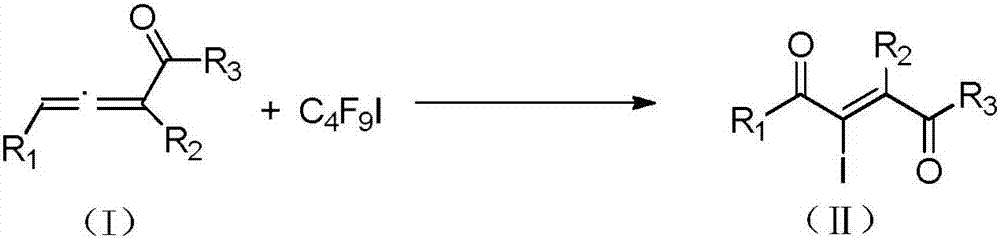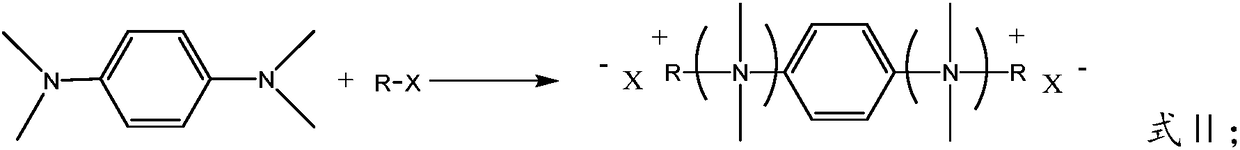Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
208results about "Organic compound preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dehydration process
ActiveUS20160229779A1Organic compound preparationPreparation by aldehyde oxidation-reductionMethacroleinOrganic chemistry
A process for preparing dry methacrolein, and a process for producing methyl methacrylate are disclosed.
Owner:ROHM & HAAS CO
Self-supporting nickel phosphide catalyst and preparation method and application thereof
ActiveCN107694584AImprove thermal conductivityGood choiceOrganic compound preparationCarboxylic acid esters preparationOxalateNickel oxide hydroxide
The invention discloses a self-supporting nickel phosphide catalyst and a preparation method and application thereof. The self-supporting nickel phosphide catalyst is a nickel phosphide catalyst obtained by in-situ growing of a nickel oxalate or nickel hydroxide crystal layer on a framework matrix through a hydrothermal method and performing phosphating while secondary forming is not needed, and the nickel phosphide catalyst is composed of the framework matrix and a nickel-phosphorus compound, wherein the nickel-phosphorus compound is at least one of Ni3P, Ni12P5, Ni2P and Ni5P4, total mass ratio of the nickel-phosphorus compound is 0.1-50%, and the balance is the framework matrix. Experiments show that the self-supporting nickel phosphide catalyst is high in stability and thermal conductivity, easy to form and fill, high in flux and low in pressure drop, especially has the advantages of high low-temperature activity, high dimethyl oxalate conversion rate and high methyl glycolate selectivity and can be used as a reaction catalyst for hydrogenating dimethyl oxalate to prepare methyl glycolate.
Owner:EAST CHINA NORMAL UNIV
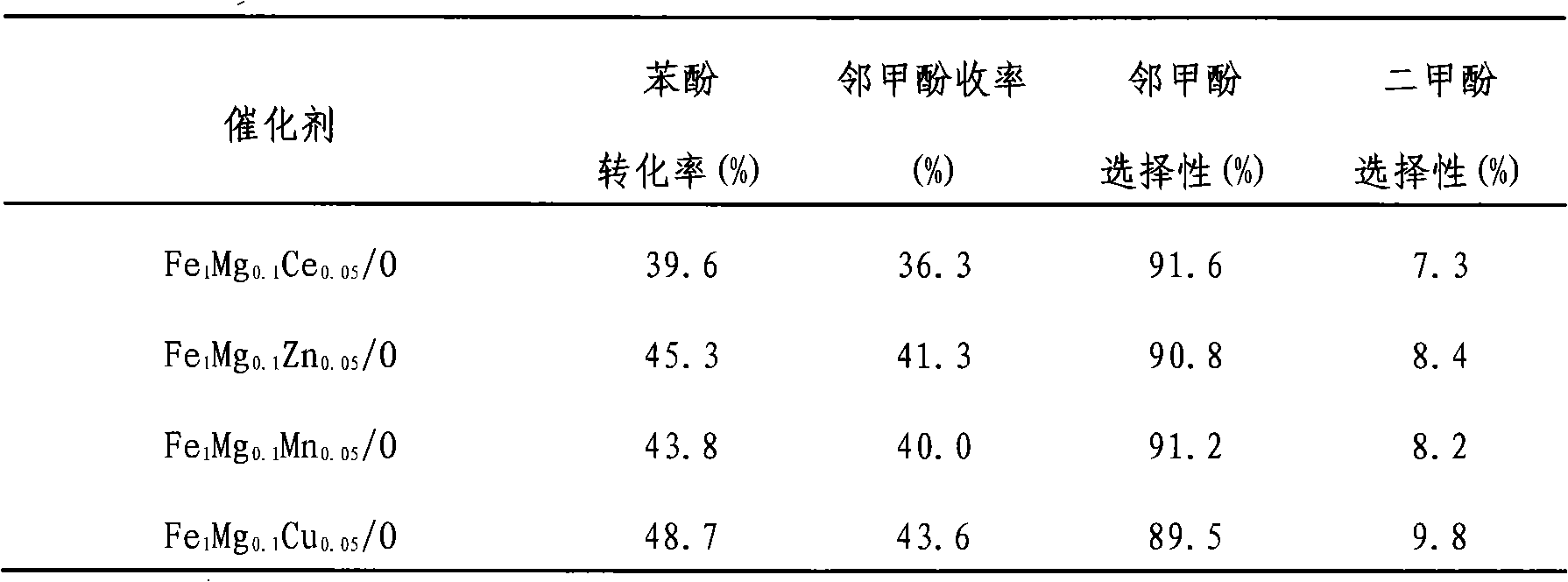
Phenol ortho-methylation catalyst and preparation method thereof
ActiveCN101513614AEasy to makeLow reaction temperatureOrganic chemistryOrganic compound preparationMagnesium saltOrtho position
Owner:HUNAN XINLING CHEM CO LTD
Valine purification method
ActiveCN101798273AEasy to operateGood choiceIon-exchange process apparatusIon-exchanger regenerationPurification methodsFiltration
The invention relates to a valine purification method, which comprises the following steps: A. adding flocculating agents into valine fermentation liquid for fast precipitating solid impurities, and obtaining primary filter liquid through filtering supernatant; B. adding activated carbon into the primary filter liquid obtained in the step A, wherein the added activated carbon accounts for 1+ / -0.5weight percent of the filter liquid, carrying out stirring and decoloration at 60 + / - 5 DEG C, and then, obtaining secondary filter liquid through filtration; C. using an industrial chromatographic column for separating inorganic salt and heteroacid, and then, using a sodium filter membrane for removing pigment and small molecular impurities to obtain a clear and colorless valine solution; D. concentrating the filter liquid for crystallization to obtain crude valine products; and E. shaking a transmission belt and raising the temperature to 220 + / - 10 DEG C, and carrying out sublimation to remove alanine and isoleucine to obtain pharmaceutical grade competitive valine products. The method has the characteristics of simple operation, good selectivity, cleanness and environment protection, so the yield and the quality of the valine are obviously improved.
Owner:ZHAODONG XINGHU BIOTECHNOLOGY CO LTD
Method for synthesizing citric acid ester type compound
InactiveCN101830803AHigh catalytic activityRich sourcesOrganic compound preparationCarboxylic acid esters preparationChemical synthesisBenzene
The invention discloses a method for synthesizing a citric acid ester type compound, which belongs to the technical field of chemical synthesis. The method comprises the following steps of: using citric acid and fatty alcohol as main raw materials, and using benzene sulfonic acid or amino benzene sulfonic acid as a catalyst; and performing esterification and the purification processes of acetylation, neutralization, washing, drying, distillation and the like. The catalyst has rich sources, a low cost and high activity, can be separated from an esterification liquid easily after the neutralization, is coke-free during the distillation, has less corrosion to equipment, and is safe and environment-friendly; the water generated by the esterification is separated out by adopting a binary heterogeneous separation technique, and no water separating agent is additionally added; and acetyl citric acid ester is produced by adopting an esterification-acetylation continuous synthesis method, the flow is greatly simplified, and a synthesis process is shortened. The citric acid ester prepared by the method has the advantages of high quality, high purity, low degree of color and wide applicationrange.
Owner:NORTHWEST NORMAL UNIVERSITY
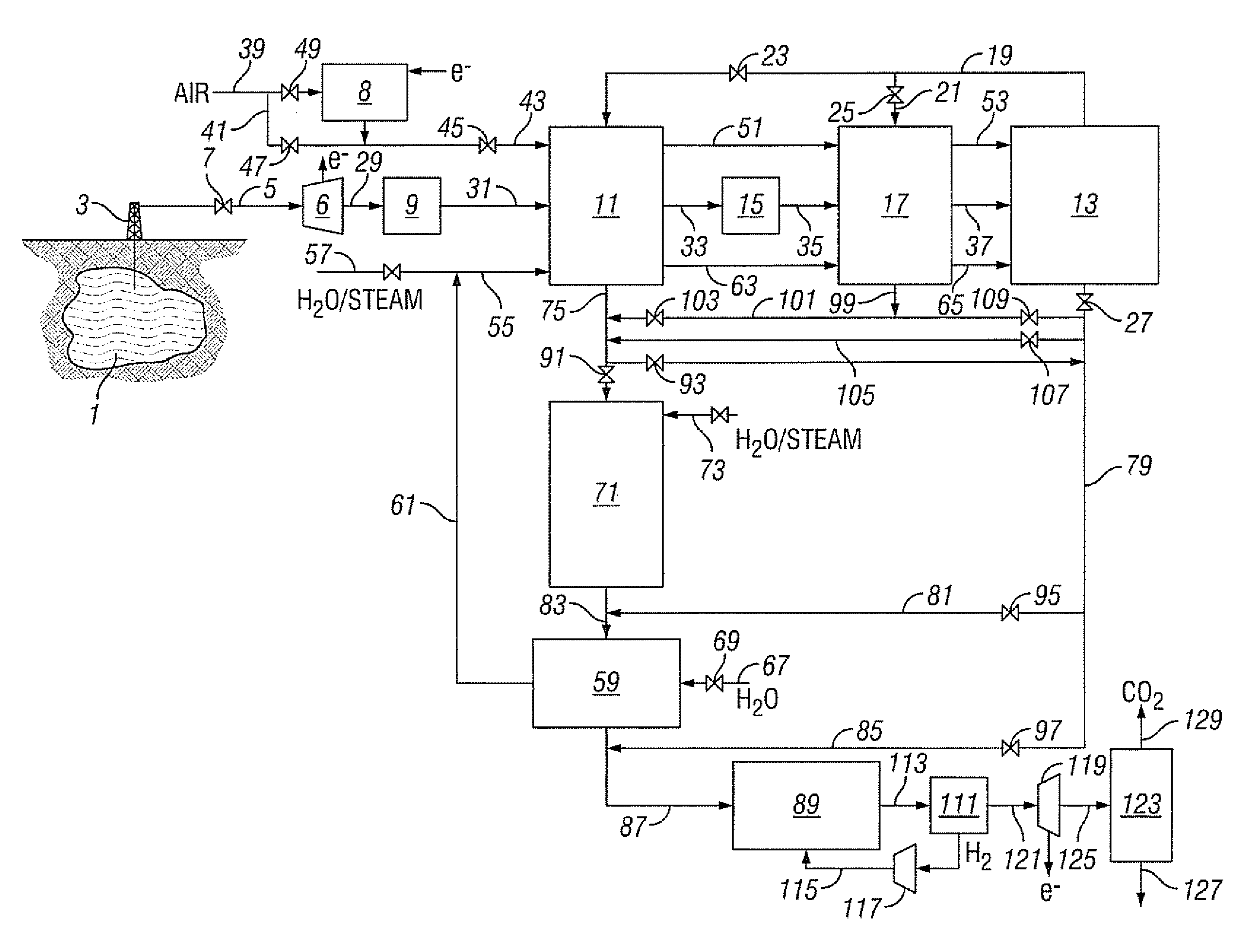
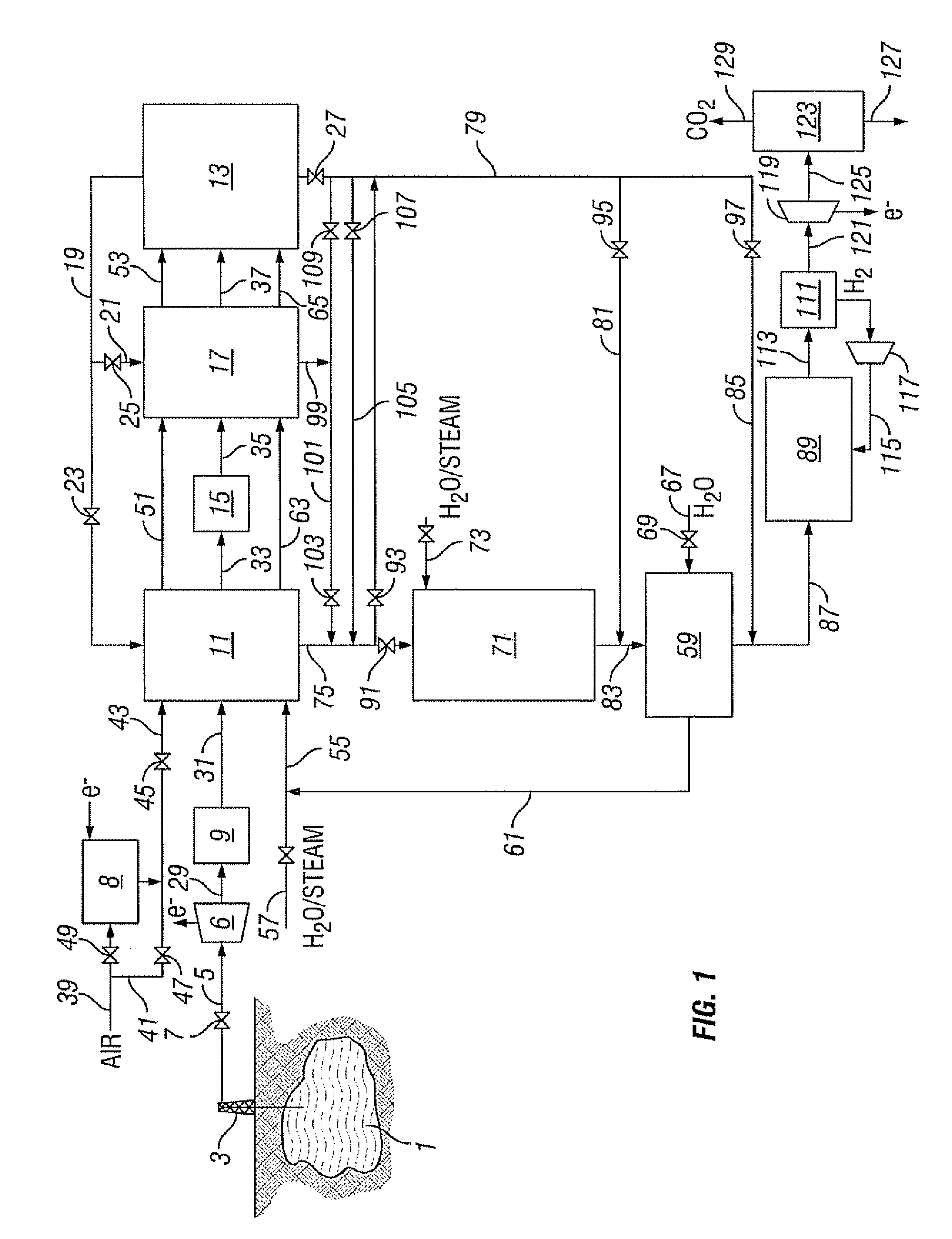
Method for recovering a natural gas contaminated with high levels of co2
Owner:SHELL OIL CO
Method for purifying citrulline from watermelon
InactiveCN101880245AReduce loadExtend your lifeUrea derivatives preparationOrganic compound preparationAcid waterCitrulline
Owner:NANJING ZELANG AGRI DEV
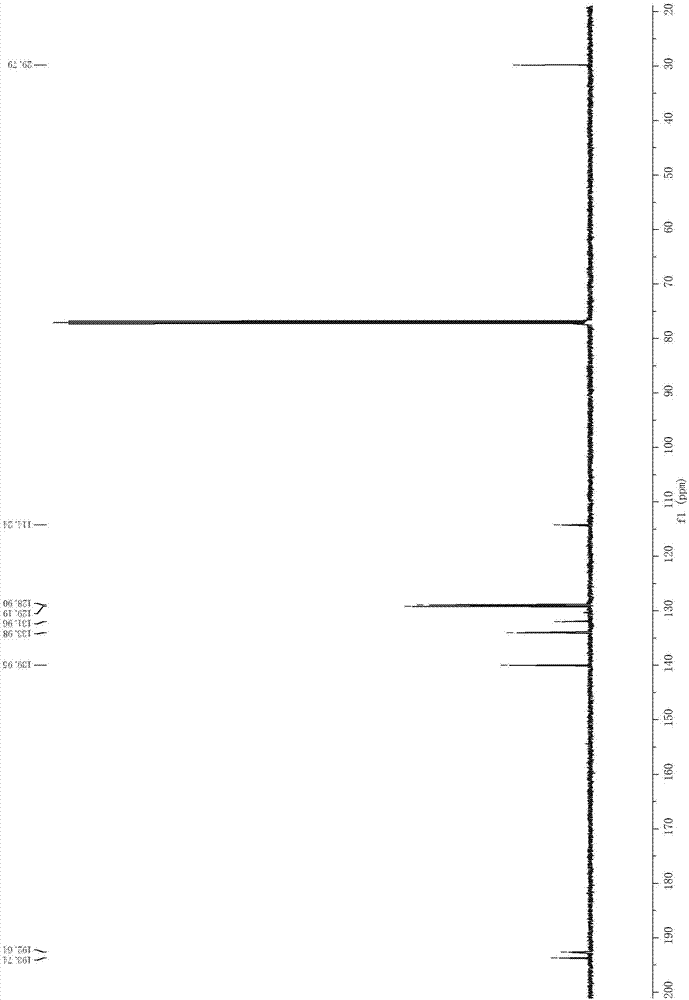
Method for preparing 2-iodine amyl -2-ene-1,4-diketone derivative by adopting visible light catalysis
ActiveCN107011145AMild reaction conditionsEasy to operateOrganic compound preparationCarboxylic acid esters preparationIodidePollution
Owner:ZHEJIANG UNIV OF TECH
USE OF PHTHALIMIDE AND/OR SULPHONAMIDE DERIVATIVES IN THE TREATMENT OF DISEASES WHICH REQUIRE REDUCING THE TNF-alpha LEVELS AND AN EXOGENOUS SOURCE OF NITRIC OXIDE, PHTHALIMIDE DERIVATIVES, SULPHONAMIDE DERIVATIVES, AND A METHOD FOR OBTAINING A SULPHONAMIDE DERIVATIVE
InactiveUS20100324107A1Improve the quality of lifeBiocideOrganic chemistryPhthalocyanine derivativesNitric oxide
Owner:UNIV ESTADUAL DE CAMPINAS UNICAMP +2
Esterification catalysts and esterification process of organic acid
ActiveCN101172253AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIonOrganic acid
Owner:CHINA PETROLEUM & CHEM CORP +1
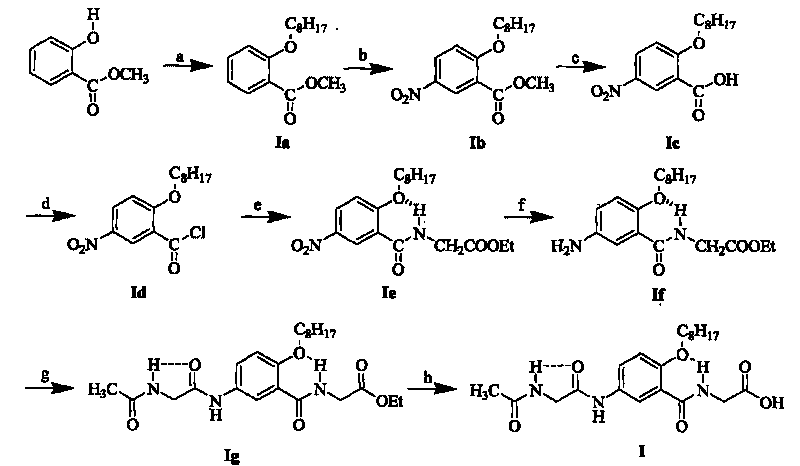
Method for preparing LCZ696
ActiveCN105330609AReduce generationPrecipitation state is goodOrganic compound preparationOrganic chemistry methodsSodium acetateReaction temperature
The invention discloses a method for preparing LCZ696. Please see the synthesis route in the specification. When sodium acetate is used as alkali in the compound III preparation process, the conversion rate is high, hydrolysis impurities are few, system stability is good, and the reaction time is greatly shortened; in the compound I preparation process, acetone and normal heptanes with the mass ratio between 5 to 1and 10 to 1 serve as cocrystallization solvent, the reaction temperature of 35-45 DEG C is adopted, a sodium hydroxide solution is dropwise added into a reaction system at a certain speed at the temperature of 35-45 DEG C, generation of hydrolysis products can be greatly reduced, the solid precipitation state is good, purity is high, aminolysis impurities and hydrolysis impurities can be effectively controlled, and the LCZ696 can directly serve as crude drug to be used for preparations.
Owner:NANJING CHIA TAI TIANQING PHARMA +1
Method for preparing solid base catalyst with high specific surface by hybrid composite precursors
InactiveCN101554596APore Structure RegulationControl areaCarboxylic acid nitrile preparationOrganic compound preparationAcetic acidBenzaldehyde
Owner:BEIJING UNIV OF CHEM TECH
Preparation method of environment-friendly type alpha-cyanoacrylate
InactiveCN104402763AOrganic compound preparationCarboxylic acid nitrile purification/separationParaformaldehydePlasticizer
Owner:SHANDONG YUWANG IND
Method for recycling methylal in glyphosate wastewater
ActiveCN103288606AReduce security risksEfficient recyclingOrganic chemistryOrganic compound preparationAlcoholDistillation
Owner:HUBEI TAISHENG CHEM
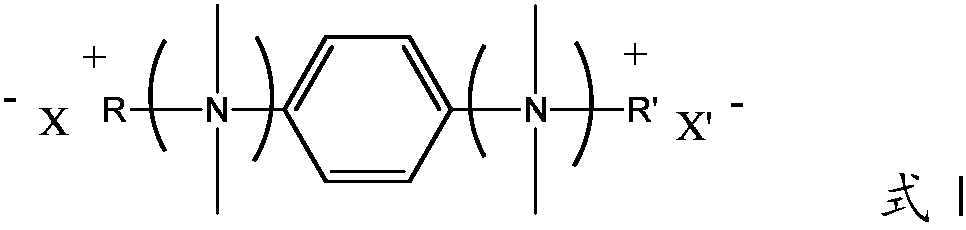
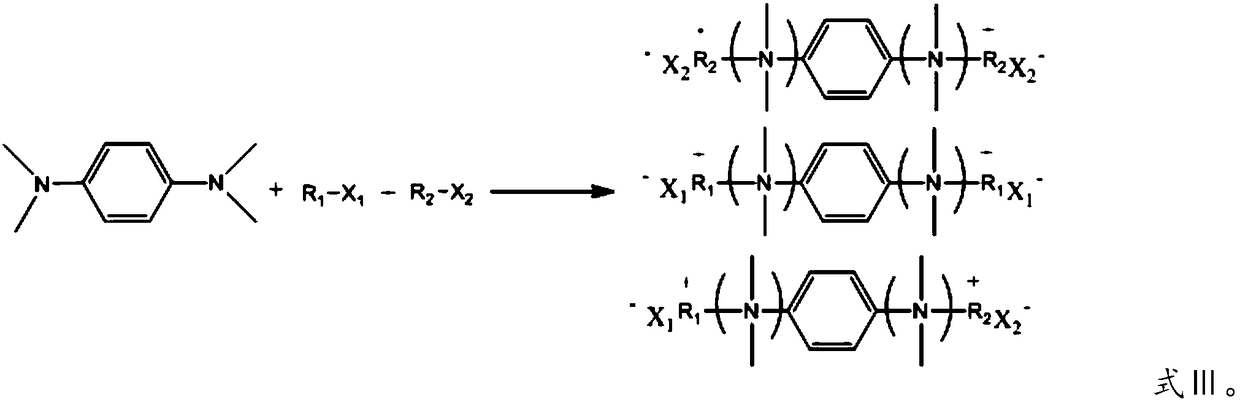
Quaternary ammonium salt concrete anti-mud agent, and preparation method and application thereof
ActiveCN108545978AImprove securityThe production process is simpleOrganic compound preparationAmino compound preparationDistillationOrganic solvent
The invention provides a quaternary ammonium salt concrete anti-mud agent, and belongs to the technical field of building materials. The molecular structure of the quaternary ammonium salt concrete anti-mud agent is as shown in formula I, wherein R or R' is a hydrocarbyl group with a molecular weight of 200 or below, or a derivative of the hydrocarbyl group, and X or X' is selected from Cl, Br andI. The invention also provides a preparation method of the anti-mud agent. The preparation method comprises the following steps: 1) dissolving N,N,N,N-tetramethyl-1,4-phenylenediamine and a halogenated hydrocarbon into an inert organic solvent at an inert atmosphere; 2) controlling the temperature of the system, carrying out stirring, and carrying out reflux condensation; and 3) carrying out reduced pressure distillation to remove the organic solvent, adding distilled water to obtain an aqueous solution of the quaternary ammonium salt type concrete anti-mud agent. According to the anti-mud agent provided by the invention, epoxy monomers are not used as raw materials, the safety is high, and the production process is simple and convenient. When the usage amount of the anti-mud agent provided by the invention accounts for 0.05-0.25 wt% of the total mass of the concrete glue material, the addition amount of a water reducing agent of high-mud-containing mechanism sand concrete can be reduced by 0.5% or more, and the newly mixed concrete has no fluidity loss in 3 hours.
Owner:广西中建西部建设有限公司 +3
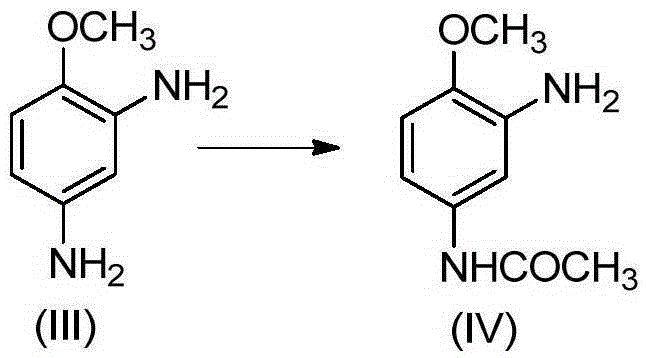
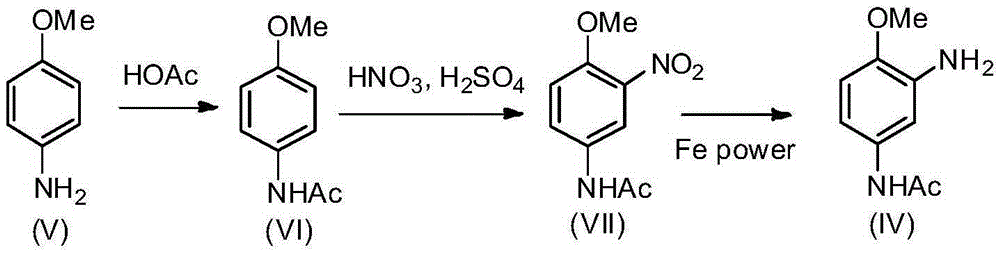
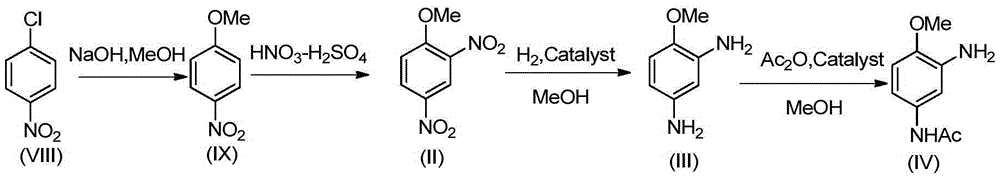
2-amino-4-acetamino anisole synthesis process
InactiveCN105348132AOrganic compound preparationCarboxylic acid amides preparationAcetic anhydrideEther
Owner:安徽奥瑞化工有限公司
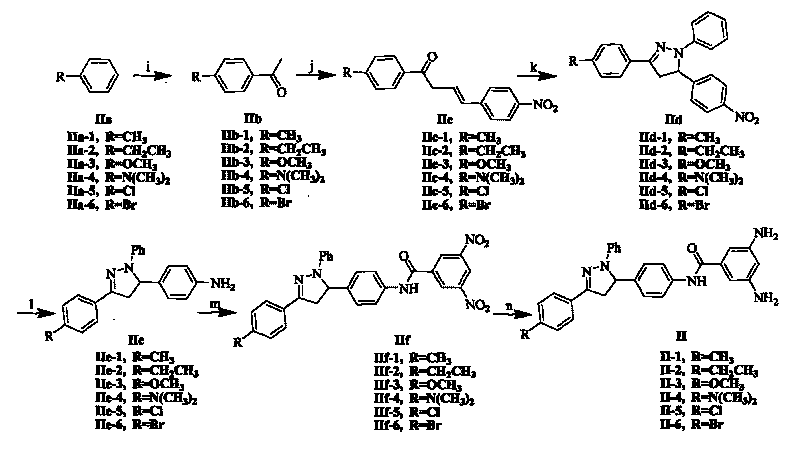
Hydrogen-bond self-assembly super-molecular blue-fluorescence polymer and symmetric method thereof
InactiveCN101693763ANo secondary repulsionImprove bindingOrganic compound preparationCarboxylic acid amides preparationChemistryRepulsion force
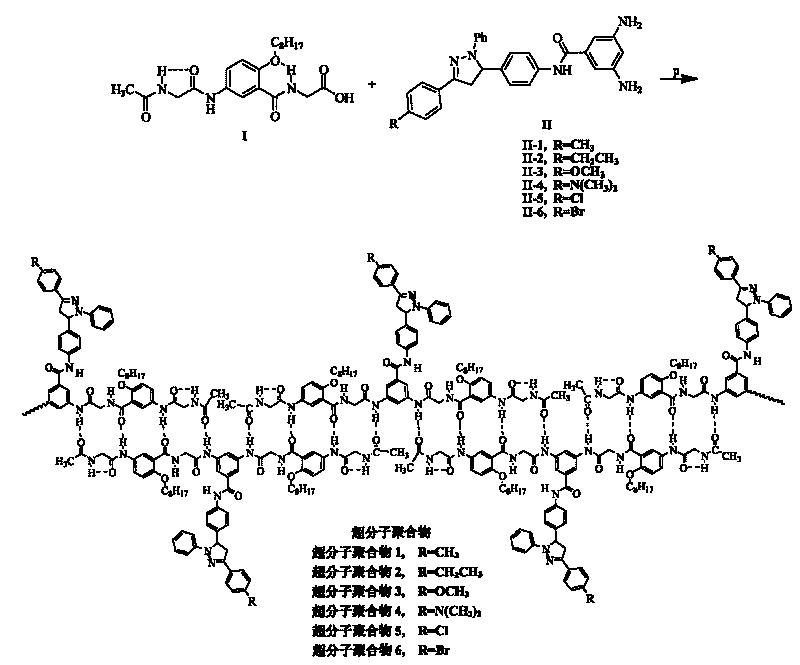
The invention relates to a pyrazoline-type hydrogen-bond self-assembly super-molecular polymer with blue-fluorescence property. The invention adopts an oligo-polyamide strip without secondary repulsion force as a hydrogen-bond bonding part and increases the number of hydrogen-bond donors and hydrogen-bond receptors to 8 from 4 so as to greatly improve the connection strength of the polymer. In addition, a pyrazoline derivative is introduced in a super-molecular polymer monomer as a blue-fluorescence unit to obtain a super-molecular blue-fluorescence polymer with a higher fluorescence quantum yield. The obtained six novel super-molecular polymer blue-fluorescence materials can emit pure-blue fluorescence in a solution state and have emission wavelengths of 440-455nm, semi-peak widths of 61-70nm and better color purity and can emit pure-blue fluorescence in a solid state and have emission wavelengths of 451-474nm, semi-peak widths of 71-79nm and better color purity.
Owner:SICHUAN UNIV
Coproduction method of 1, 2-propanediamine and dimethyl piperazine
InactiveCN102718661AReduce consumptionLow costOrganic compound preparationAmino compound preparationPorosityContact time
Owner:XIAN MODERN CHEM RES INST
Method of preparing 4-amino-3-phenyl butyric hydrochloride
InactiveCN101033195AHigh yieldHigh product contentOrganic compound preparationAmino-carboxyl compound preparationChemistryFormate
This invention relates to a preparation method for 4-amido-phenyl butyrate including: mixing benzene formate and methyl alcohol with nitromethane to be reacted at heat preservation, cooled and crystallized, separated for solid and liquid to be dried to get nitrobenzene ethane, which is put into a methanol solution together with diethyl malonate to be reacted to generate alpha-carbomethoxy-beta-phenyl-gamma-nitryl methyl butyrate, then Ni and H2 are added into a methanol solution to carry out hydrogenation reaction to get alpha-carbomethoxy-beta-phenyl-gamma tetrahydroketopyrrolidine, which is added with HCl to be hydrolyzed dried to get a product of 4-amido-phenyl butyrate.
Owner:安徽省郎溪县科联实业有限公司 +1
Separation method used for preparing isopropanol via hydrogenation of acetone
ActiveCN103772145ASimple processLess investmentOrganic compound preparationHydroxy compound preparationExtractive distillationAqueous solution
Owner:CHINA PETROLEUM & CHEM CORP +1
Process and plant for the production of urea with high conversion yield and low energy consumption
Owner:UREA CASALE SA
Simple method for extracting natural taurine from abalone viscera
ActiveCN103342668ASimple processReduce manufacturing costOrganic chemistryOrganic compound preparationBiotechnologyActivated carbon
Owner:JIMEI UNIV
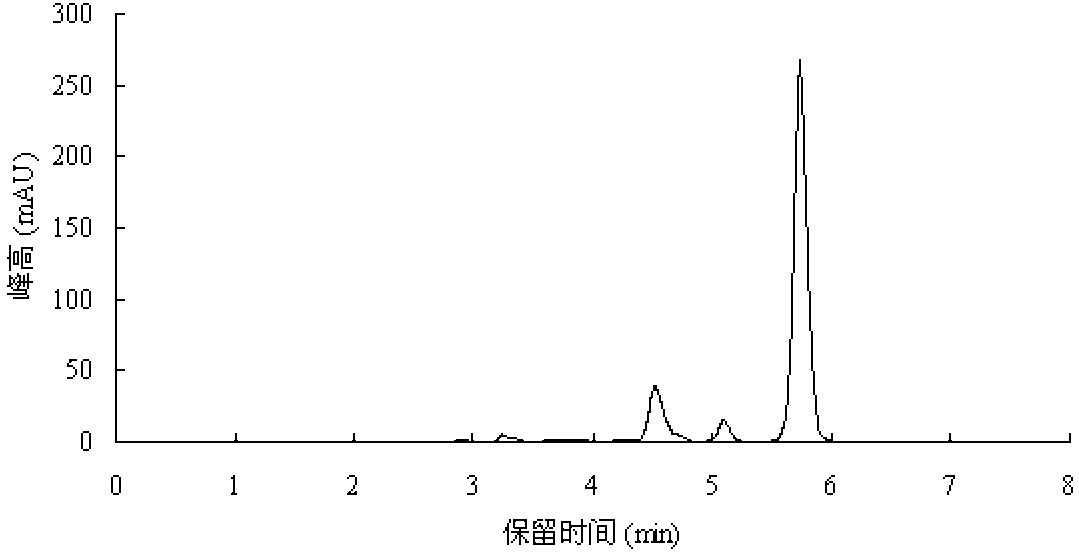
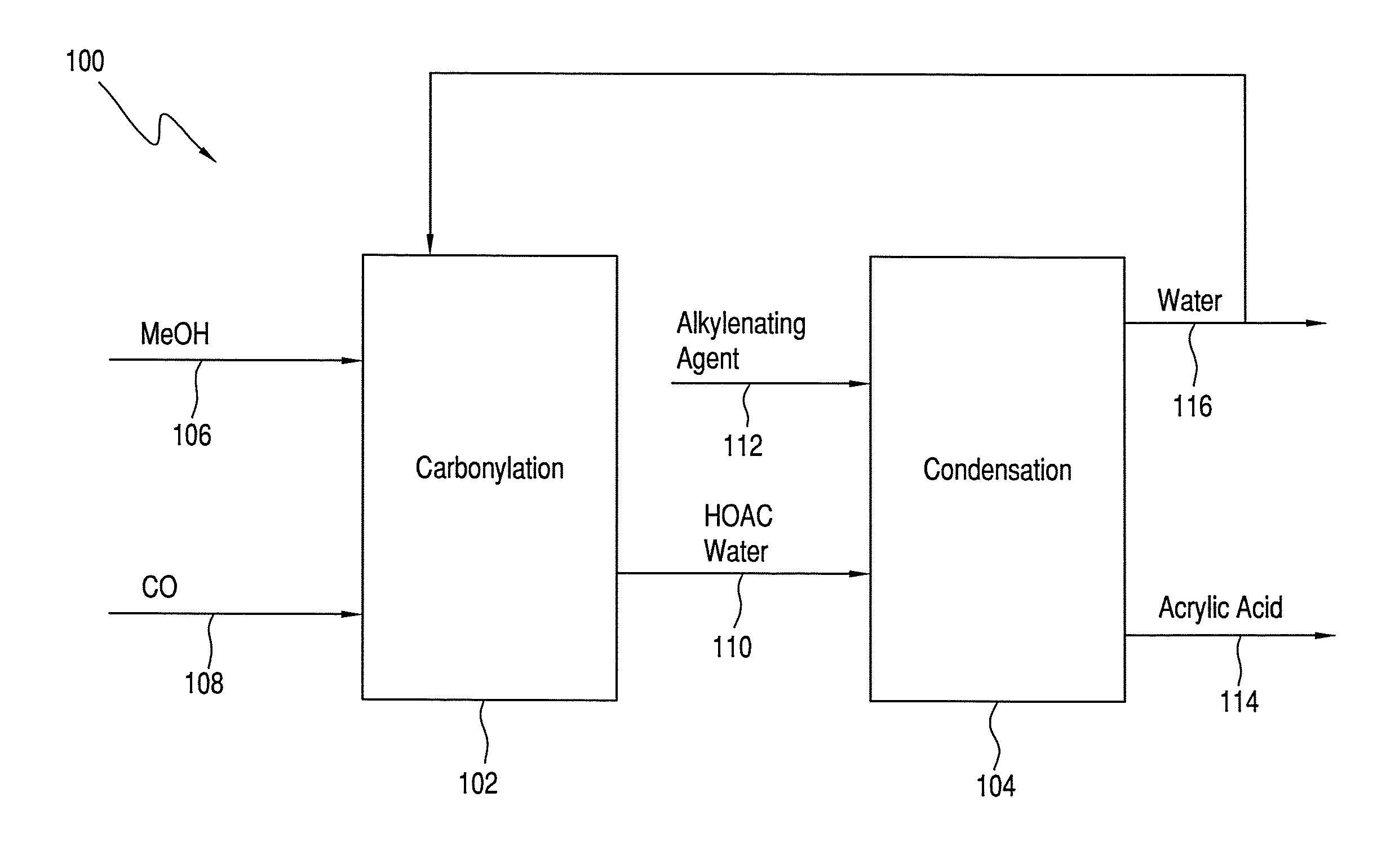
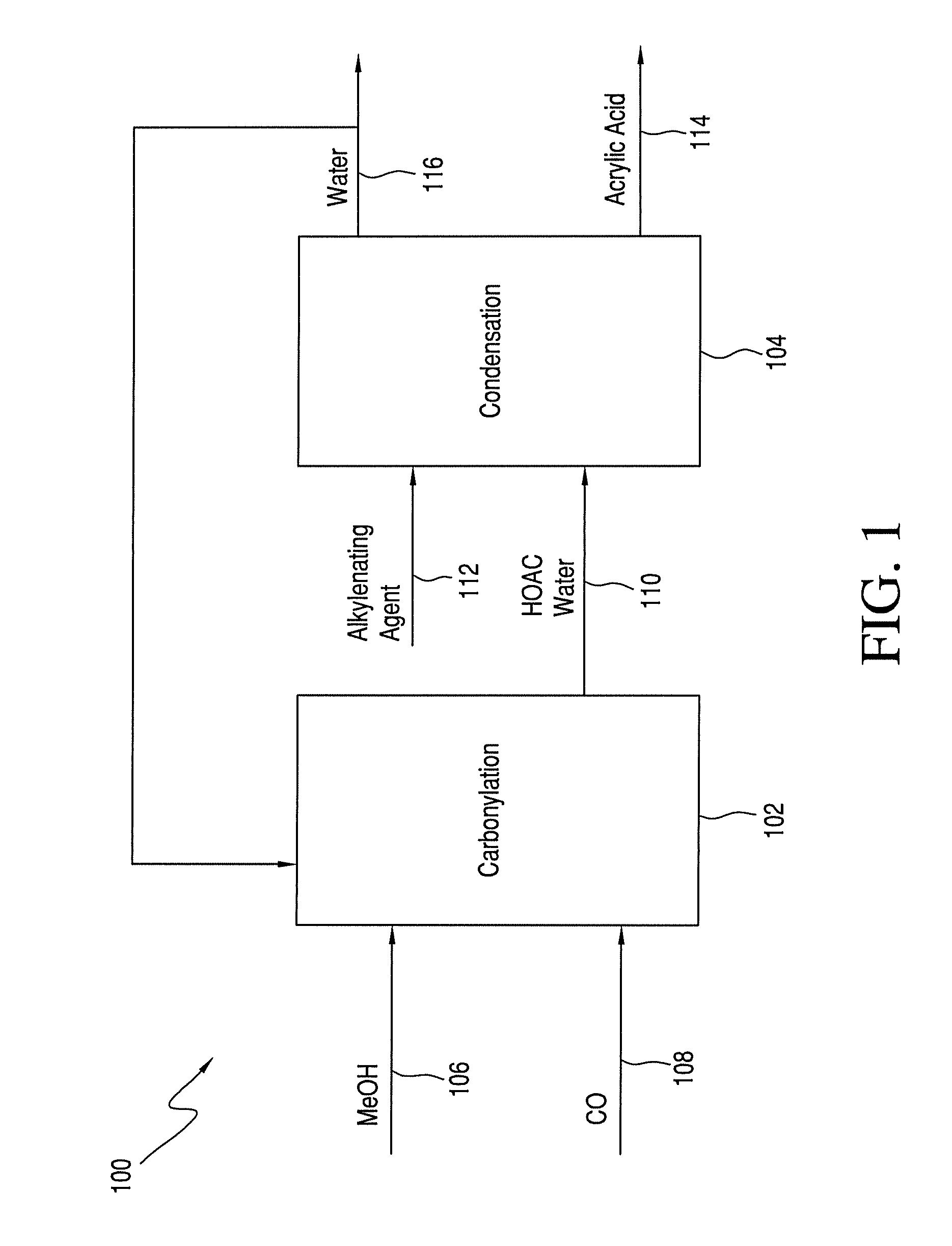
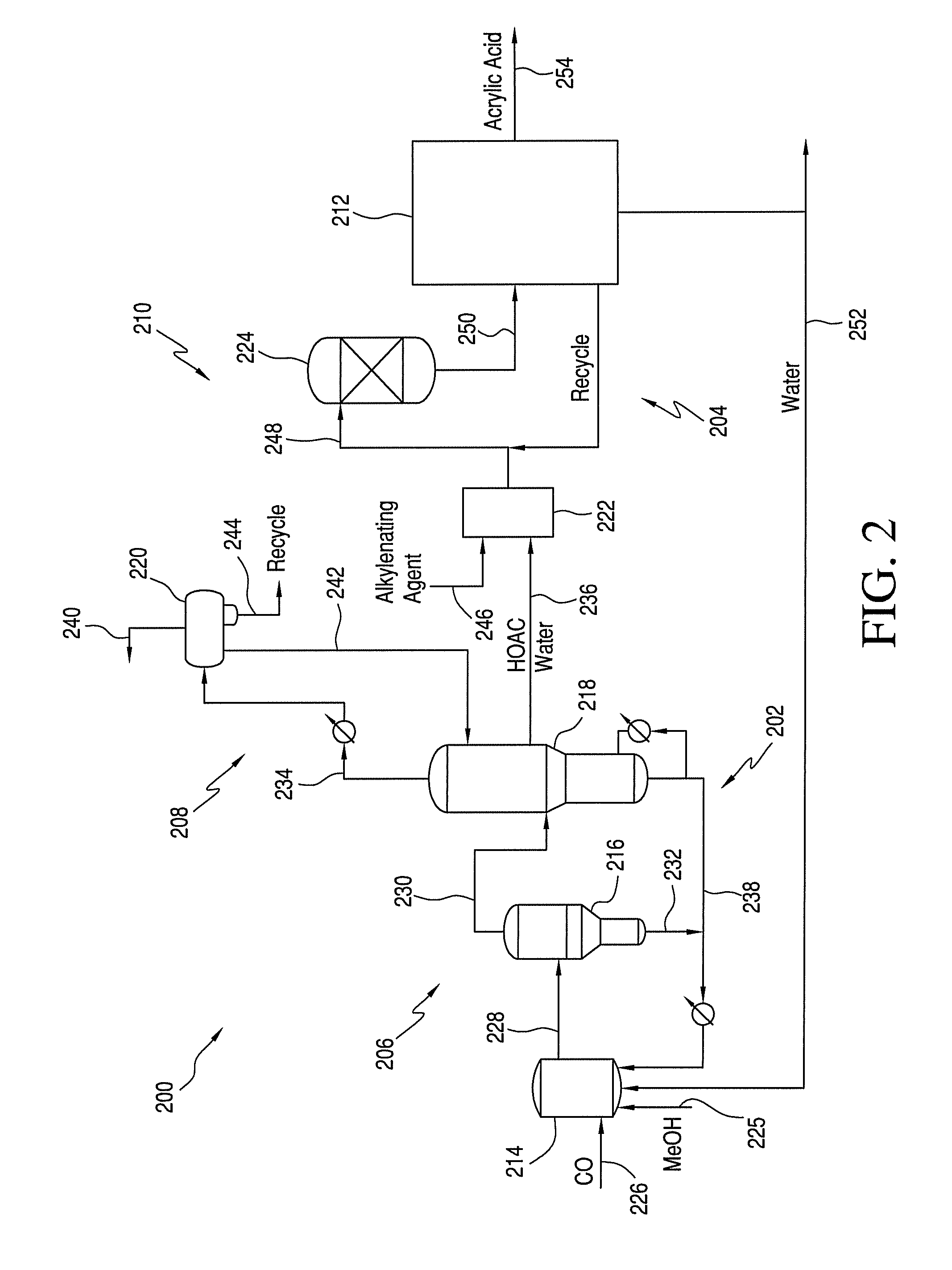
Process for Making Acrylic Acid by Integrating Acetic Acid Feed Stream from Carbonylation Process
InactiveUS20140073812A1Organic compound preparationCarboxylic preparation from carbon monoxide reactionProtein carbonylCarbonylation
Owner:CELANESE INT CORP
Fluorinated isocyanate and method for preparing fluorinated isocyanate-acrylate copolymer emulsion
ActiveCN103992242AGood compatibilityThe reaction system is stableCarbamic acid derivatives preparationOrganic compound preparationIsocyanateEmulsion
The invention discloses a vinyl-containing fluorinated isocyanate monomer. The preparation method of the fluorinated isocyanate monomer comprises the following steps: reacting diisocyanate with fluorinated alcohol to prepare a fluorinated isocyanate intermediate and then reacting the fluorinated isocyanate intermediate with (meth) hydroxyalkyl acrylate to obtain the fluorinated isocyanate monomer. The organic fluorine-modified polyurethane-acrylate copolymer emulsion with the characteristics of organic fluorine, polyurethane and acrylate resin is synthesized by carrying out emulsion polymerization on the fluorinated isocyanate monomer and the conventional (meth) acrylate. Compared with the prior art, the method for preparing fluorinated isocyanate-acrylate copolymer emulsion has the advantages that since the fluorinated isocyanate monomer is prepared in advance, when an emulsion copolymerization reaction is carried out on the fluorinated isocyanate monomer and acrylates monomer in an aqueous system, the compatibility between the monomers is better, the reaction system is more stable, the reaction is controlled easier and the synthesized copolymer emulsion has better stability.
Owner:BEIHANG UNIV
Nickel catalyst, process for the preparation thereof, process for hydrogenation of m-dinitro benzene to m-phenylene diamine
InactiveUS20050070740A1Speed up the processLong catalyst lifeMolecular sieve catalystsOrganic compound preparationSolventSilicon dioxide
Owner:COUNCIL OF SCI & IND RES
Method for composite phosphotungstate catalyzed synthesis of citrate ester
InactiveCN106008207AAchieve reuseHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsEsterification reactionFatty alcohol
The invention relates to a method for composite phosphotungstate catalyzed synthesis of citrate ester. The citrate ester is synthesized through an esterification reaction of citric acid and fatty alcohol with composite phosphotungstate as a catalyst. The structure formula of the composite phosphotungstate adopted in the invention is (NH4)xM(3-x-y) / 4HyPW12O40, wherein M is Ti or Zr, x is 0.4-1, and y is 0.4-1. The method has the following advantages: the catalyst has low cost, is easy to prepare, has a high catalysis efficiency, can be simply separated from the above product, and has excellent performances when being reused.
Owner:SHAOYANG UNIV
High-shear reactor with feed distribution device
InactiveCN101947427AAvoid cloggingUniform particle size distributionIsocyanic acid derivatives preparationOrganic compound preparationDrive shaftSolid particle
Owner:TIANJIN UNIV +1
Organic bottom antireflective coating composition for nanolithography
ActiveUS20150185614A1Strong Gap Filling CapabilityMinimizing contentOrganic compound preparationCarboxylic acid esters preparationNanolithographyAnti-reflective coating
Owner:ROHM & HAAS ELECTRONICS MATERIALS LLC
Process for producing arginine by microbial fermentation
InactiveCN103695487AQuality improvementHigh yieldOrganic chemistryOrganic compound preparationBiotechnologyMicroorganism
Owner:滨州市生物技术研究院有限责任公司
Vanadium phosphorus oxygen catalyst for preparing acrylic acid and acetic acid by oxidation of propane and preparation method thereof
InactiveCN1557549ASmall particlesEvenly distributedPhysical/chemical process catalystsOrganic compound preparationIsobutanolPolyethylene glycol
The VPO catalyst is prepared with V2O2 and phosphoric acid in mixed isobutanol-benzyl alcohol solvent, and has polyglycol as dispersant. It has P / V atom rati of 1.1, specific surface area as high as 70-78 sq m / g and main material phase of vanadyl pyrophosphate. It is used the catalyst for oxidizing propane with air to preparing acrylic acid and acetic acid, and has single pass converting rate at the reaction temperature range of 380-400 deg.c of 37-72 % typically, acrylic acid selectivity of 5-35 % and total acrylic acid and acetic acid selectivity of 19-83 %. Under proper reaction condition, it has the propane converting rate of 40.3 %, acrylic acid selectivity of 33.8 %, acetic acid selectivity of 49.4 %, acrylic acid yield of 13.6 %, total acrylic acid and acetic acid yield of 33.5 % and space-time yield obviously higher than that with available similar catalyst. The preparation process of the catalyst is also disclosed.
Owner:NANJING UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap