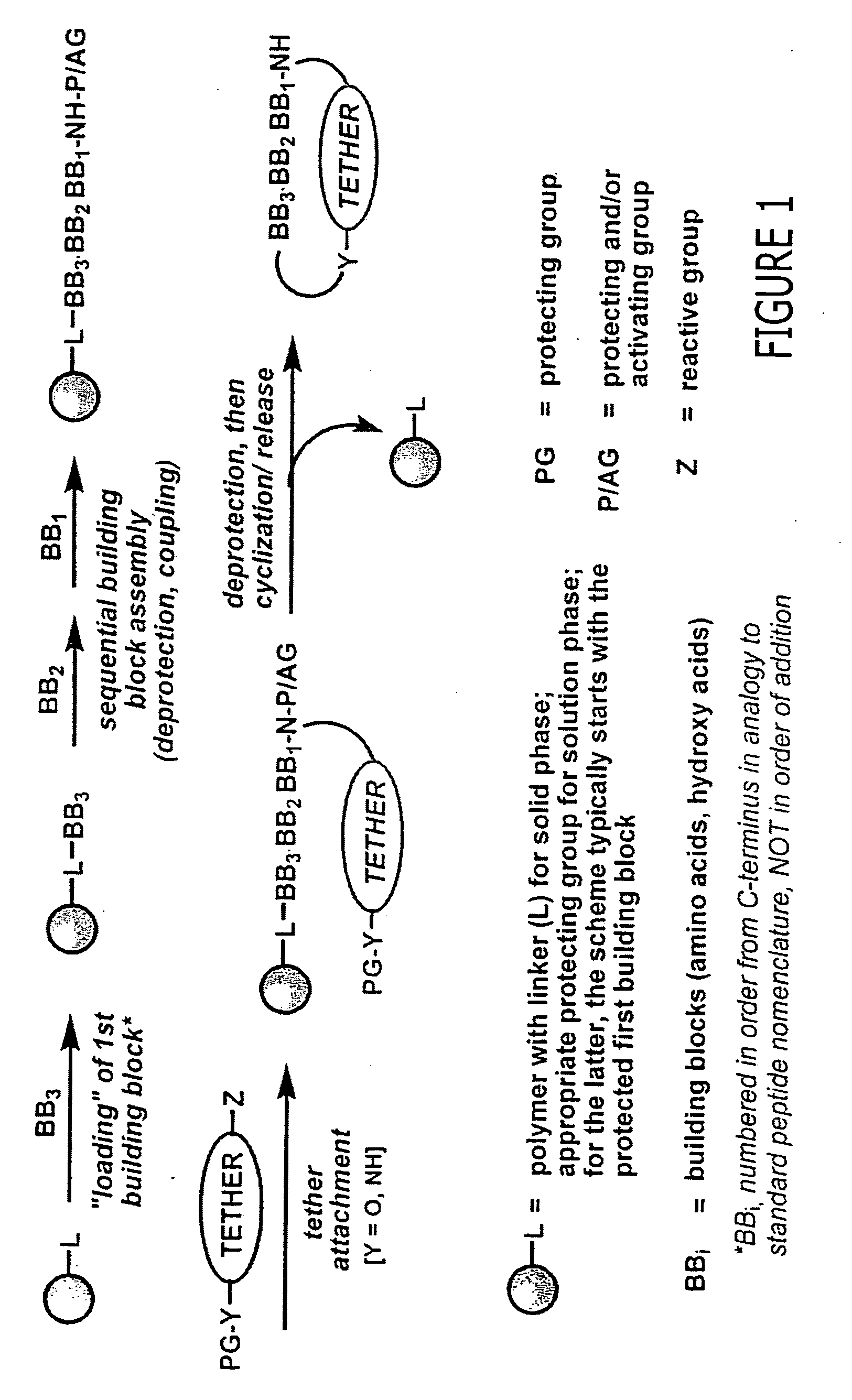
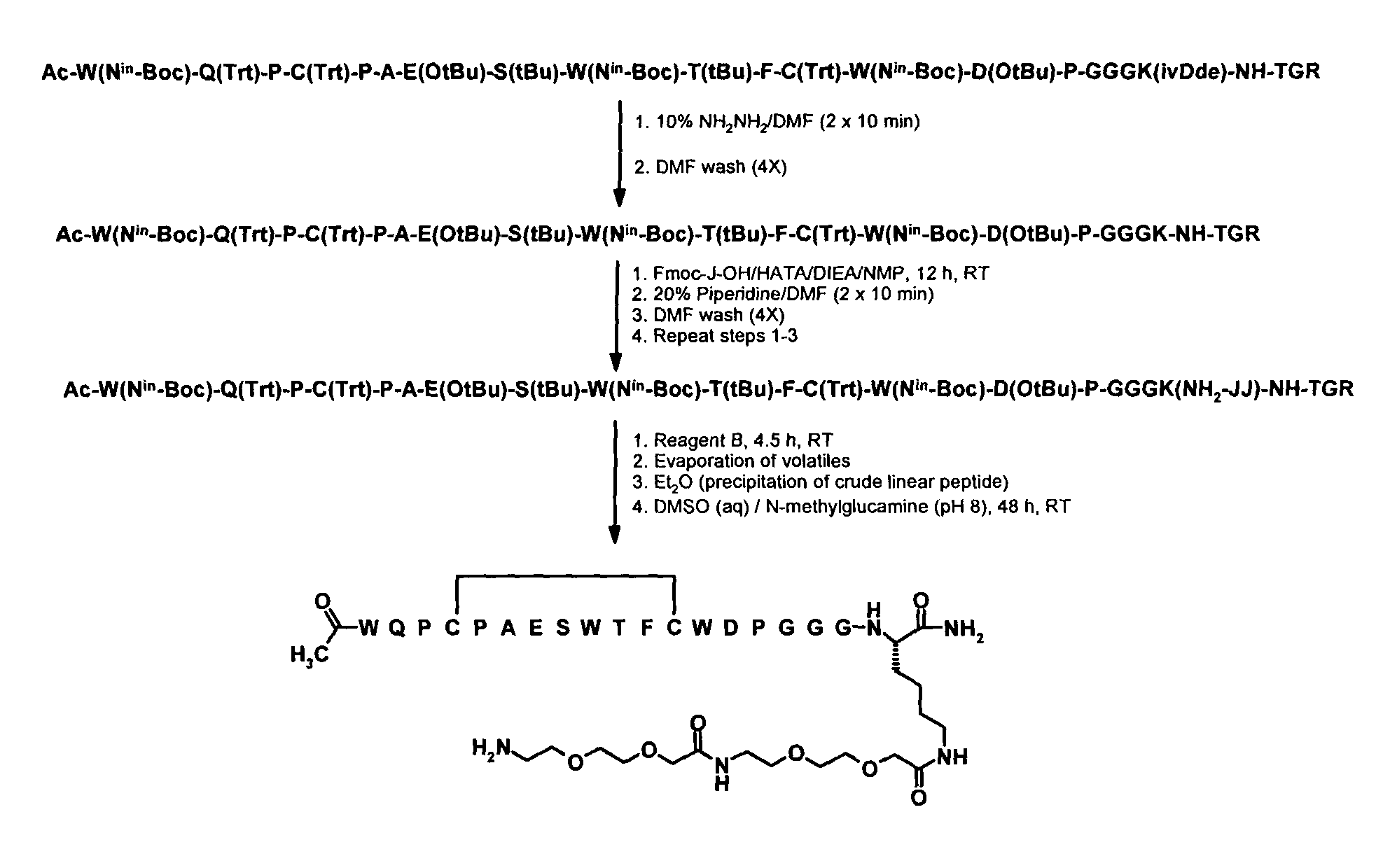
Methods and compositions related to peptides and proteins with c-terminal elements
ActiveUS20090226372A1Powder deliveryMicrobiological testing/measurementCell selectivityPeptide sequence
Disclosed are compositions and methods useful for targeting and internalizing molecules into cells of interest and for penetration by molecules of tissues of interest. The compositions and methods are based on peptide sequences that are selectively internalized by a cell, penetrate tissue, or both. The disclosed internalization and tissue penetration is useful for delivering therapeutic and detectable agents to cells and tissues of interest.
Owner:SANFORD BURNHAM MEDICAL RES INST
Caustic stable chromatography ligands
ActiveUS20100221844A1Cost-effectiveComponent separationBiological material analysisImmunoglobulin bindingImmunoglobulin class
The present invention relates to chromatography ligands having improved caustic stability, e.g., ligands based on immunoglobulin-binding proteins such as, Staphylococcal protein A, as well as methods of making and using such ligands.
Owner:EMD MILLIPORE CORP
Non-mammalian GnRH analogs and uses thereof in the immune system
InactiveUS20050043245A1Effective supervisionHigh affinityPeptide/protein ingredientsLuteinising hormone-releasing hormoneDiseaseD-Arginine
Specially designed non-mammalian GnRH, its analogs, or biometics resistant to degradation by peptidase, are disclosed. The GnRH analogs are further defined as analogs of GnRH II or salmon GnRH. These non-mammalian analogs incorporate D-arginine, D-leucine, D-tBu-Serine, D-Trp or other active D amino acids at position 6 and ethylamide, aza-Gly-amide or other Gly amide at position 10. The D-Arg (6)—GnRH II-ethylamide, D-Arg (6)—GnRH II-aza-Gly (10)-amide, the D-Arg (6)—salmon GnRH ethylamide, and D-Arg (6)—salmon GnRH-aza-Gly (10)-amide analogs are also provided, and demonstrate preferential binding to immune system non-mammalian GnRH receptors. These non-mammalian GnRH or its analogs, or long-acting preparation, biometics or their antibodies may be used in pharmaceutical preparation, and specifically in treatment of various immune system disorders. The non-mammalian GnRH or its analogs are also provided in pharmaceutical preparations that may be used clinically for treating immune system disorders when used in very low doses and administered in pulsatile fashion. The aza-Gly (10) amide non-mammalian analogs are yet other embodiments of the non-mammalian GnRH or its analogs provided as a part of the invention. The use of agents that regulate the production or antibodies or In addition, the detection of non-mammalian GnRH or GnRH II or the non-mammalian GnRH receptors may be used as a diagnostic tool.
Owner:SILER KHODR THERESA
Fluorescent compounds
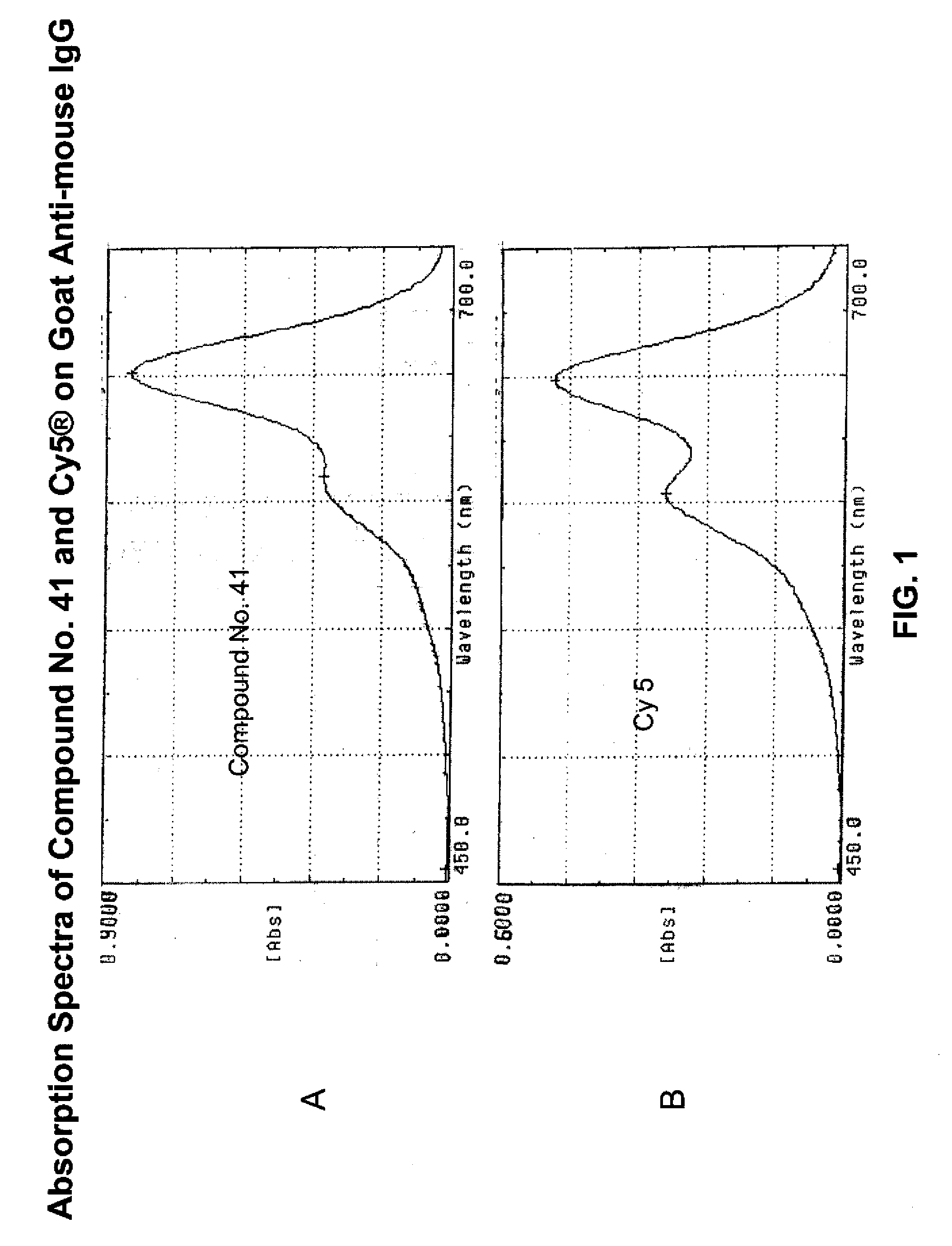
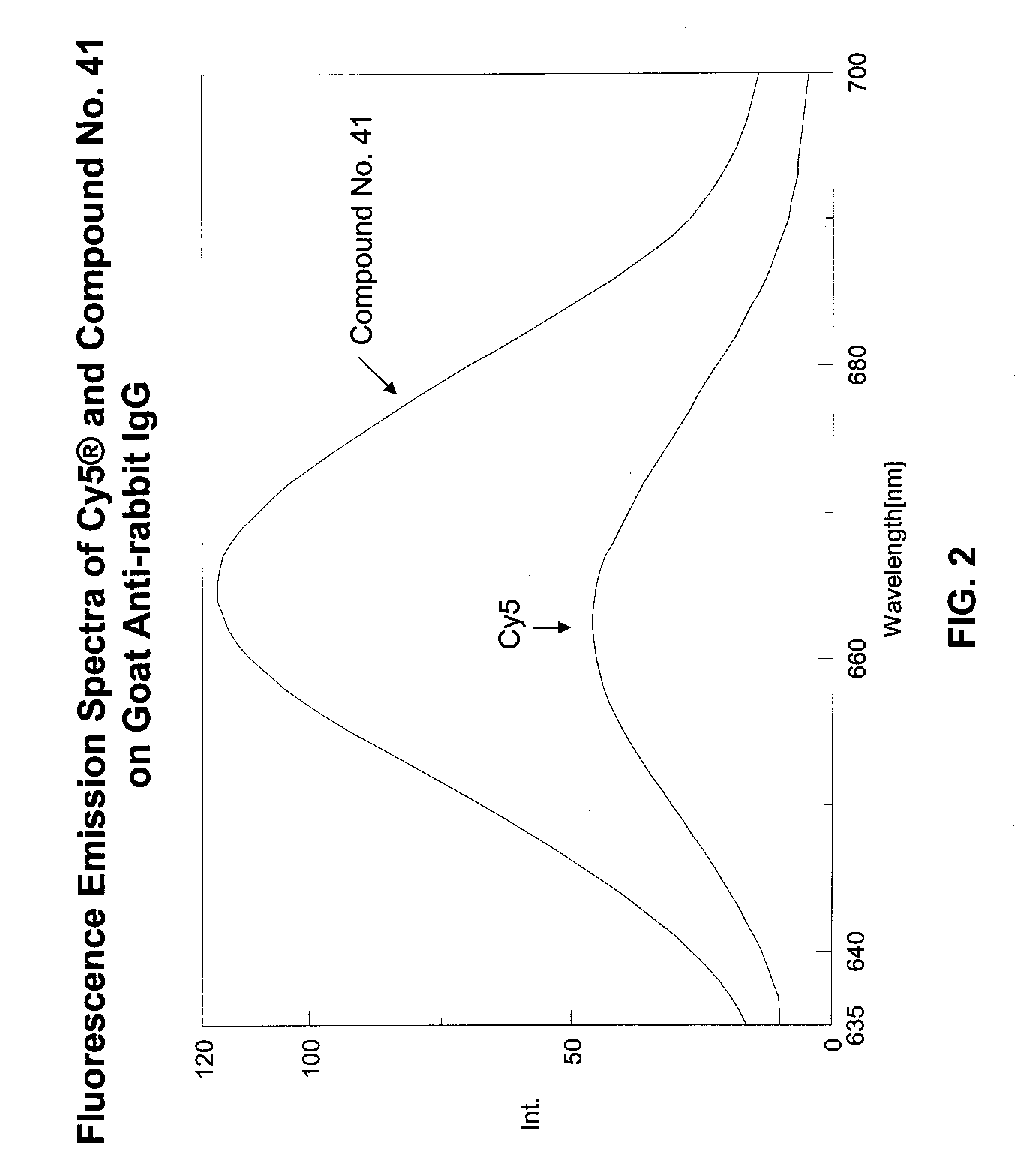
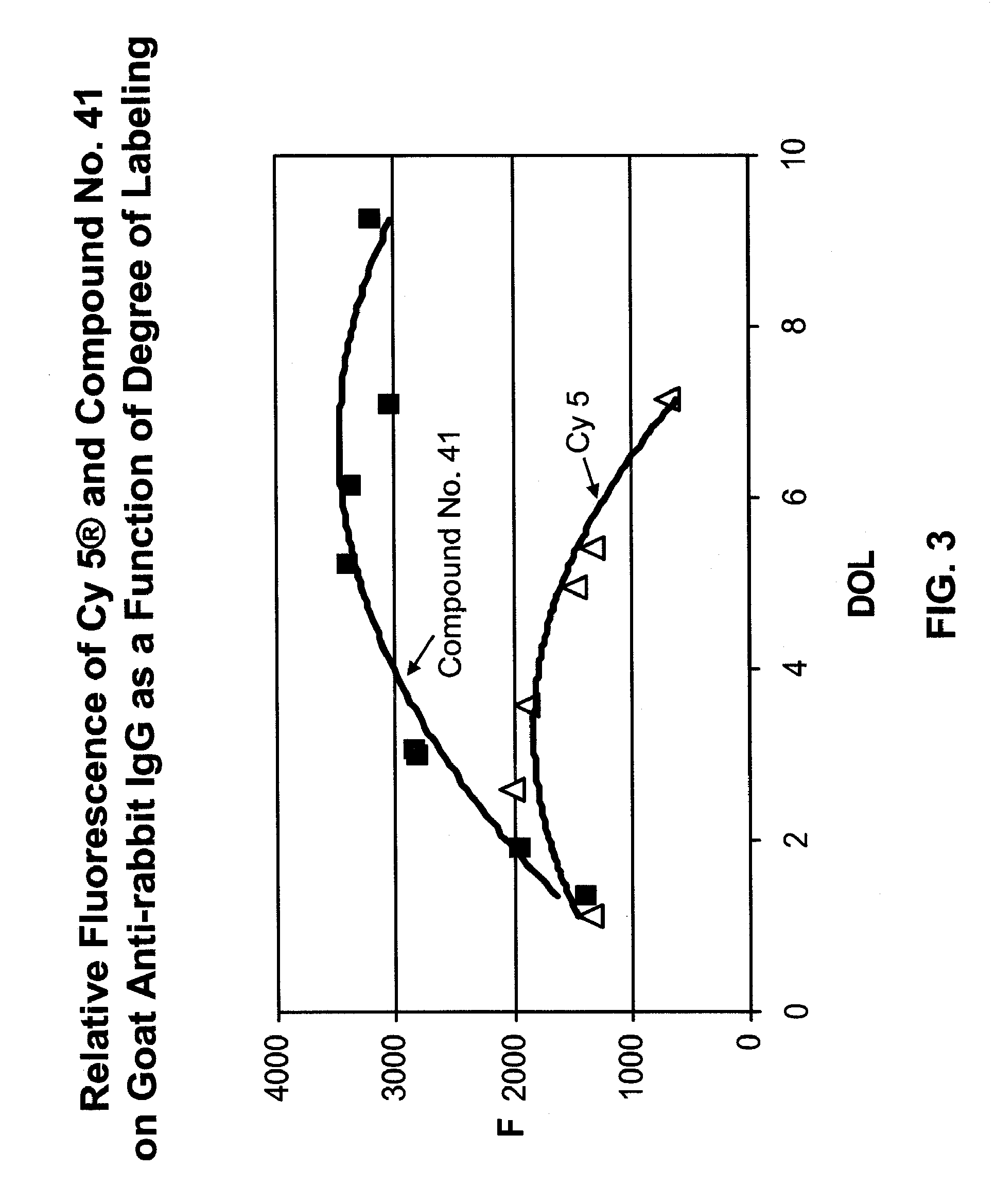
ActiveUS20090305410A1Convenient and effective labelingMethine/polymethine dyesPeptide/protein ingredientsBiotechnologyDisease
Owner:BIOTIUM INC
Macrocyclic modulators of the ghrelin receptor
ActiveUS20060025566A1Promote gastrointestinal motilityModulating activity of receptorDigestive systemImmunoglobulinsInflammationCentral nervous system
Owner:OCERA THERAPEUTICS INC
Genes differentially expressed in cancer cells to design cancer vaccines
Owner:GENZYME CORP
Method of Delivering Rna Interference and Uses Thereof
InactiveUS20080153737A1Limiting potential side effectQuantity minimizationFusion with RNA-binding domainAntibacterial agentsGeneticsDouble strand
Owner:CHILDRENS MEDICAL CENT CORP
Filler for affinity chromatography
ActiveUS20130085199A1High dynamic binding capacityGood alkali resistanceSolid sorbent liquid separationImmunoglobulinsEpoxyPorous particle
Owner:JSR CORPORATIOON
Methods for the treatment of lada and other adult- onset autoimmune using immunosuppressive monoclonal antibodies with reduced toxicity
InactiveUS20100015142A1Low toxicityDamage autoimmunityMetabolism disorderAntibody ingredientsDiseaseAutoimmune responses
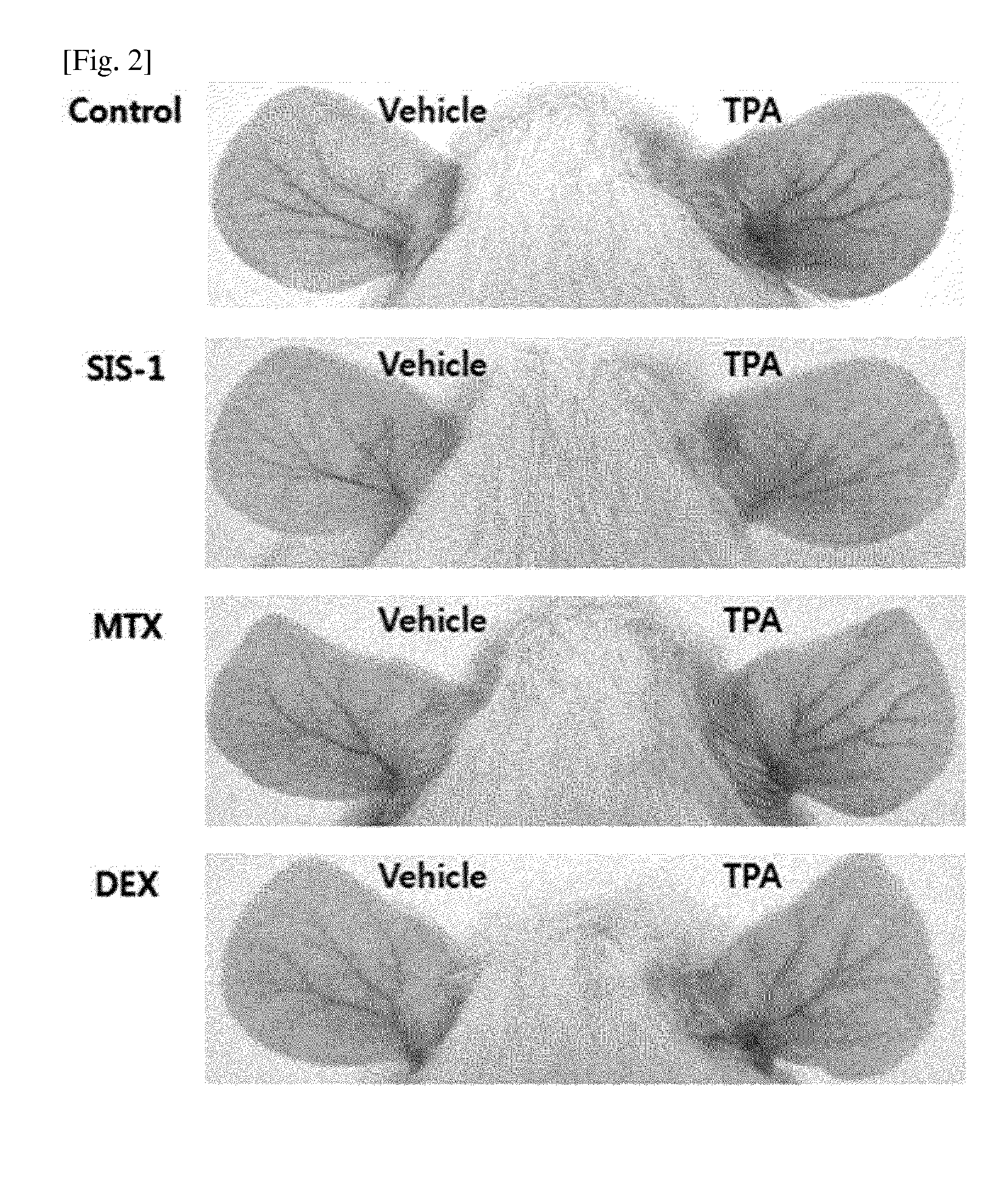
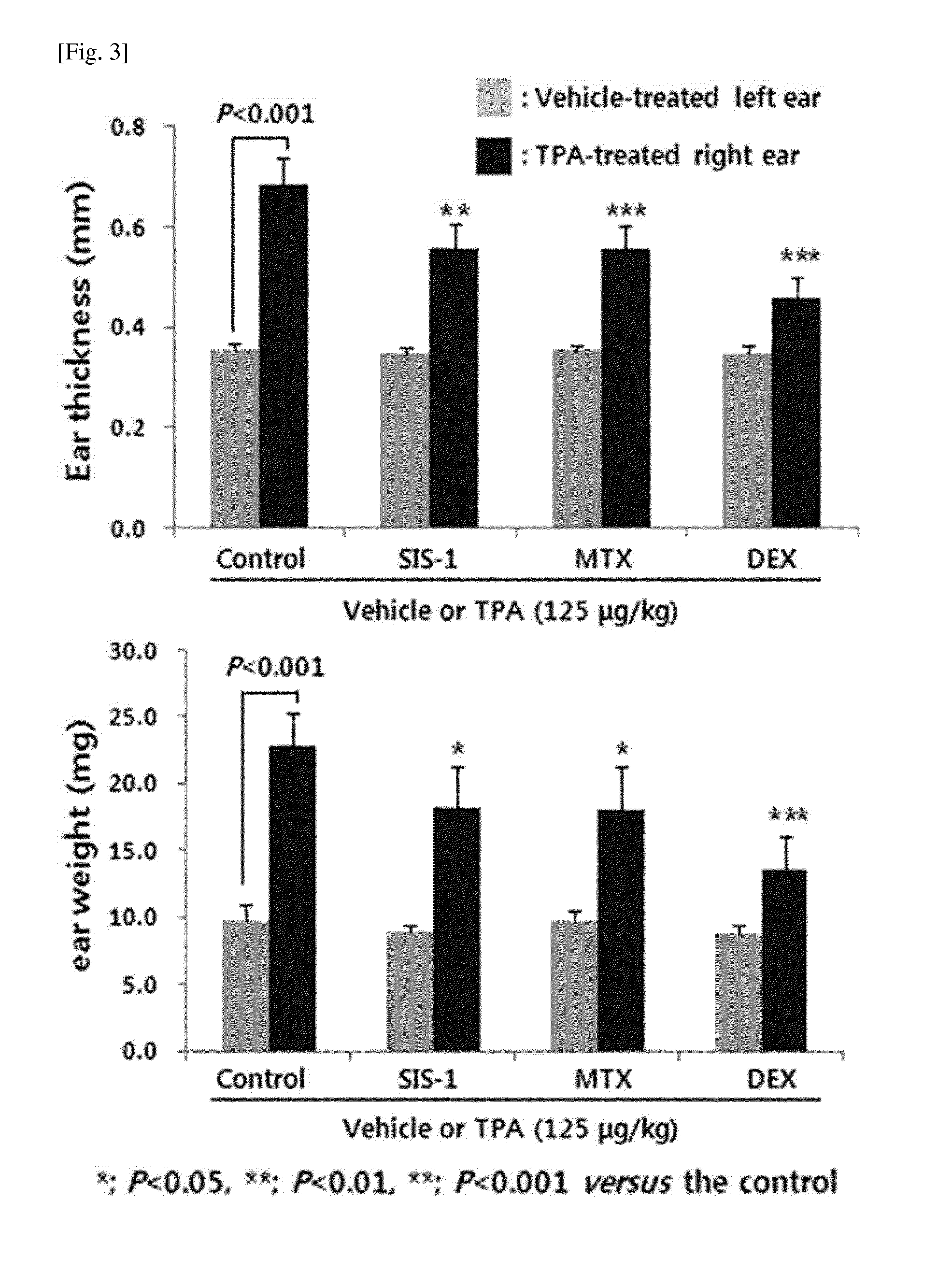
The present invention provides methods of treating, preventing or ameliorating the symptoms of Latent Autoimmune Diabetes in Adults (LADA) and adult-onset type 1 diabetes through the use of anti-human CD3 antibodies. In particular, in invention provides methods of preventing or delaying insulin requirement in patients diagnosed with LADA. The methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the invention provides for modification of the anti-human CD3 antibodies such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-human CD3 antibodies.
Owner:MACROGENICS INC
Method for retarding unhealth manifestations brought by ageing of human beings
InactiveUS20090053200A1Reduce functionReduced stress resistancePeptide/protein ingredientsHydrolasesDna antibodyBlood plasma
Owner:CLS THERAPEUTICS
Adenovirus vector containing a heterologous peptide epitope in the hi loop of the fiber knob
InactiveUS7297542B2Efficient transductionRaise transfer toBiocideAntibody mimetics/scaffoldsHeterologousEpitope
The present invention provides means to modify the tropism of recombinant adenoviral vectors using genetic methods to alter the adenoviral fiber cell-binding protein. The present invention generates an adenovirus with modified fiber gene such that novel tropism is achieved. This recombinant adenovirus has a fiber gene modified in the HI loop domain.
Owner:UAB RES FOUND
Macrocyclic modulators of the ghrelin receptor
InactiveUSRE42013E1Promote gastrointestinal motilityModulating activity of receptorMetabolism disorderPeptide preparation methodsGrowth hormone-releasing peptideInflammation
Owner:OCERA THERAPEUTICS INC
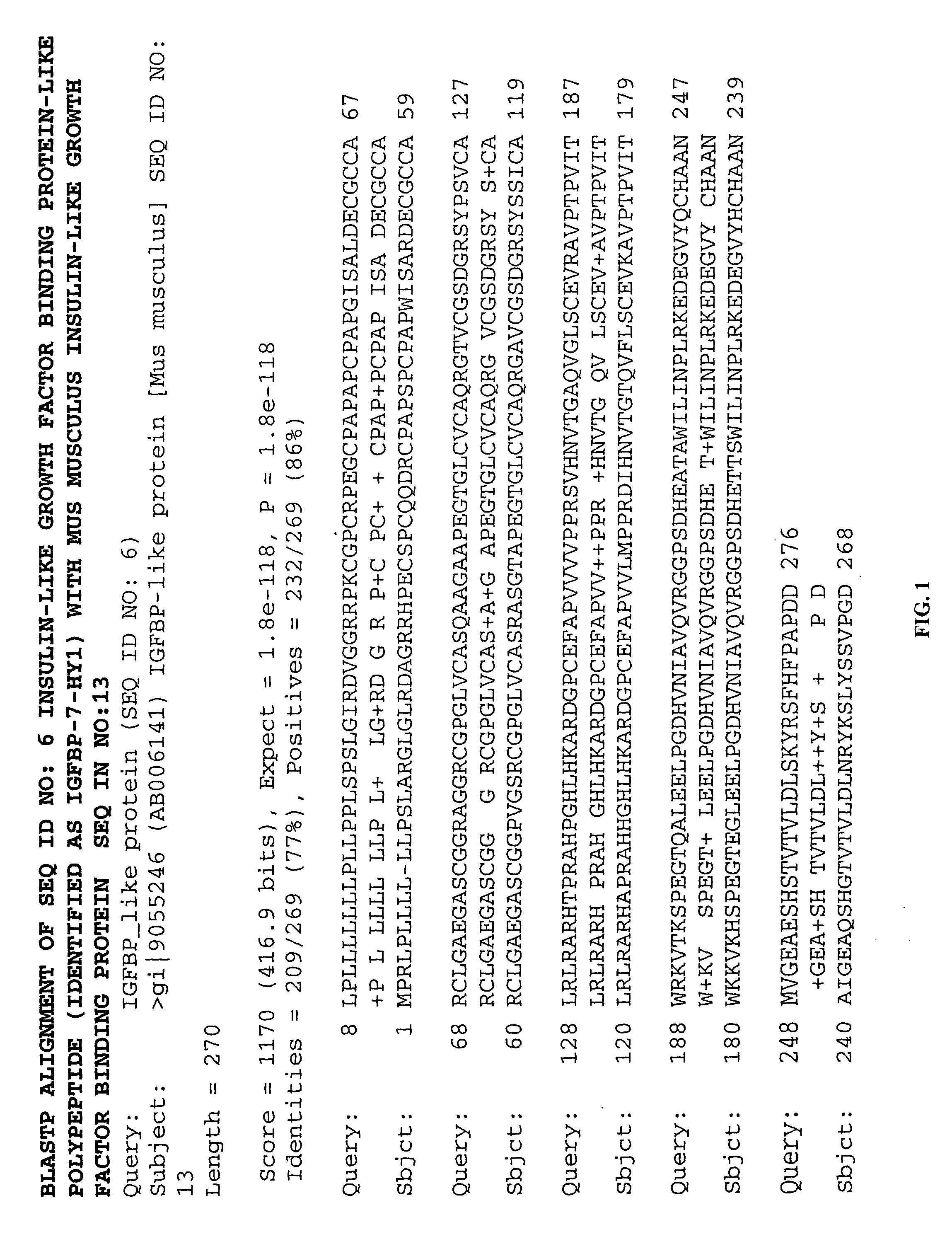
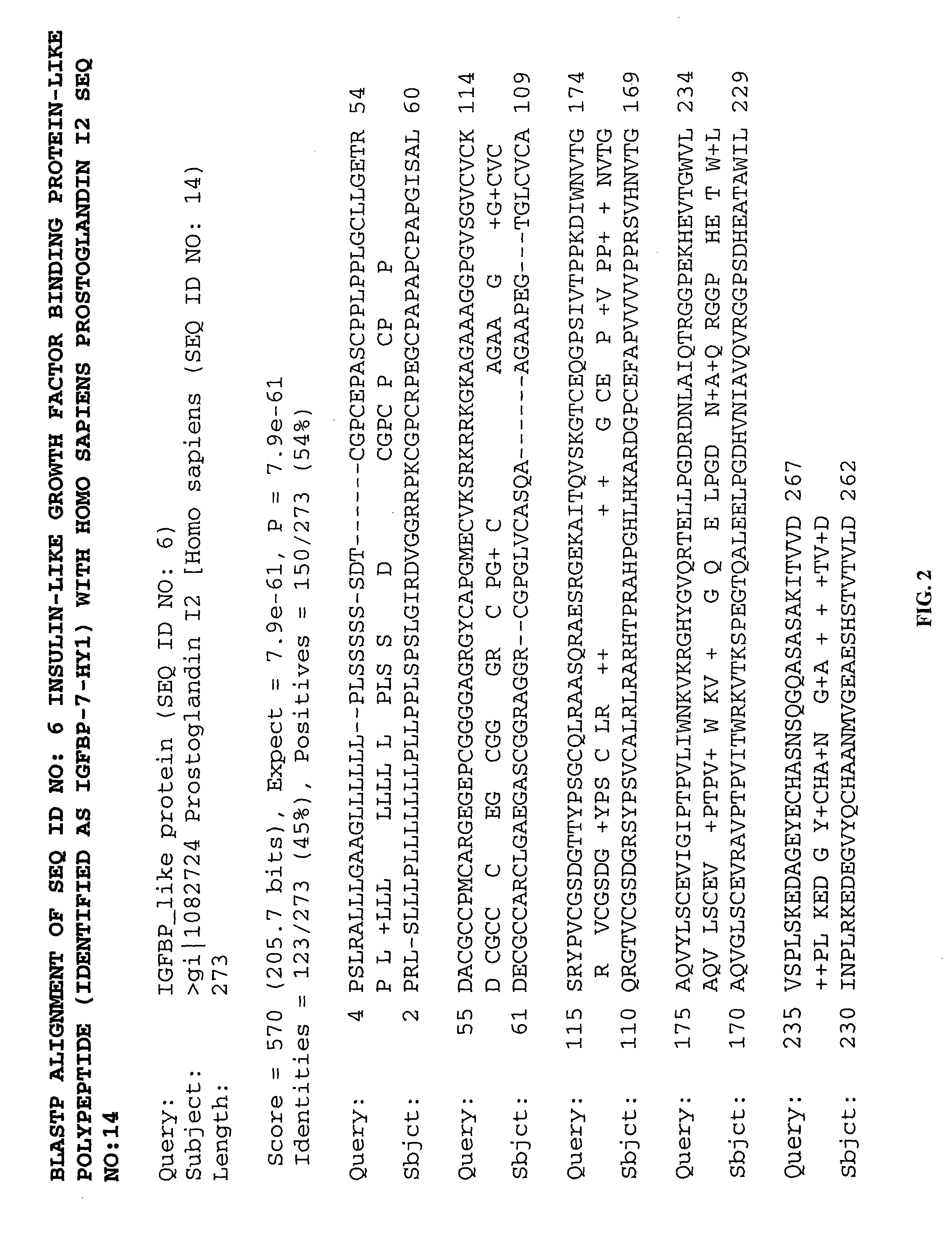
Methods of therapy and diagnosis using insulin-like growth factor binding protein-like polypeptides and polynucleotides
InactiveUS20060073514A1Promote wound healingReduced activityPeptide/protein ingredientsReceptors for hormonesNucleotideMutant
Owner:NUVELO INC
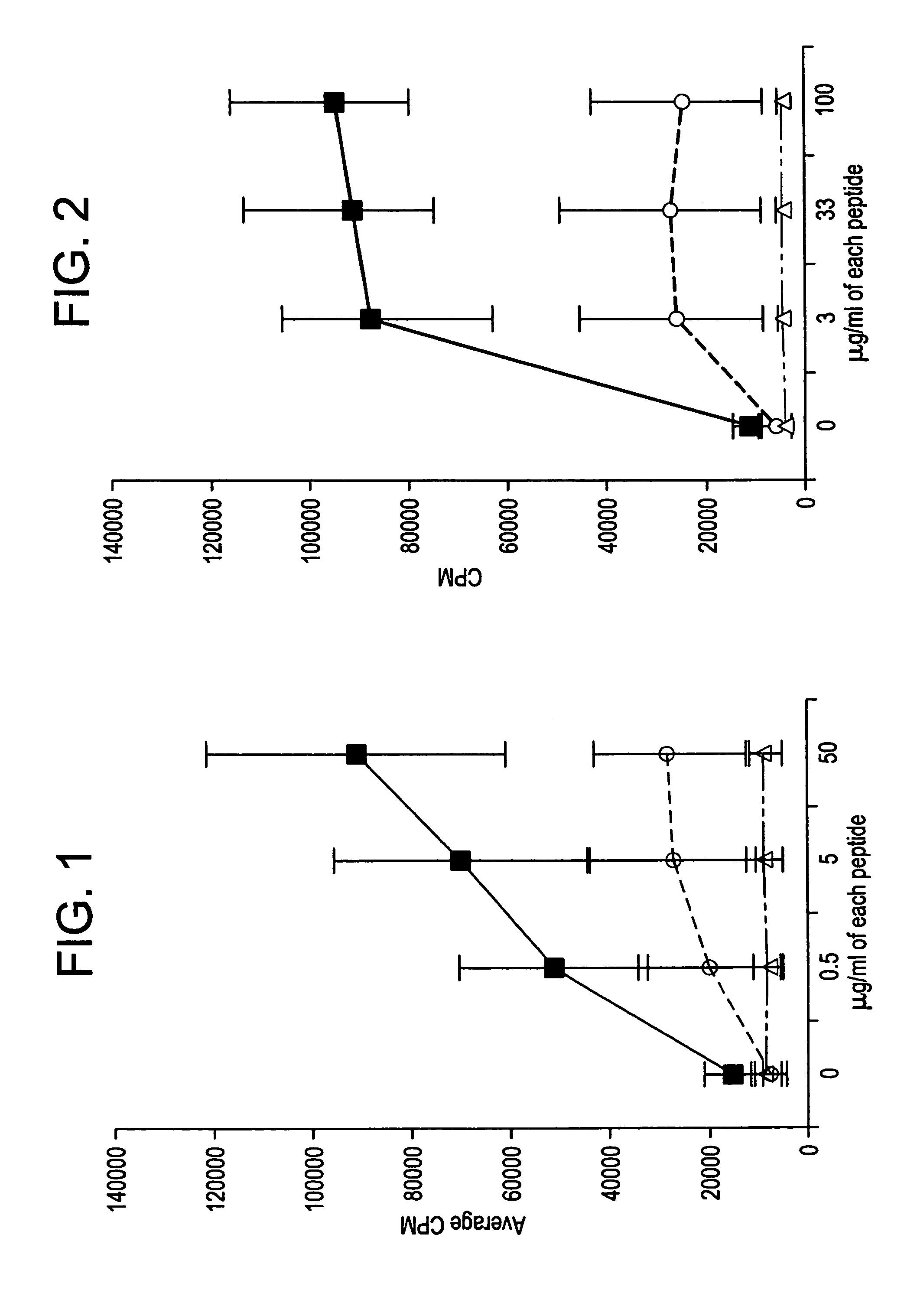
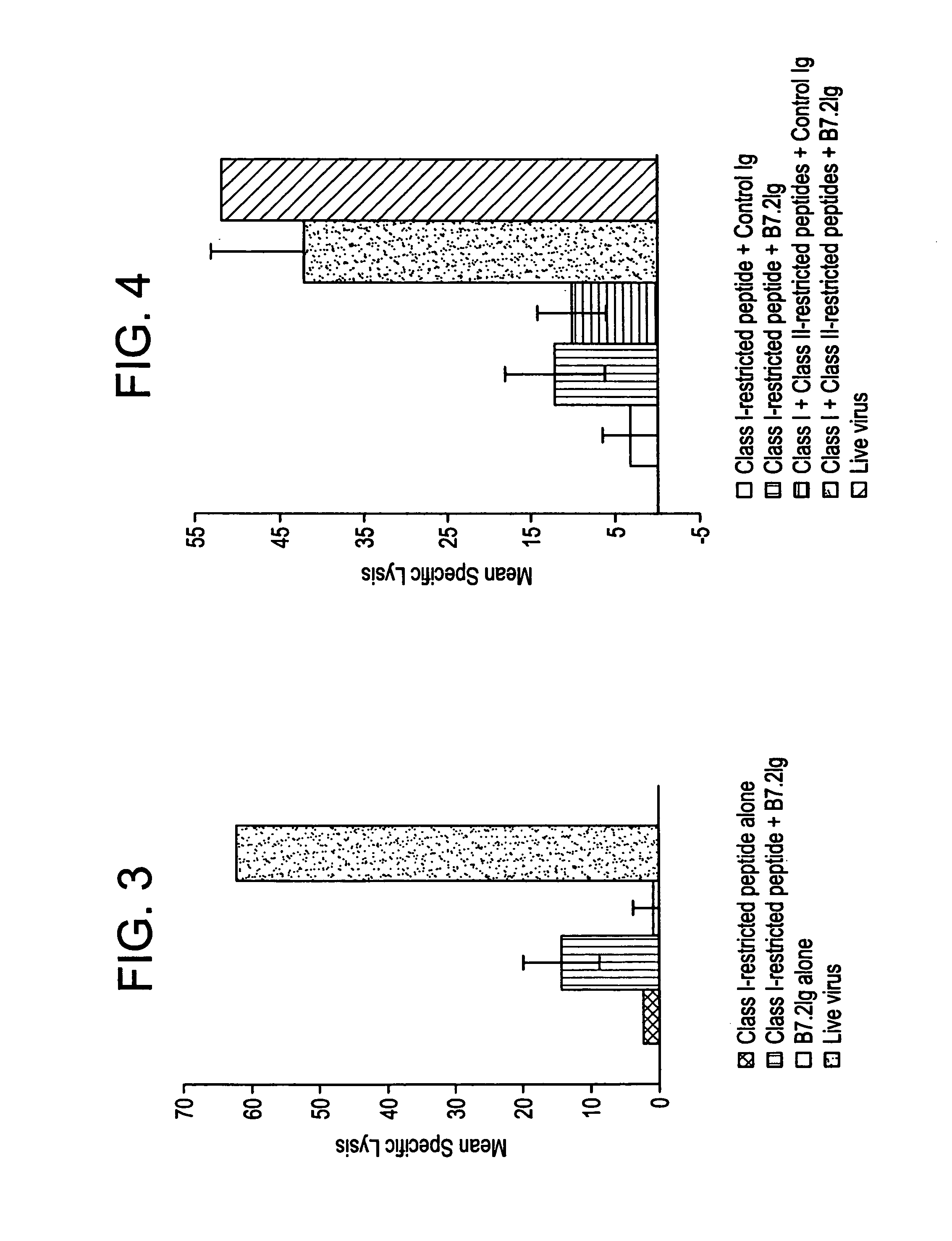
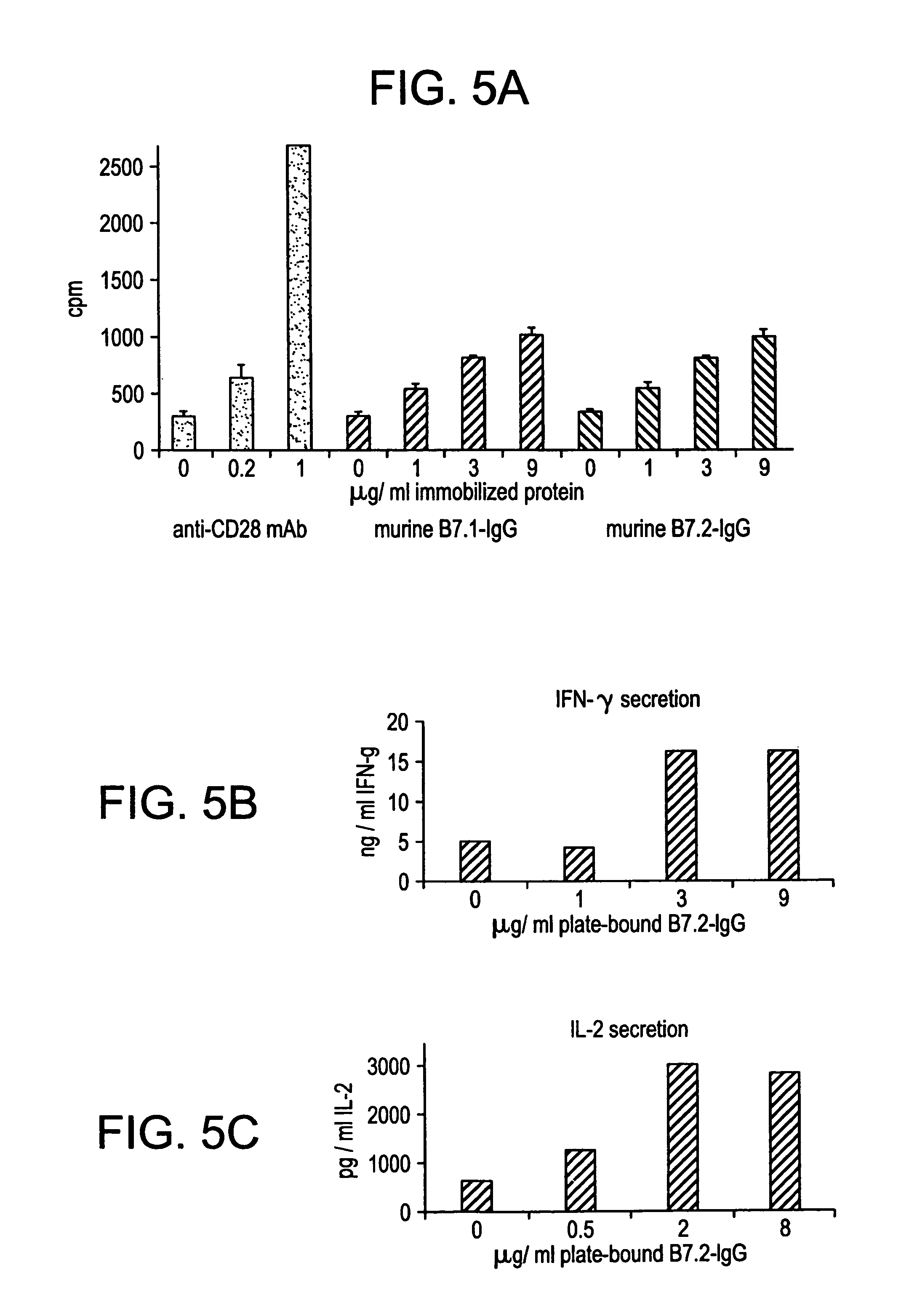
Enhancing immune responses with B7-1 or B7-2 in the absence of a crosslinking agent
InactiveUS7011833B1Enhance immune responseCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsAntigenInfectious agent
Owner:GENETICS INST INC
Fibrin-Binding Peptides and Conjugates Thereof
ActiveUS20100158814A1High degreeSuperior fibrin specific bindingUltrasonic/sonic/infrasonic diagnosticsCompound screeningBinding peptideCompanion animal
Owner:BRACCO IMAGINIG SPA
Arylalkyl and Heteroarylalkyl Derivaties of Cyclosporine a for the Treatment and Prevention of Viral Infection
This invention provides compounds of general formula (T): (I) wherein A, B, R1, R2, and X are as defined in this specification, and pharmaceutical compositions prepared from the same, for use in treatment of hepatitis C virus and / or human immunodeficiency virus.
Owner:SCYNEXIS INC
Soybean Cultivar 6035184
Owner:STINE SEED FARM +1
Peptide for inhibiting dipeptidyl-peptidase iv
InactiveUS20140193463A1Metabolism disorderTetrapeptide ingredientsGelatin hydrolysateEnzymatic digestion
Owner:CHINA MEDICAL UNIVERSITY(TW)
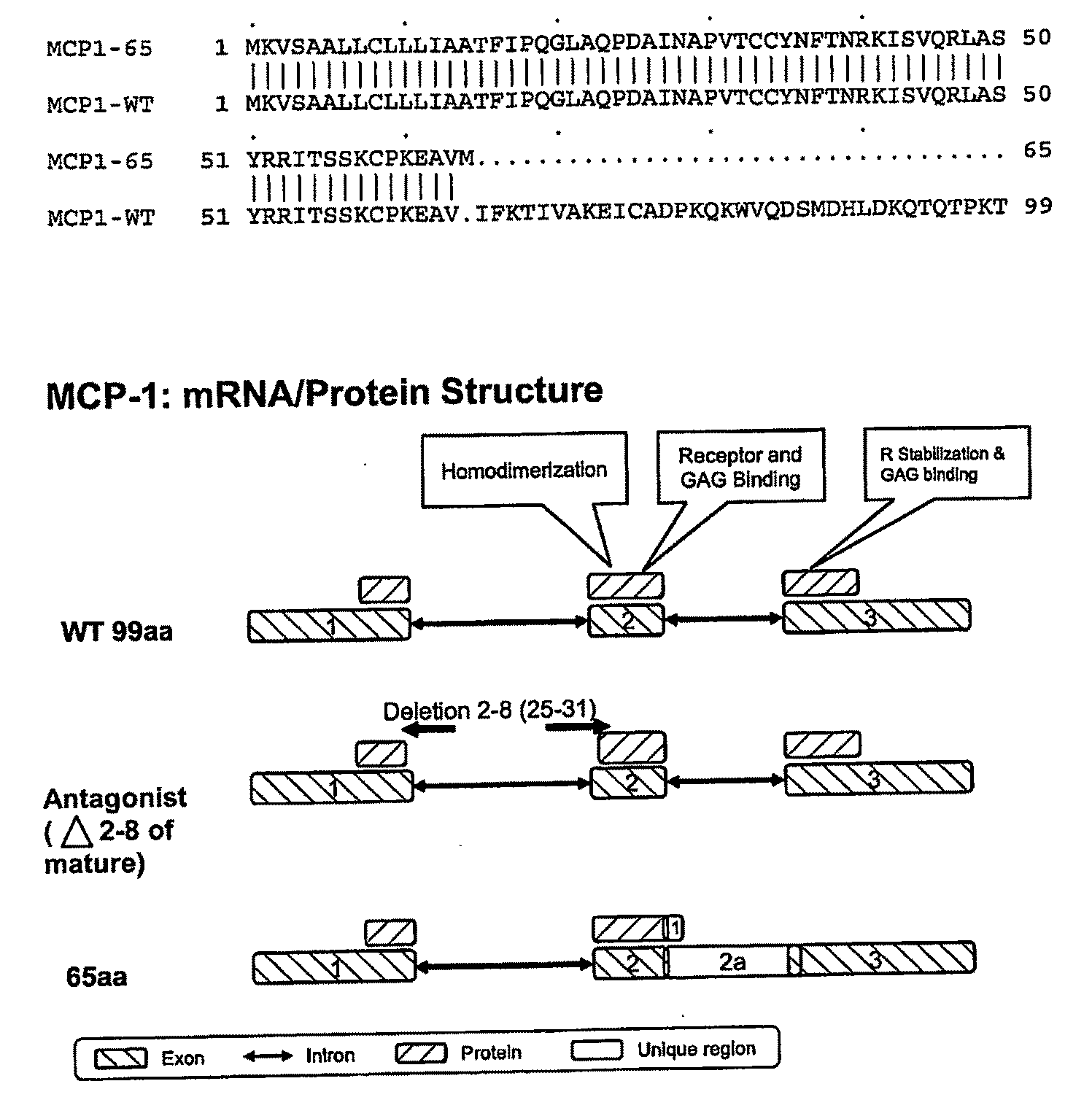
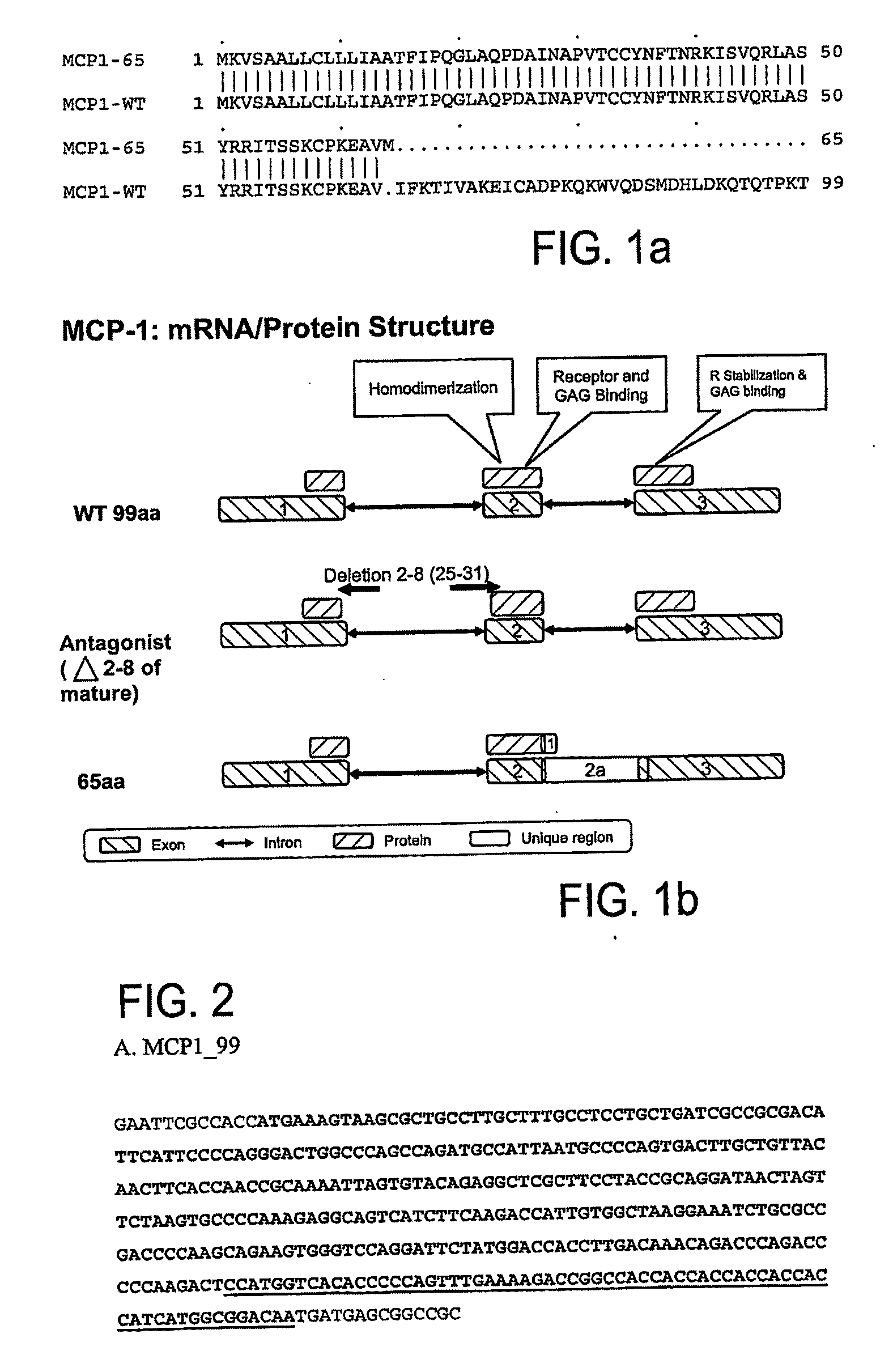
Mcp-1 splice variants and methods of using same
Owner:COMPUGEN
Methods and compositions for promoting localization of pharmaceutically active agents to bone
Owner:AFFINERGY INC +1
Site specific pegylated hemoglobin, method of preparing same, and uses thereof
The present invention provides pegylated hemoglobins comprising a maleimide polyethylene glycol (PEG) conjugated to a thiol moiety of a cysteine residue of hemoglobin, methods of preparing the pegylated hemoglobins, compositions and blood substitutes comprising the pegylated hemoglobins, and methods of treating a subject which comprise administering to the subject blood substitutes comprising vasoinactive pegylated hemoglobins.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Inhibitors of the Trypsin-Like Site of the Proteasome and Methods of Use Thereof
Owner:LEIDEN UNIVERSITY +1
Method for forming protein crystal
InactiveUS20130196160A1Easily taken outEasy to handlePackage sterilisationLaboratory glasswaresCross-linkProtein solution
Owner:RIKEN
Y-shaped polyethylene glycol modified g-csf, the preparation and use thereof
ActiveUS20110280826A1Good treatment effectExtended half-lifePeptide/protein ingredientsDepsipeptidesMedicinePolyethylene glycol
Owner:BIOSTEED GENE EXPRESSION TECH
Formulation
InactiveUS20130344102A1Mitigates excessive productionIncreased activationHydrolysed protein ingredientsMammal material medical ingredientsMedicine
Owner:SHOTTON DAVID JOHN +2
Dipropyl phthalic acid artificial antigen and its preparation method
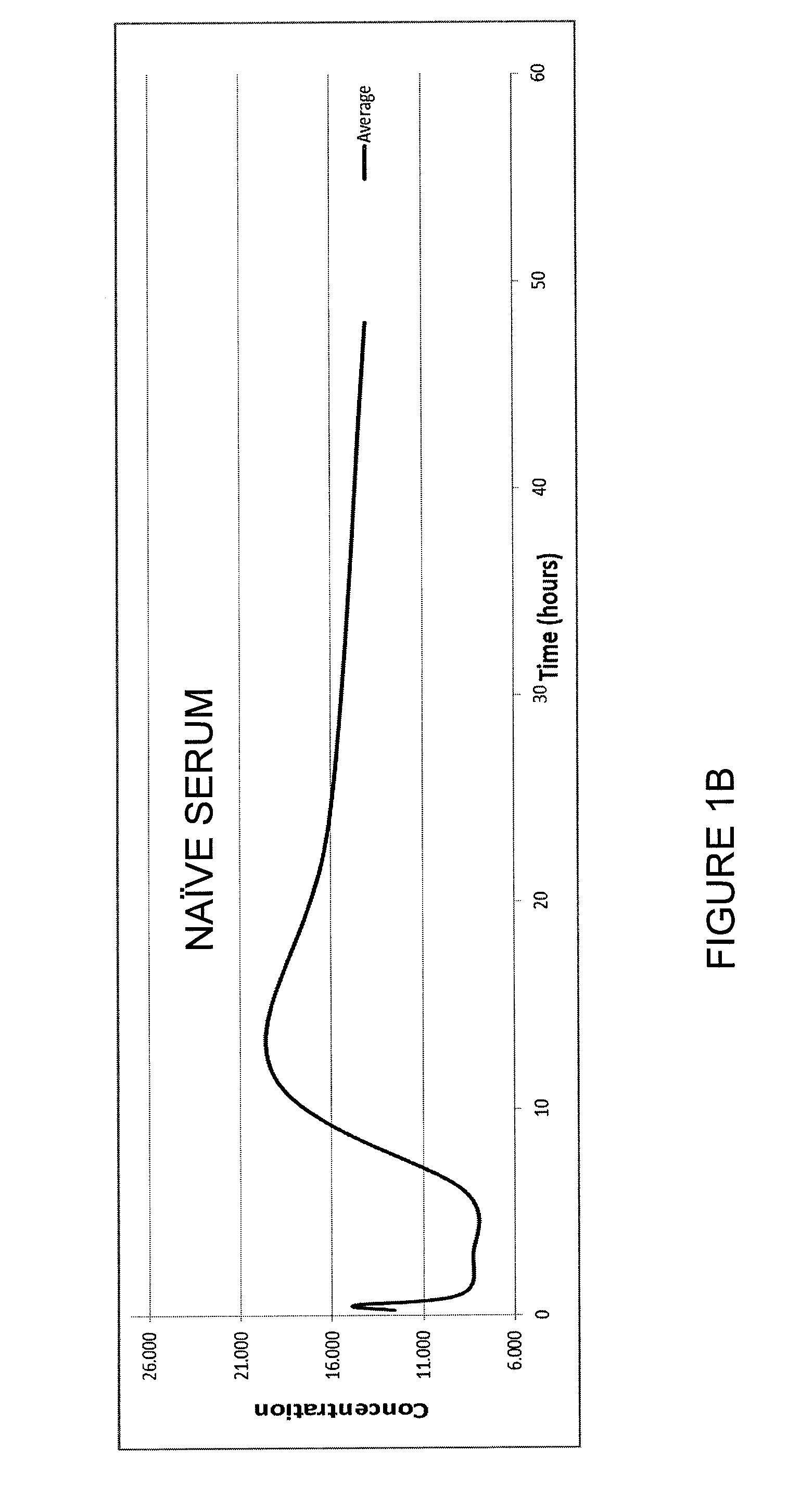
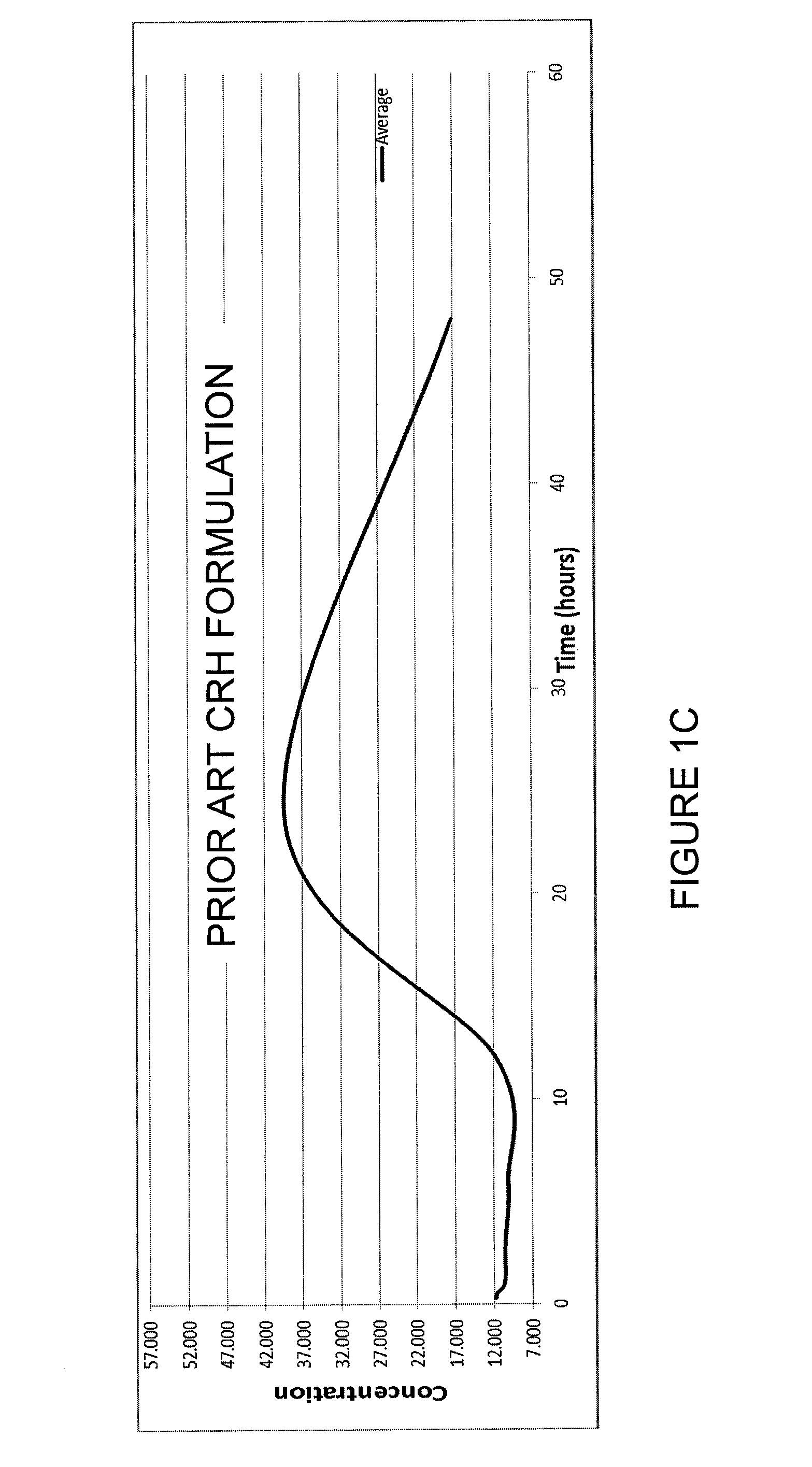
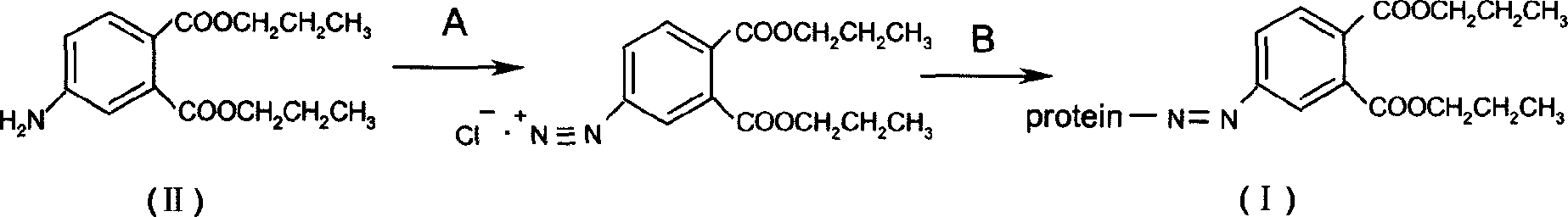
InactiveCN1763103AEasy to preparePracticalImmunoglobulinsCarrier-bound/immobilised peptidesAntigenCarrier protein
Owner:DONGHUA UNIV
Methods and pharmaceutical composition for the preservation of vascular endothelial cell barrier integrity
InactiveUS20130023473A1Reduce infarct sizeImprove therapeutic efficacyOrganic active ingredientsPeptide/protein ingredientsVascular endotheliumPharmaceutical drug
The invention relates to an ANGPTL4 polypeptide for use in the preservation of vascular endothelial cell barrier integrity and reduction in no-reflow phenomenon with myocardial infarction.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Controlled surface topography for enhanced protein crystallization rates
InactiveUS20080119642A1Prevent uncontrolled evaporationPolycrystalline material growthFrom normal temperature solutionsProtein solutionTopography
Owner:ALFRED UNIVERSITY
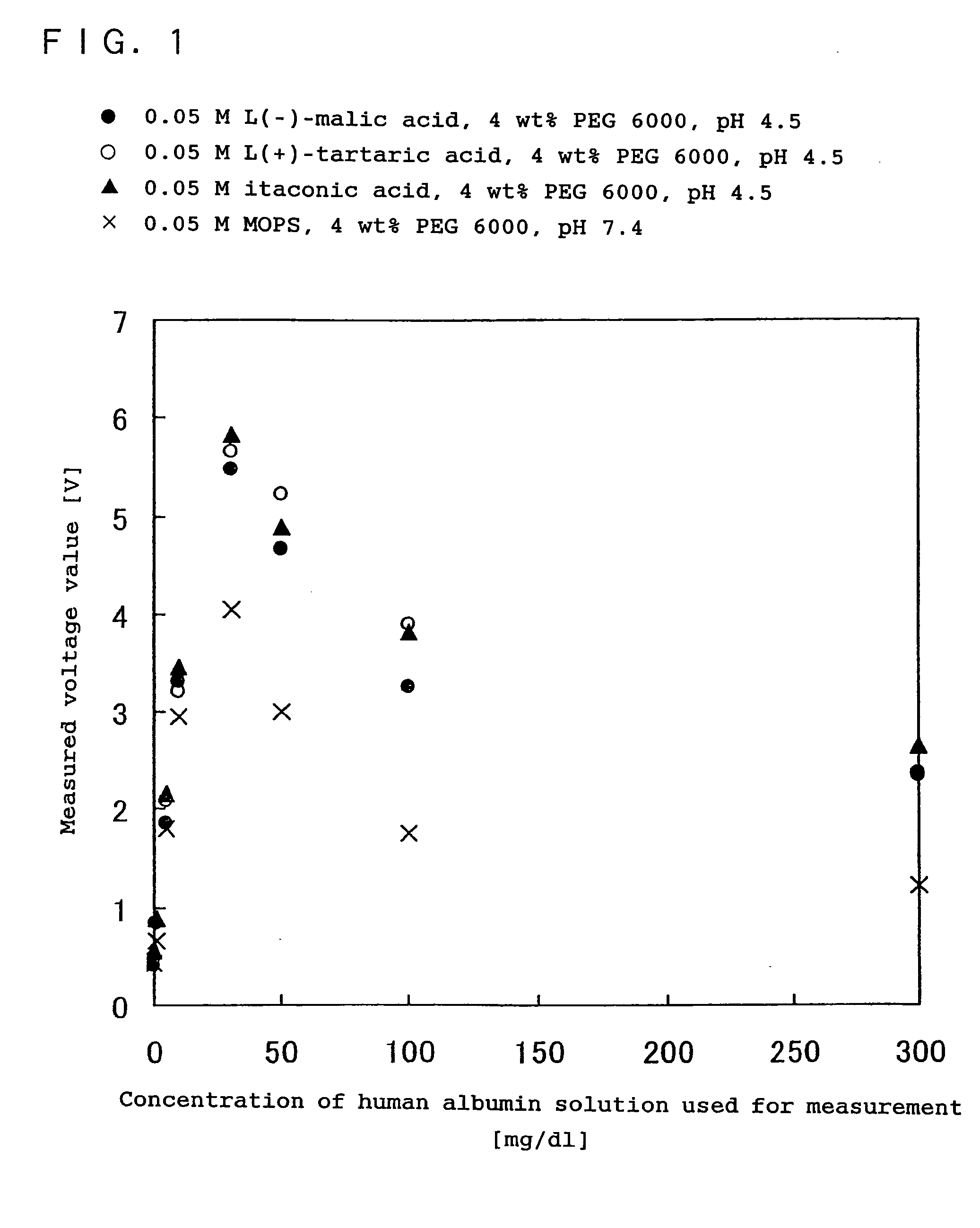
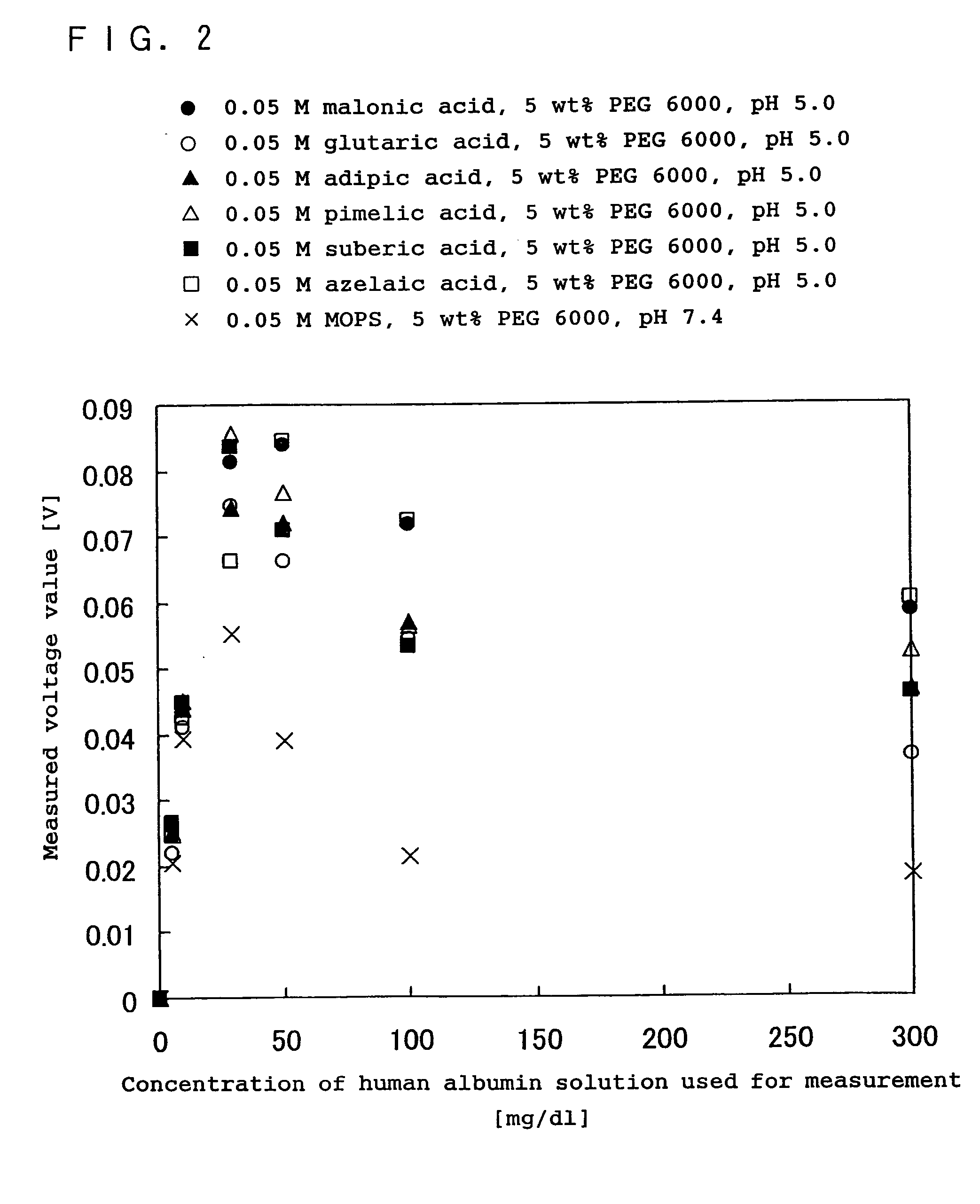
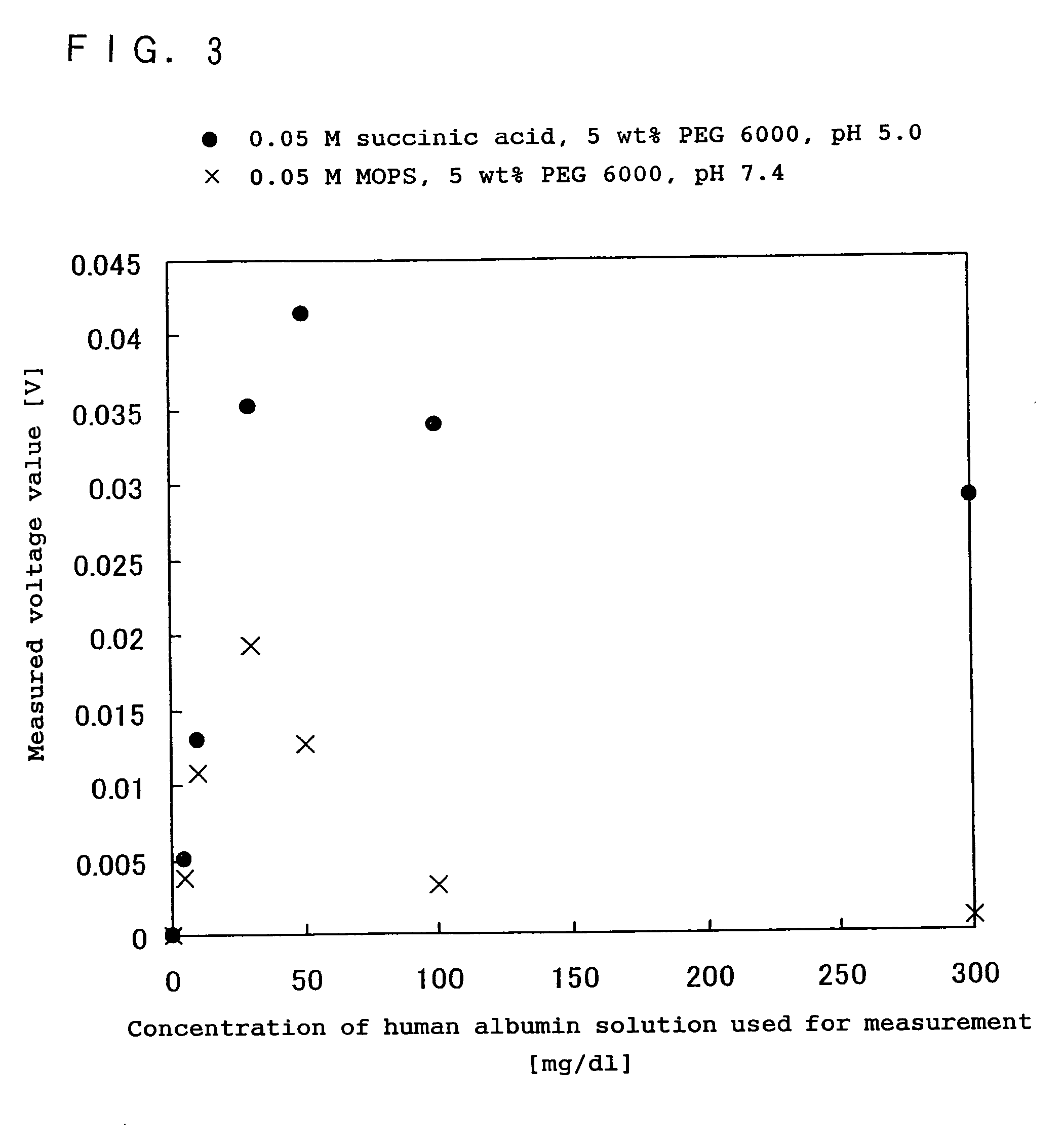
Immunoreaction measurement method
Owner:PHC HLDG CORP
Novel peptides and use thereof
ActiveUS20150175662A1Good anti-inflammatory effectLess side effectsSenses disorderUrea derivatives preparationPolynucleotideDisease cause
Owner:SOOKMYUNG WOMENS UNIV IND ACADEMIC COOPERATION FOUND +2
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap