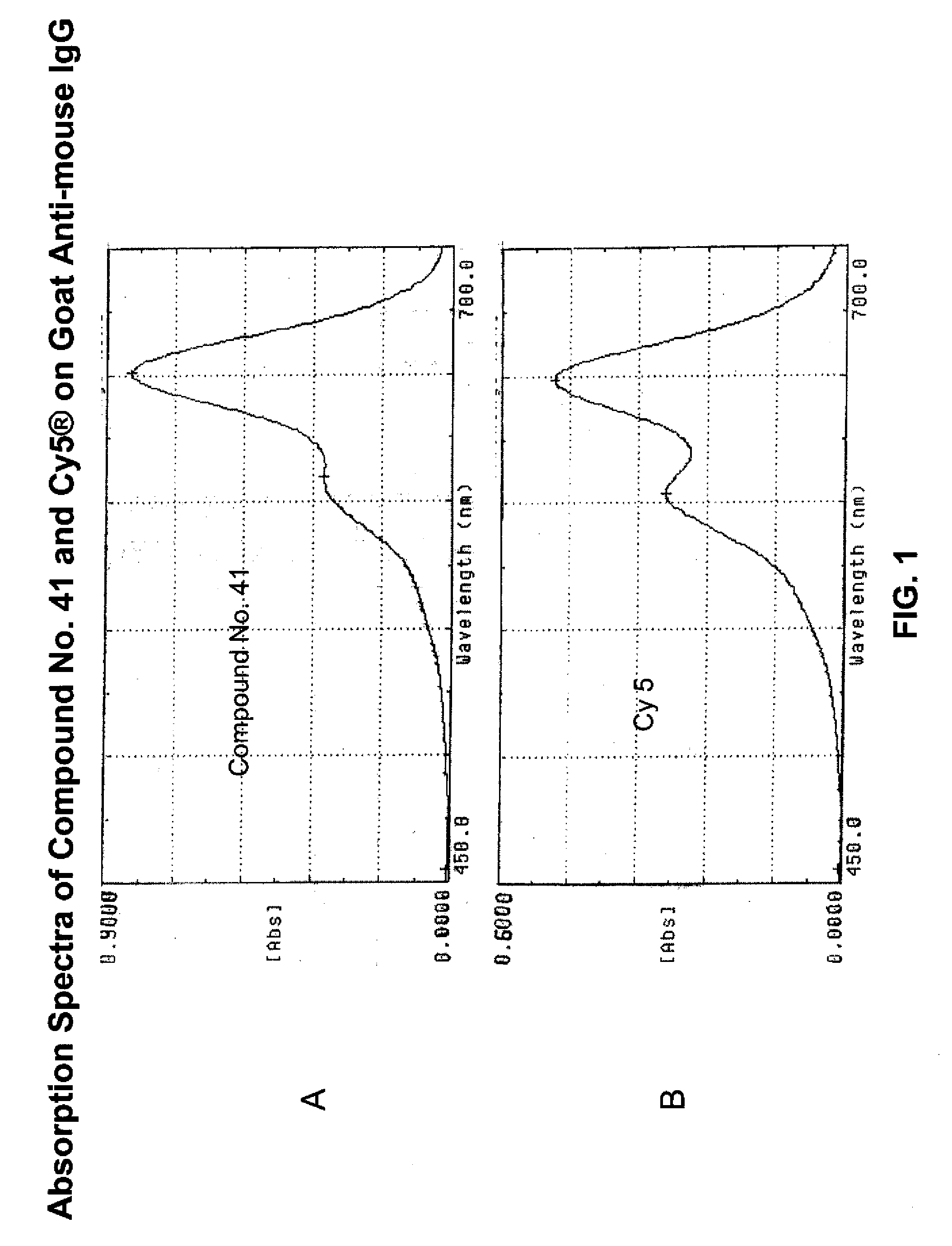
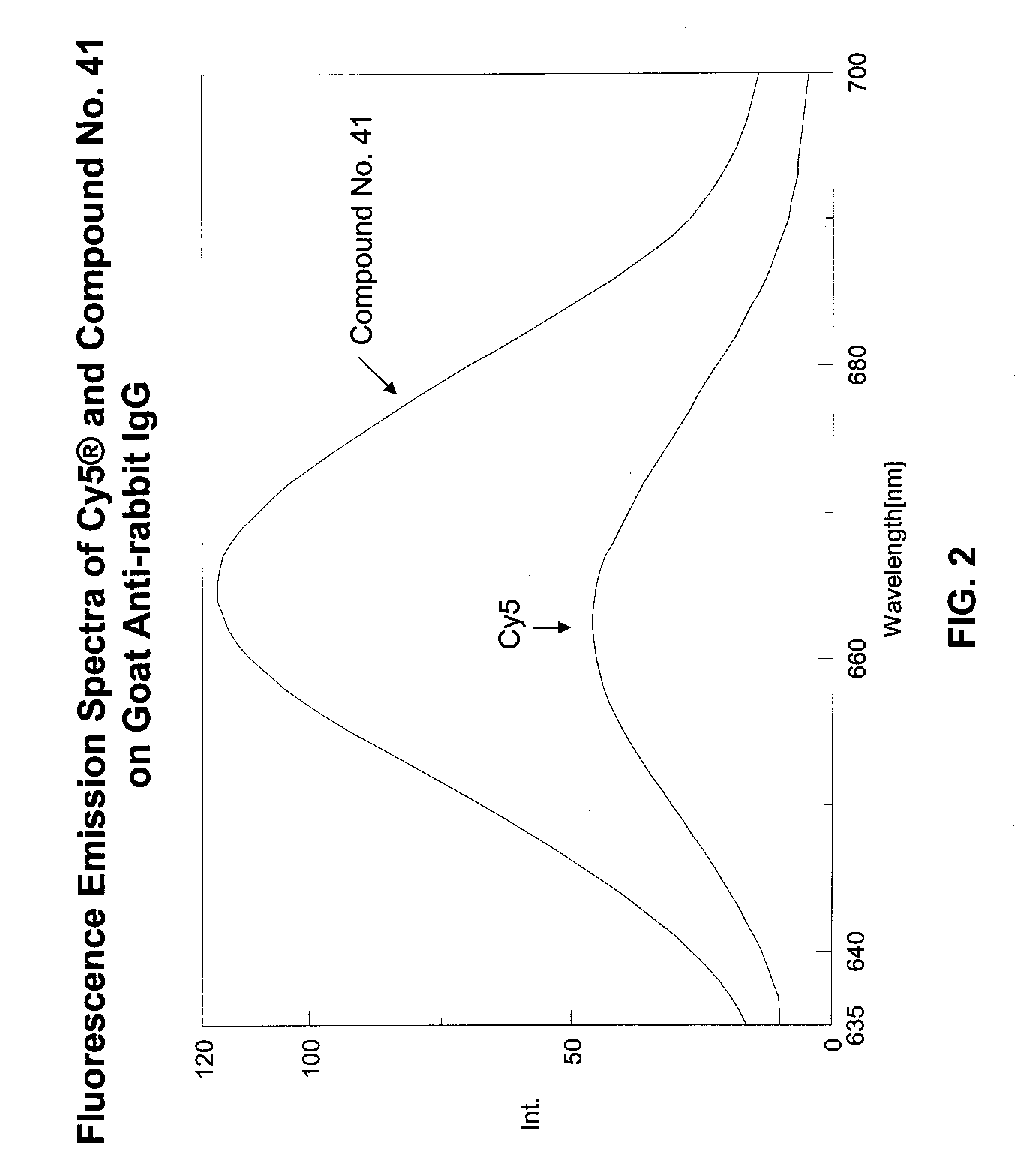
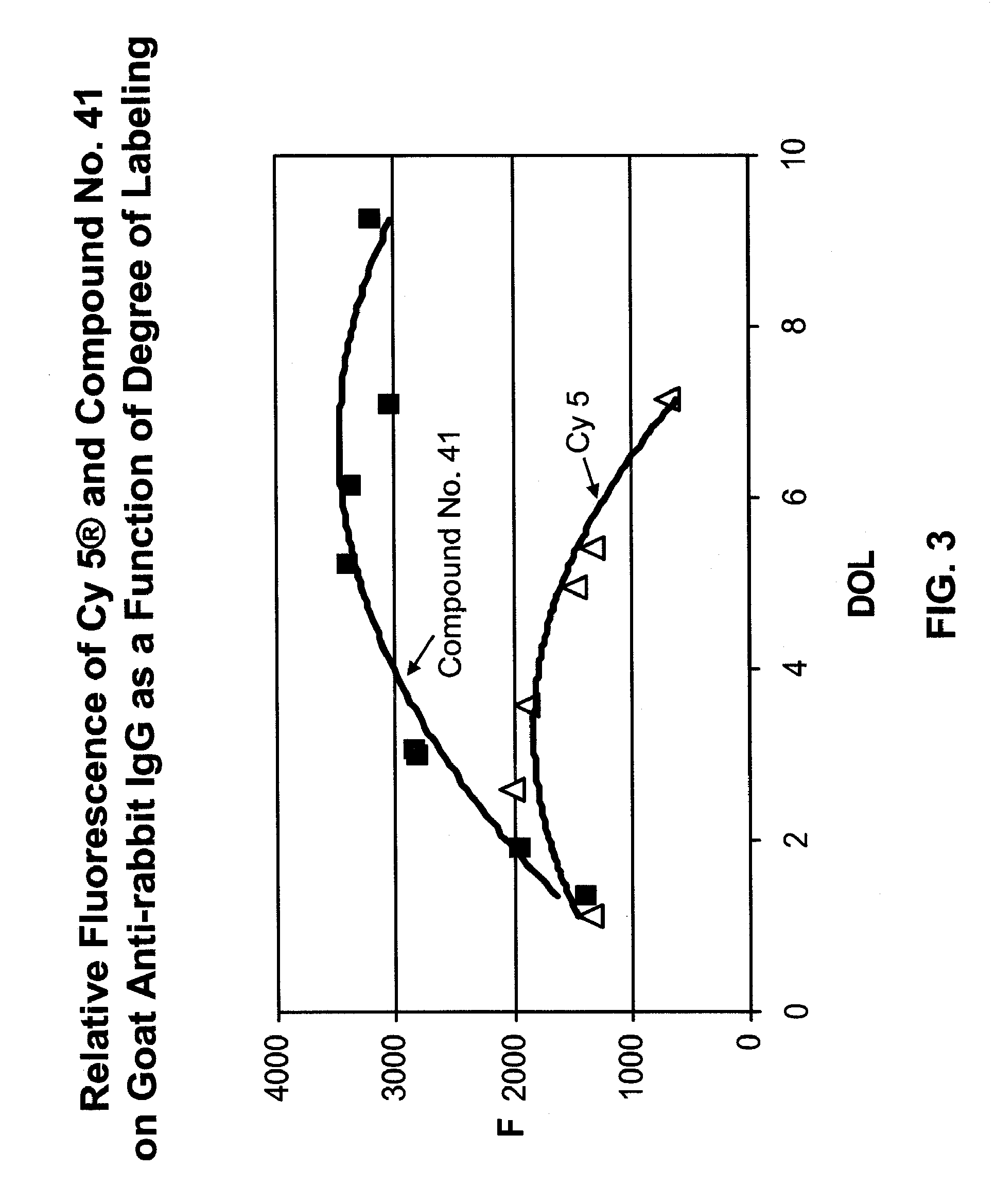
Fluorescent compounds
a technology of fluorescence compounds and fluorescent dyes, applied in the field of fluorescence compounds, can solve the problems of reducing the effective brightness of dyes, prone to inter-dye quenching of conventional fluorescent dyes, and affecting the effect of labeling, so as to achieve convenient and effective labeling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 2-(anilinovinyl)-1-(5-carboxypentyl)-3,3-dimethyl-5-sulfoindolium, Potassium Salt (Compound No. 1
[0385]
[0386]A mixture of 1-(5-carboxypentyl)-2,3,3-trimethylindoleninium-5-sulfonate, potassium salt (Bioconjugate Chem. 4, 105 (1993)) (5 g), N,N′-diphenylformamidine (2.3 g) and acetic anhydride (1.1 mL) was heated at 120° C. for 30 minutes. After cooling down to room temperature, the mixture was concentrated to dryness under vacuum and the residue was purified by column chromatography on silica gel (3.5 g).
example 2
Preparation of 2-(4-anilinobutadienyl)-1-(5-carboxypentyl)-3,3-dimethyl-5-sulfoindolium, Potassium Salt (Compound No. 2)
[0387]
[0388]A mixture of 1-(5-carboxypentyl)-2,3,3-trimethylindoleninium-5-sulfonate, potassium salt (10 g), malonaldehyde dianil hydrochloride (8 g), Et3N (0.356 mL) in AcOH (40 mL) was heated at 120° C. for 3 hours. After cooling down to room temperature, the mixture was concentrated to dryness under vacuum and the residue was purified by column chromatography on silica gel to dark brown gummy solid (5 g).
example 3
Preparation of Compound Nos. 3a and 3b
[0389]
[0390]To a solution of 1-(5-carboxypentyl)-2,3,3-trimethylindoleninium-5-sulfonate, potassium salt (0.1 g) in DMF (1 mL) was added Et3N (0.1 mL) and TSTU (80 mg). The mixture was stirred at room temperature for 1 hour, followed by the addition of Et3N (40 μL) and m-dPEG24 amine (0.4 g) (QuantaBiodesign, Powell, Ohio) or m-dPEG12 amine (0.2 g) (QuantaBiodesign, Powell, Ohio). The mixture was stirred at room temperature overnight and then concentrated to dryness. The residue was purified by column chromatography on silica gel to give a light brown solid (250 mg for compound No. 3a and 102 mg for compound No. 3b).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap