Immunoreaction measurement method
a measurement method and immunoglobulin technology, applied in the field of immunoglobulin measurement method, can solve the problems of agglutination complex, reaction solution becoming turbid, increase in viscosity,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0118] In the present example, an immunoreaction was measured using an antibody solution usable in measurement employing slide agglutination, turbidimetry or nephelometry, and a reagent including a buffer which contained at least one compound selected from the group consisting of dicarboxylic acids having a hydroxyl group, dicarboxylic acids having a double bond, straight-chain dicarboxylic acids expressed by the chemical formula (1): HOOC(CH2)nCOOH (n is an integer), and the salts of these dicarboxylic acids.
[0119] It is to be noted that pure water filtered with Milli-Q SP TOC (manufactured by Millipore) was used to prepare a buffer or the like described below. Any reagents, such as salts and buffers, which are not particularly described, were obtained from Wako Pure Chemical Industries. Polyethylene glycol (PEG) 6000 used here was an extra pure reagent, and other reagents used were guaranteed reagents.
(1) Preparation for Antibody Solution
[0120] As for antibody solutions prepared
example 2
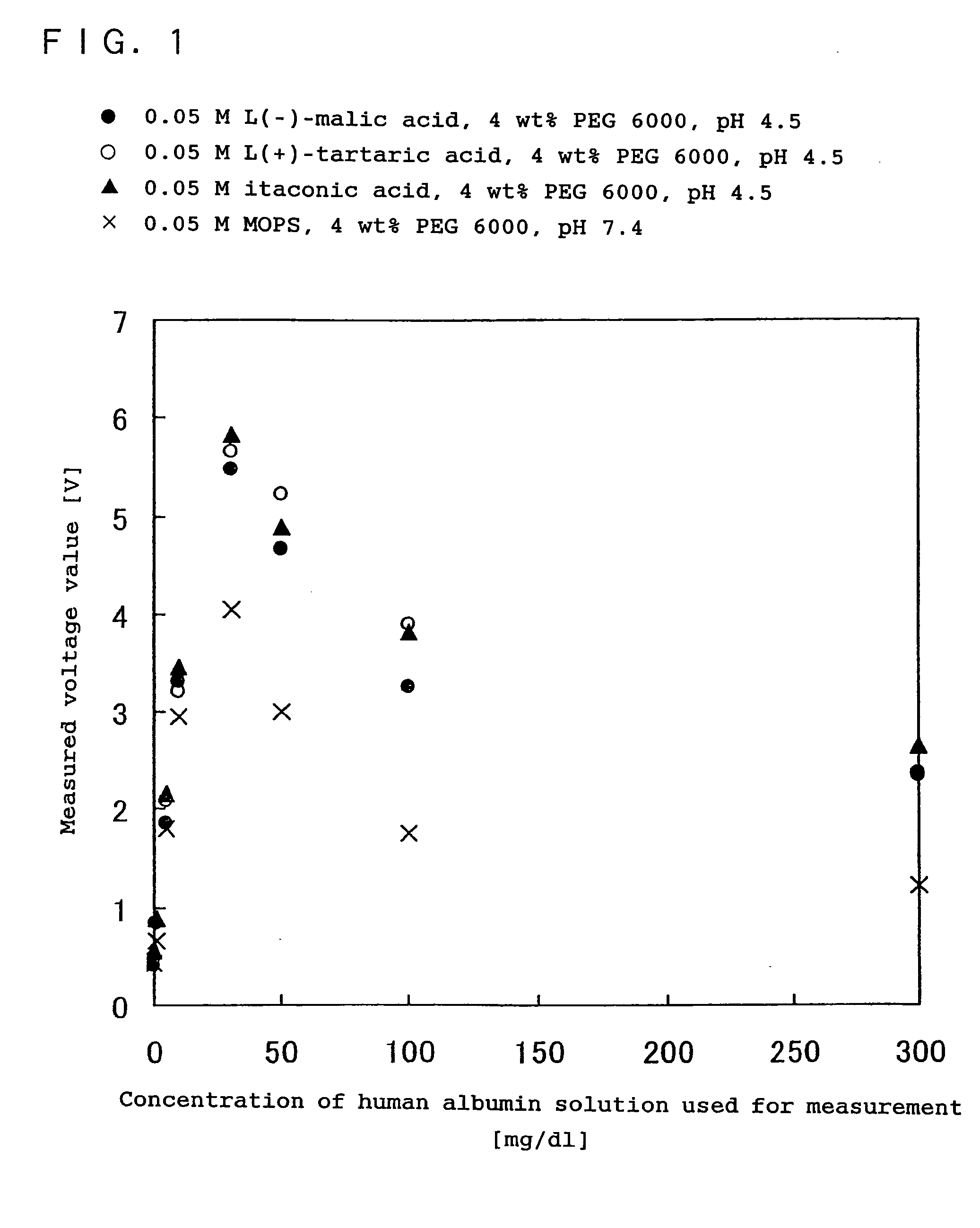
[0140] In the present example, the effect of the present invention using an acidic reaction system containing at least one compound selected from the group consisting of dicarboxylic acids having a hydroxyl group, dicarboxylic acids having a double bond, straight-chain dicarboxylic acids expressed by the chemical formula (1): HOOC(CH2)nCOOH (n is an integer), and the salts of these dicarboxylic acids, on an antigen-antibody reaction, was compared with the effect in the case of a neutral reaction system generally used in an immunoreaction measurement method. The comparison with the conventional method was made by measuring human albumin as the subject substance, using immunonephelometry.
[0141] As the reagent used was one obtained by mixing each buffer containing an L(−)-malic acid, an L(+)-tartaric acid or an itaconic acid with the antibody solution including a rabbit antihuman albumin polyclonal antibody, as in Example 1.
[0142] Further, as a buffer for comparison which formed a neutr
example 3
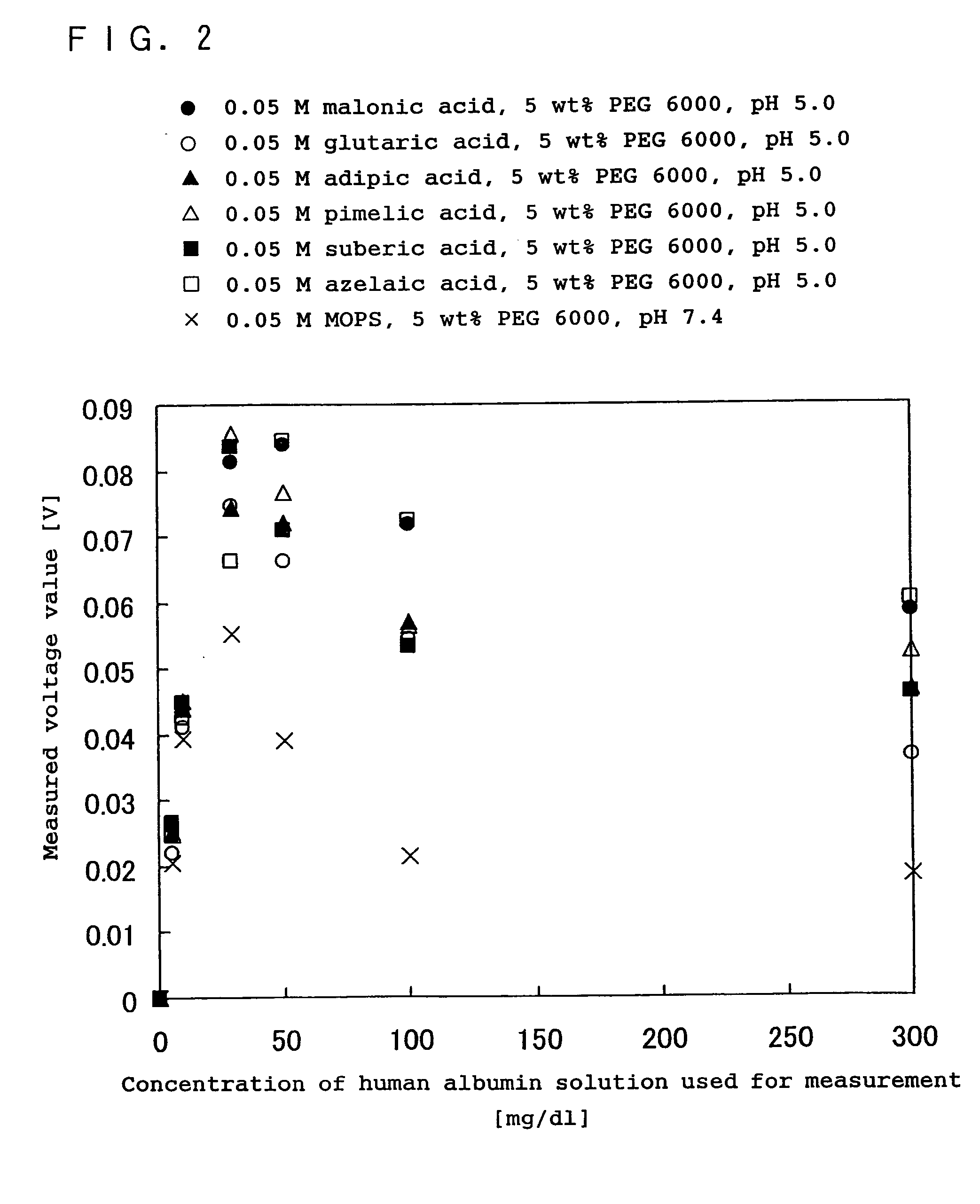
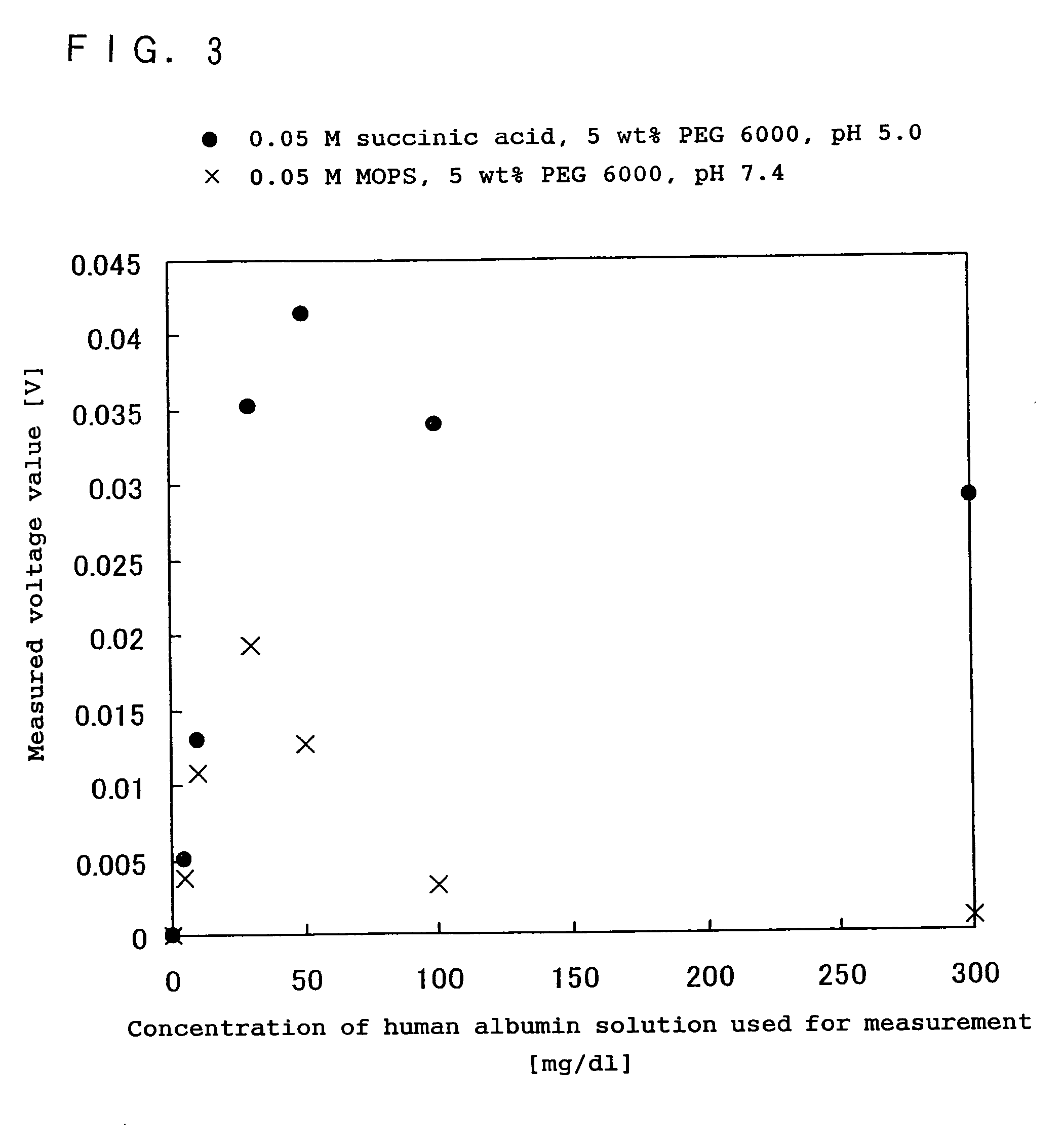
[0153] In the present example, in the same manner as in Example 2 above, the effect of an acidic reaction system containing at least one compound selected from the group consisting of dicarboxylic acids having a hydroxyl group, dicarboxylic acids having a double bond, straight-chain dicarboxylic acids expressed by the chemical formula (1): HOOC(CH2)nCOOH (n is an integer), and the salts of these dicarboxylic acids, on an antigen-antibody reaction, was examined comparing with the effect in the case of a neutral reaction system generally used in an immunoreaction measurement method. The comparison with the conventional method was made by measuring human albumin as the subject substance, using immunonephelometry.
[0154] As the reagent used was one obtained by combining each buffer containing a malonic acid, a glutaric acid, an adipic acid, a pimelic acid, a suberic acid or an azelaic acid, with the antibody solution including a rabbit antihuman albumin polyclonal antibody, as in Example 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap