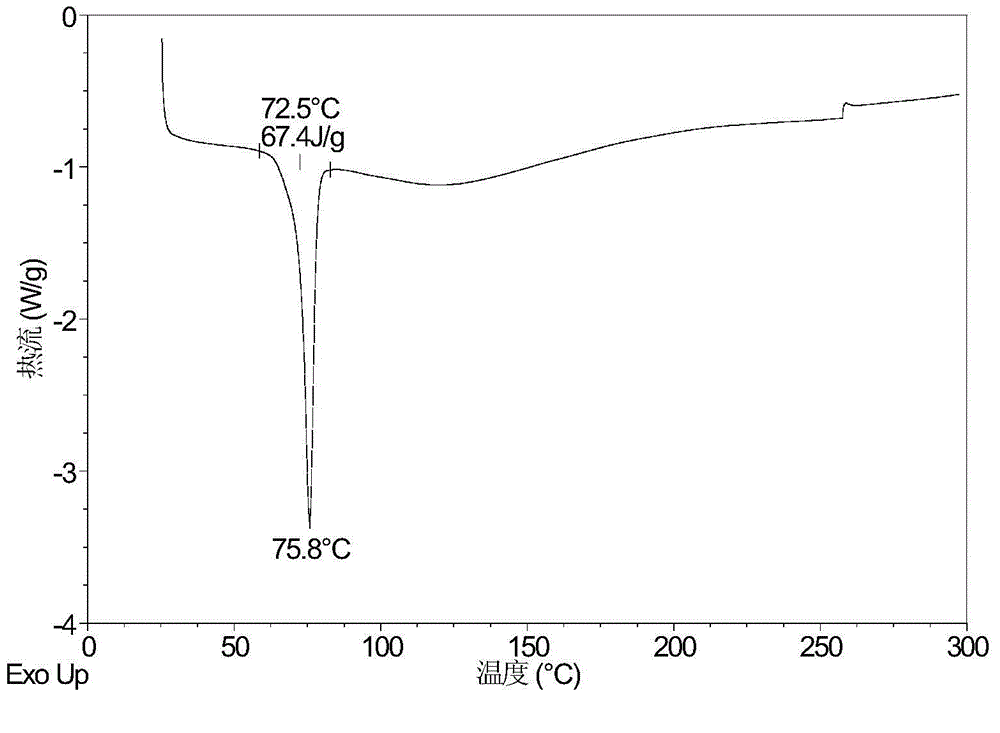
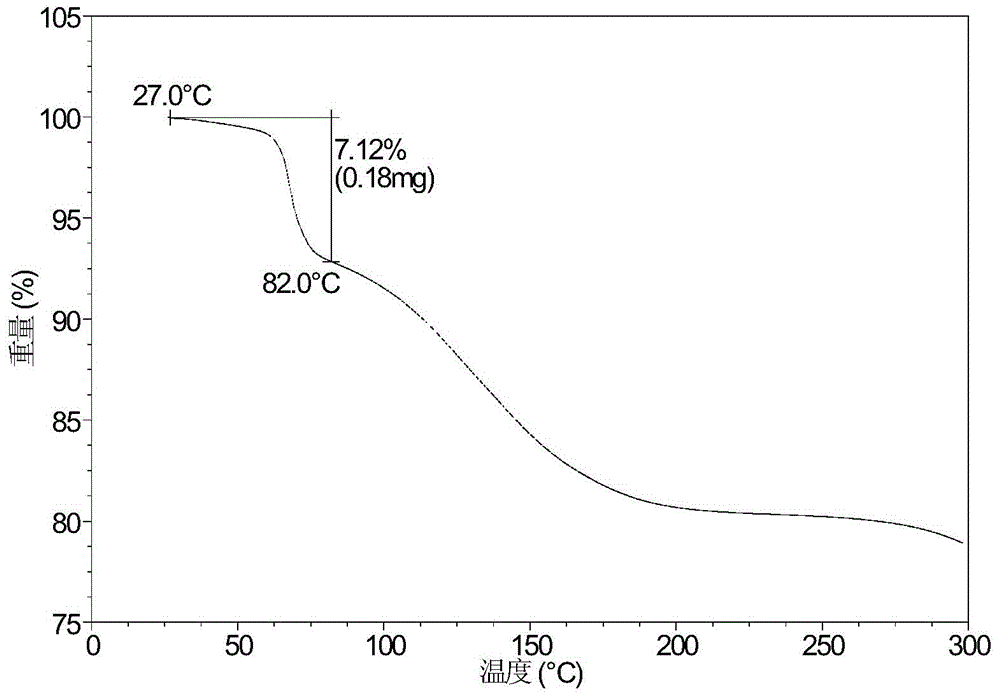
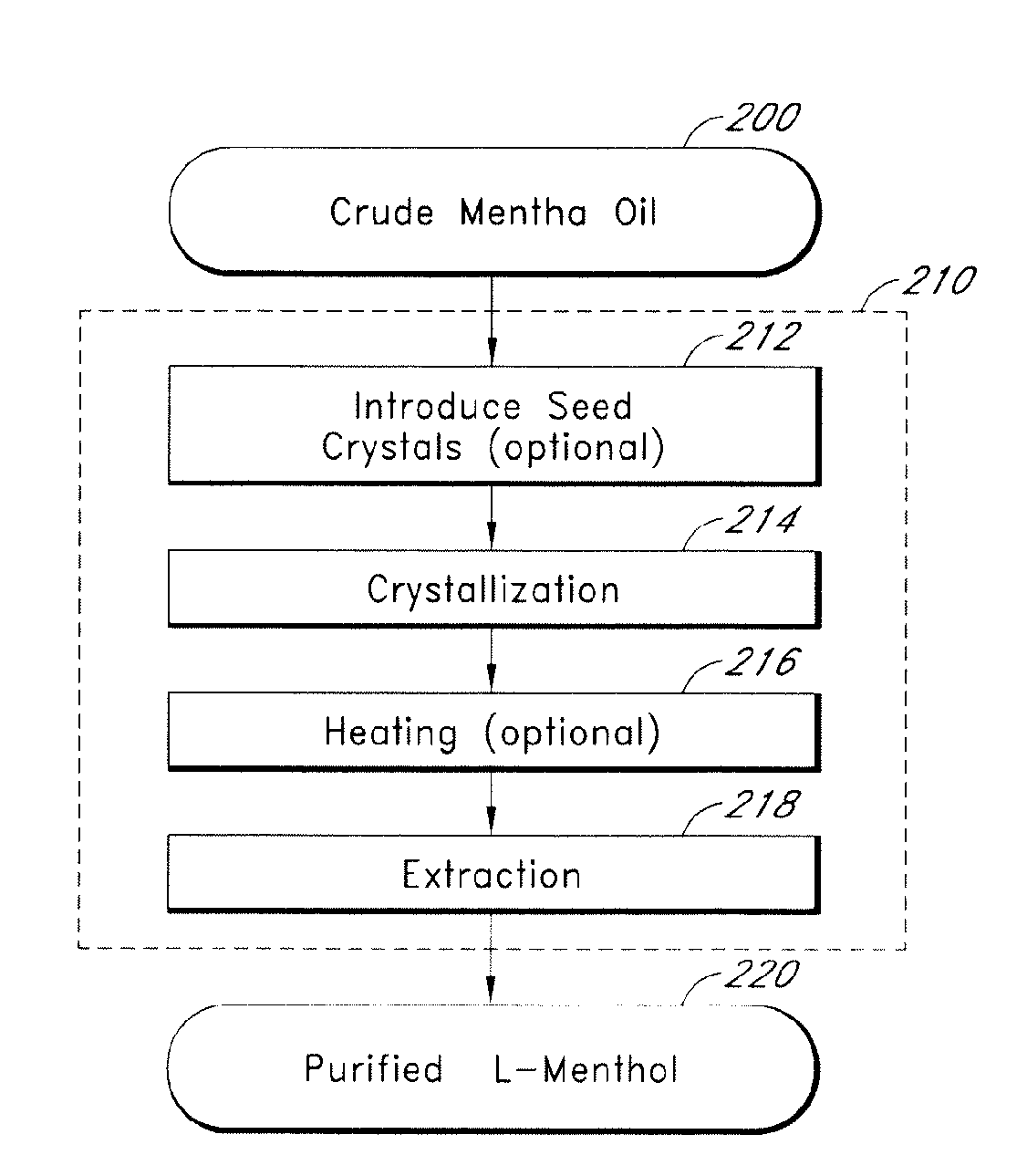
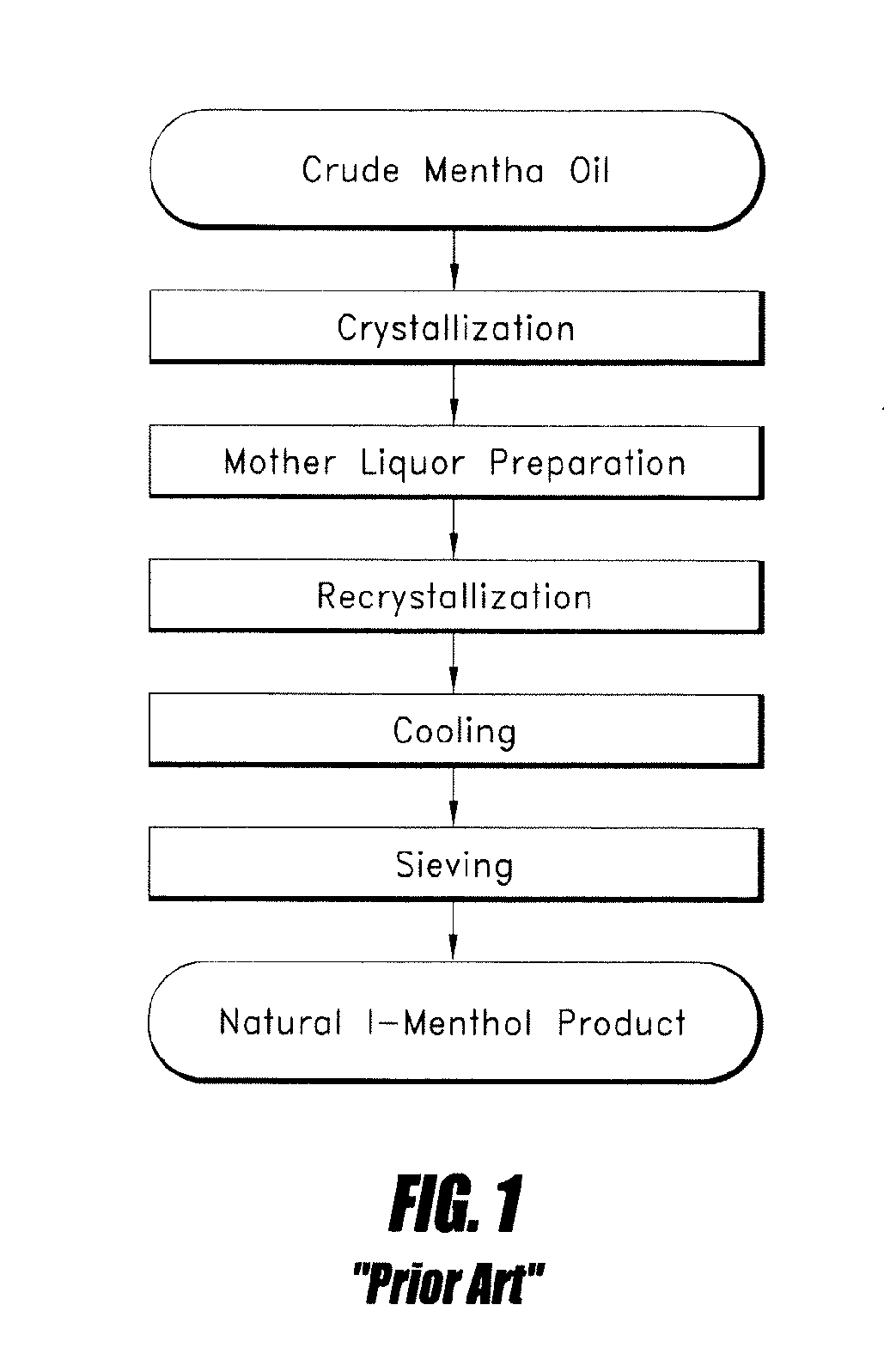
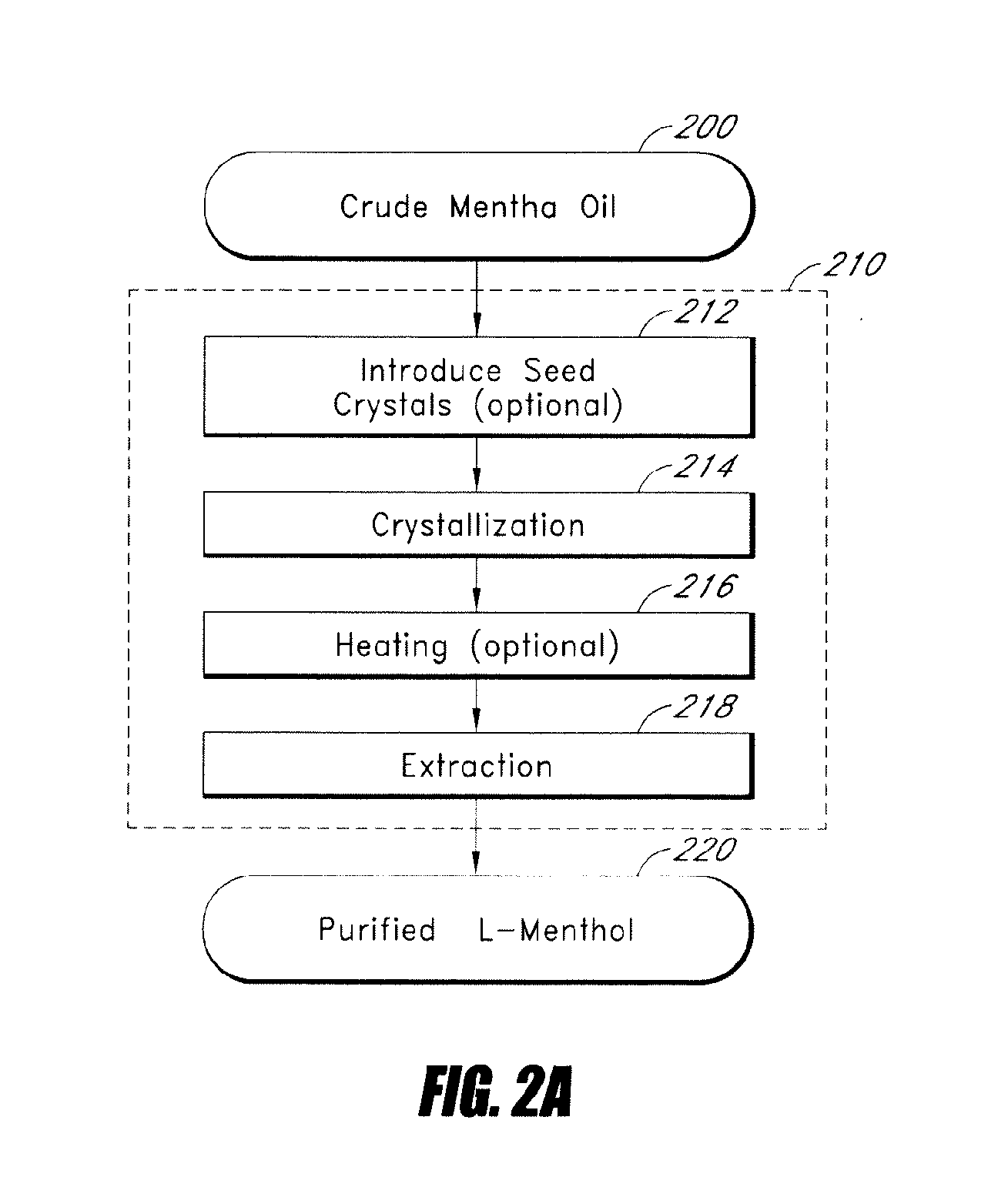
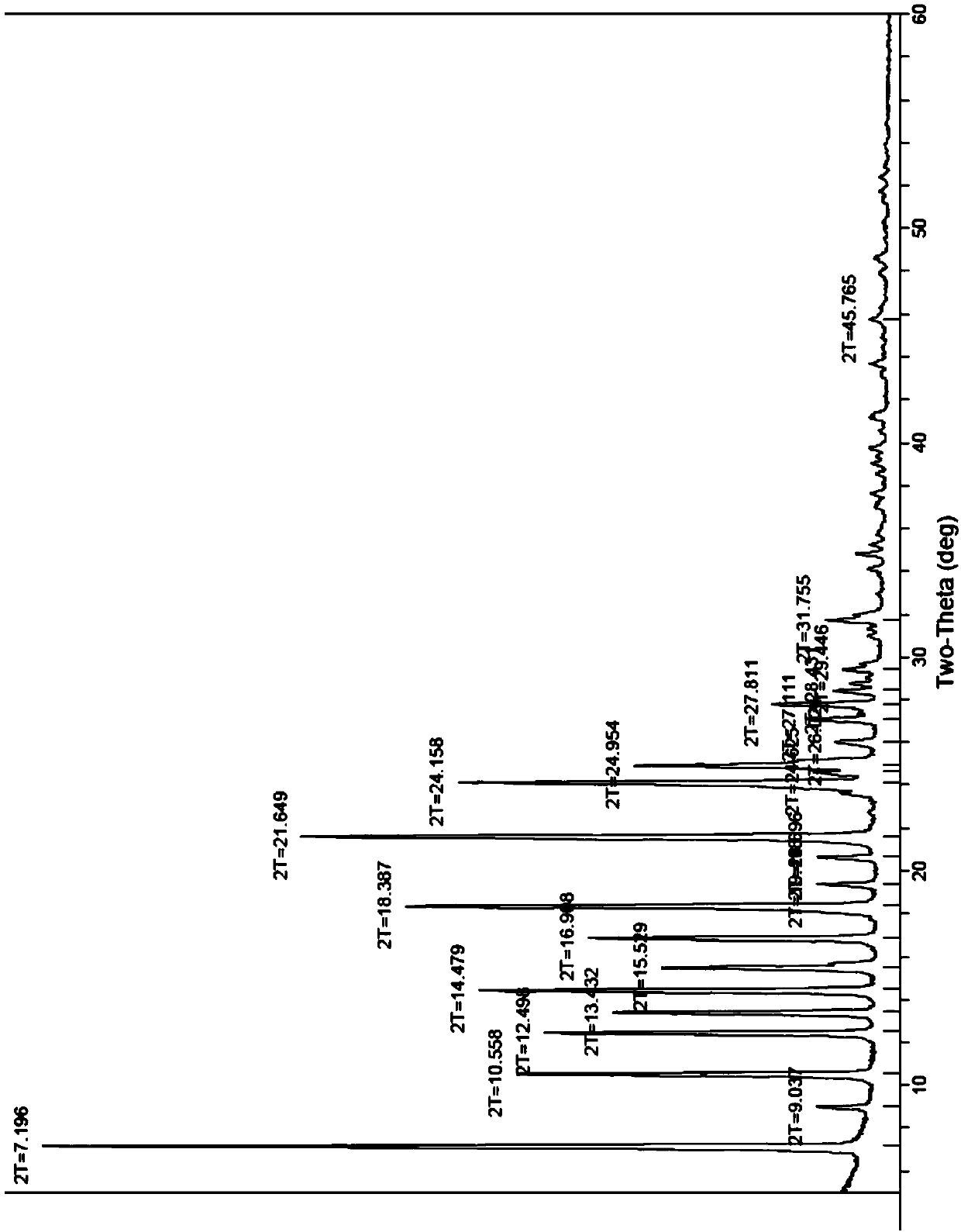
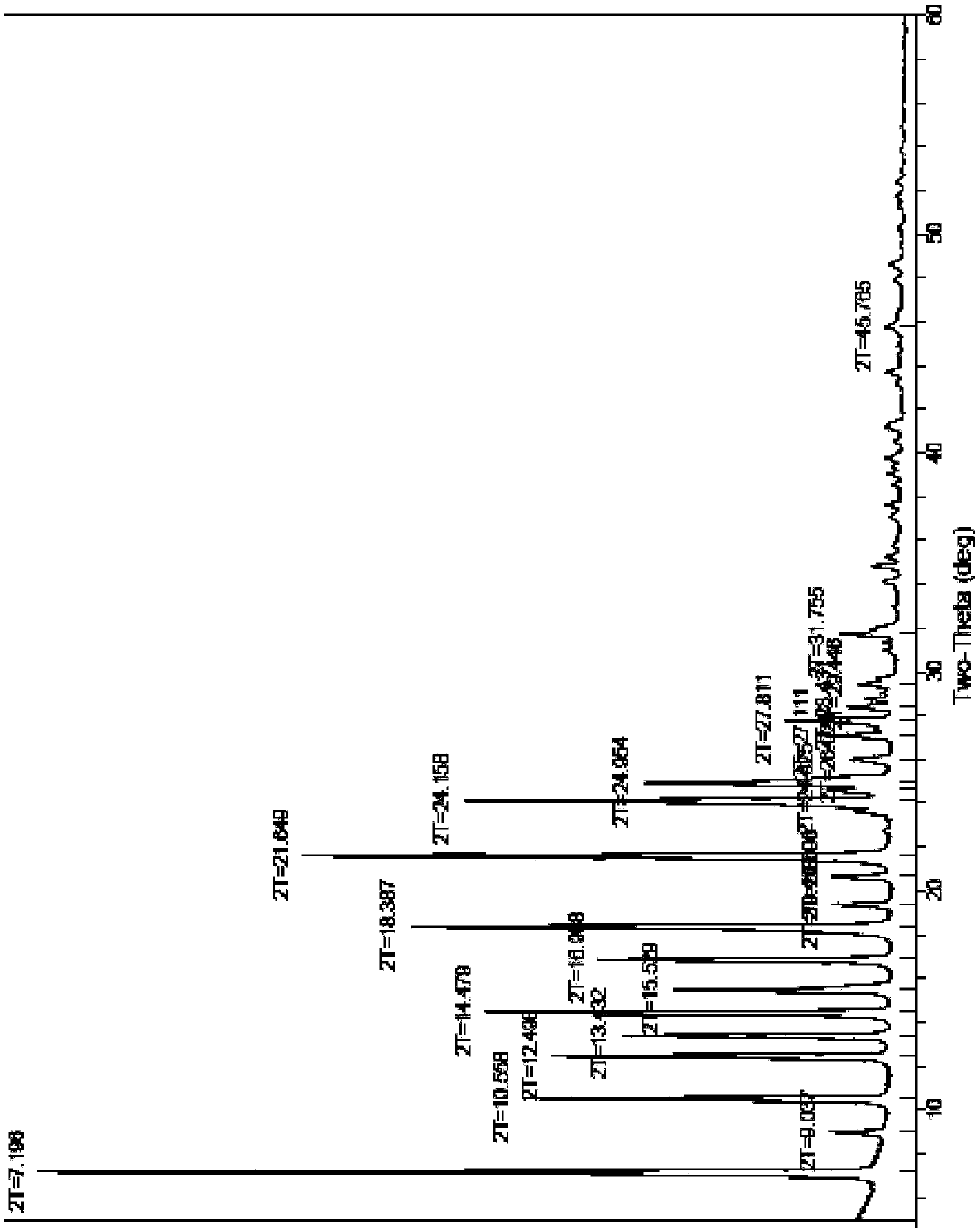
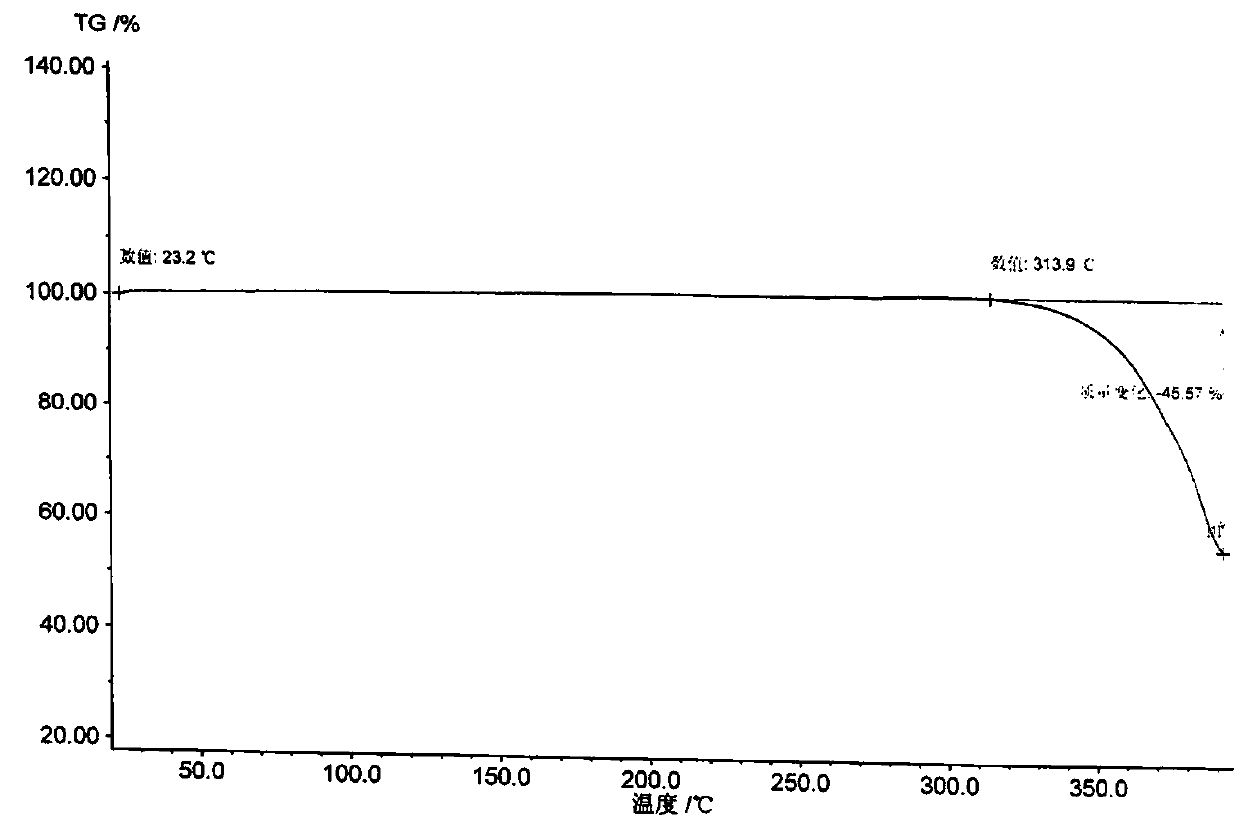
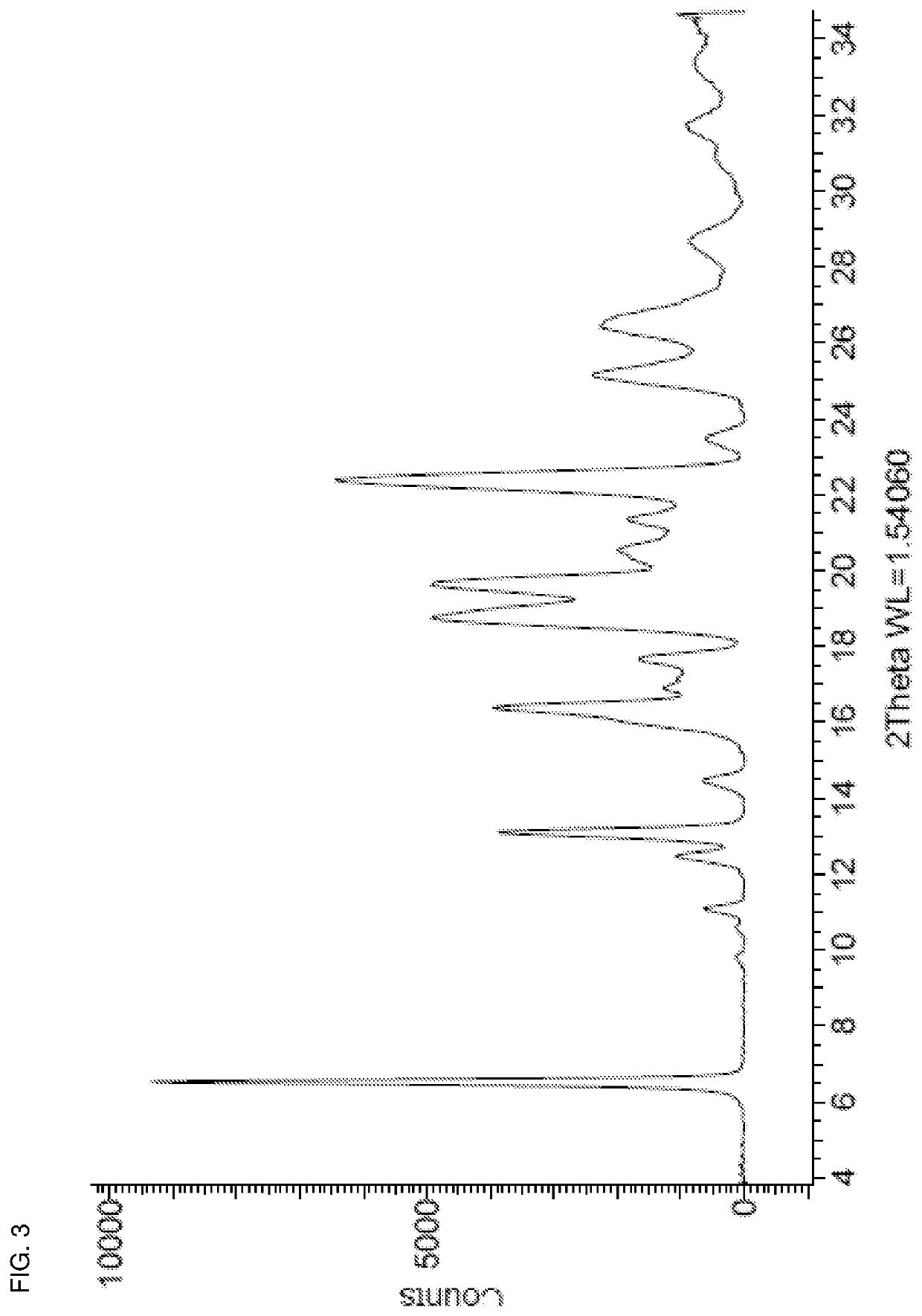
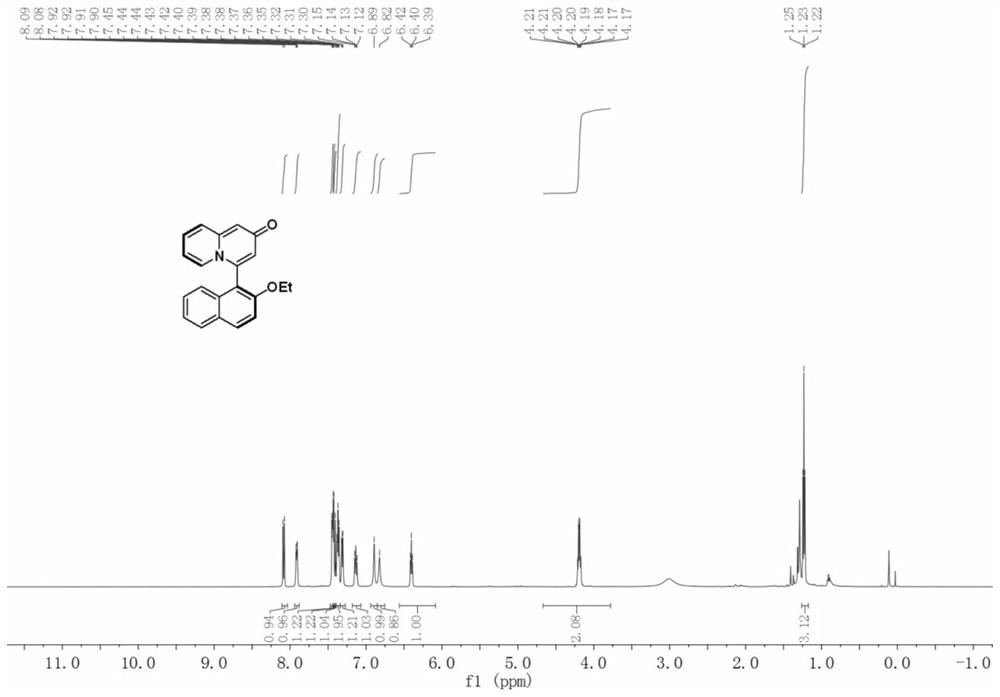
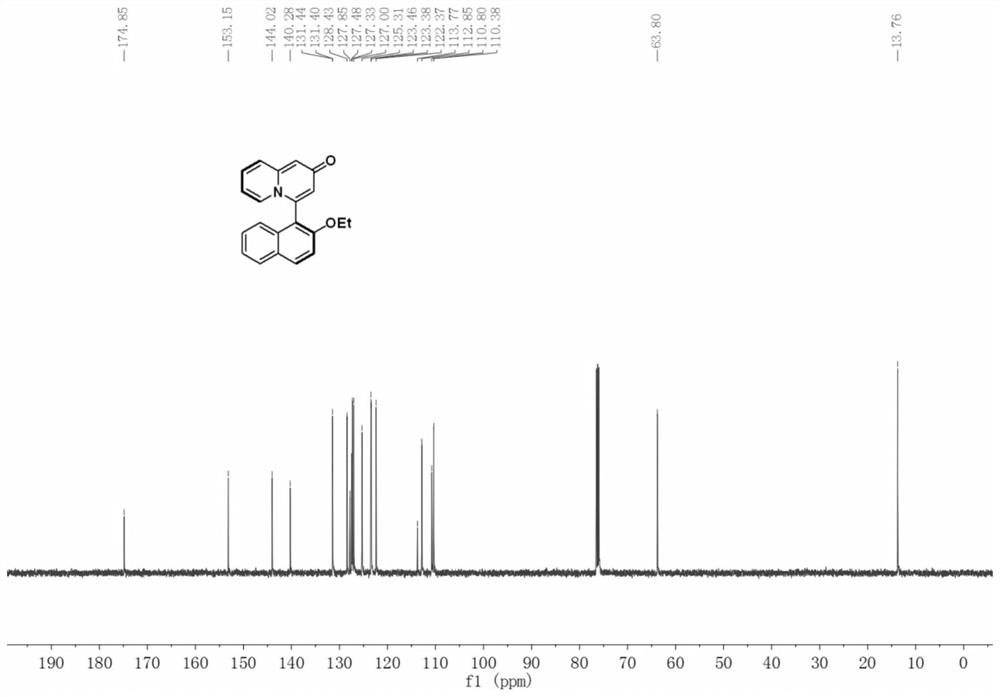
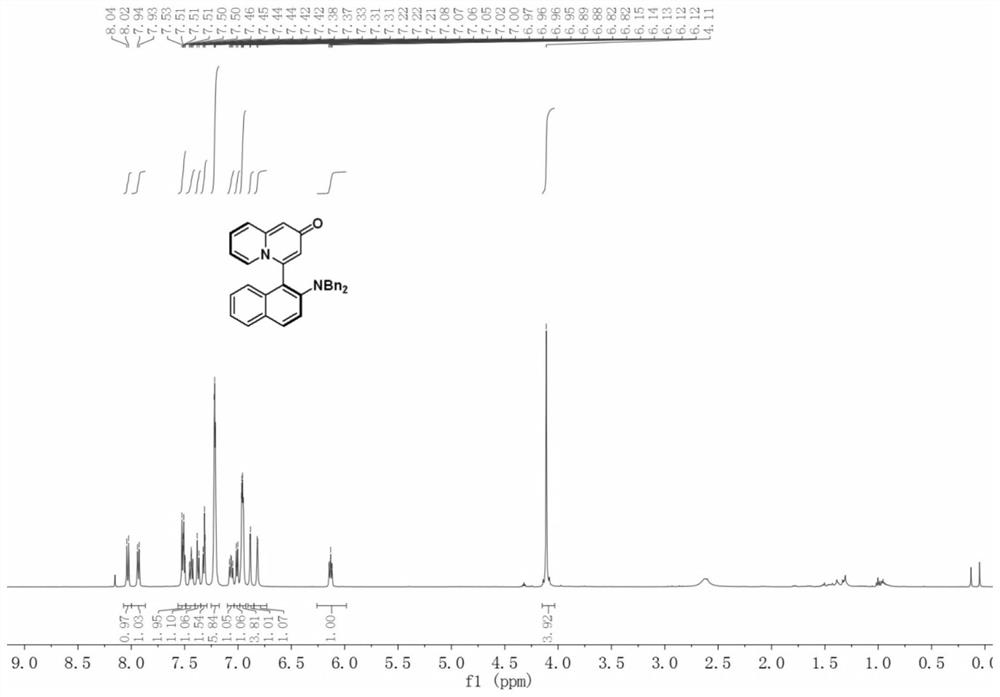
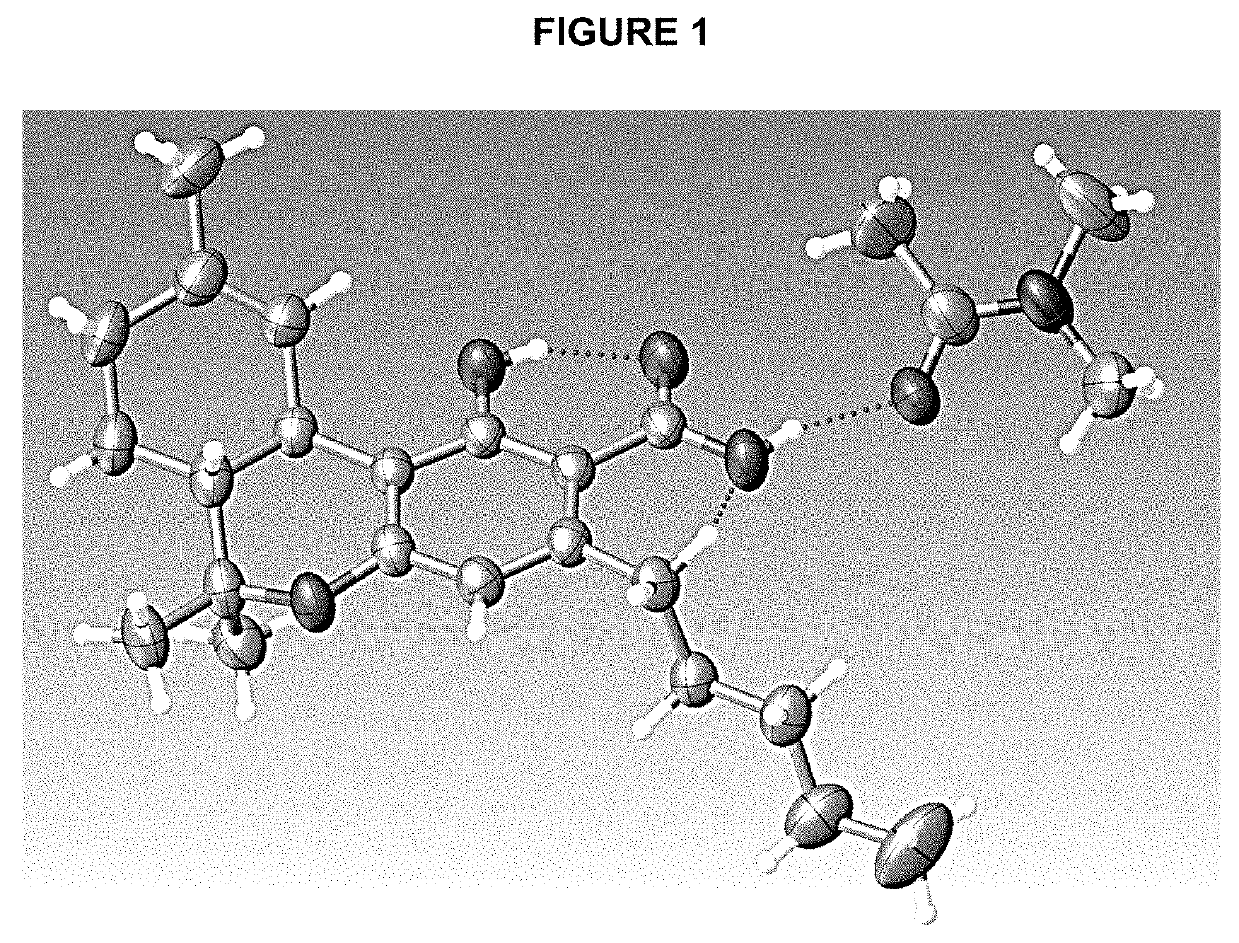
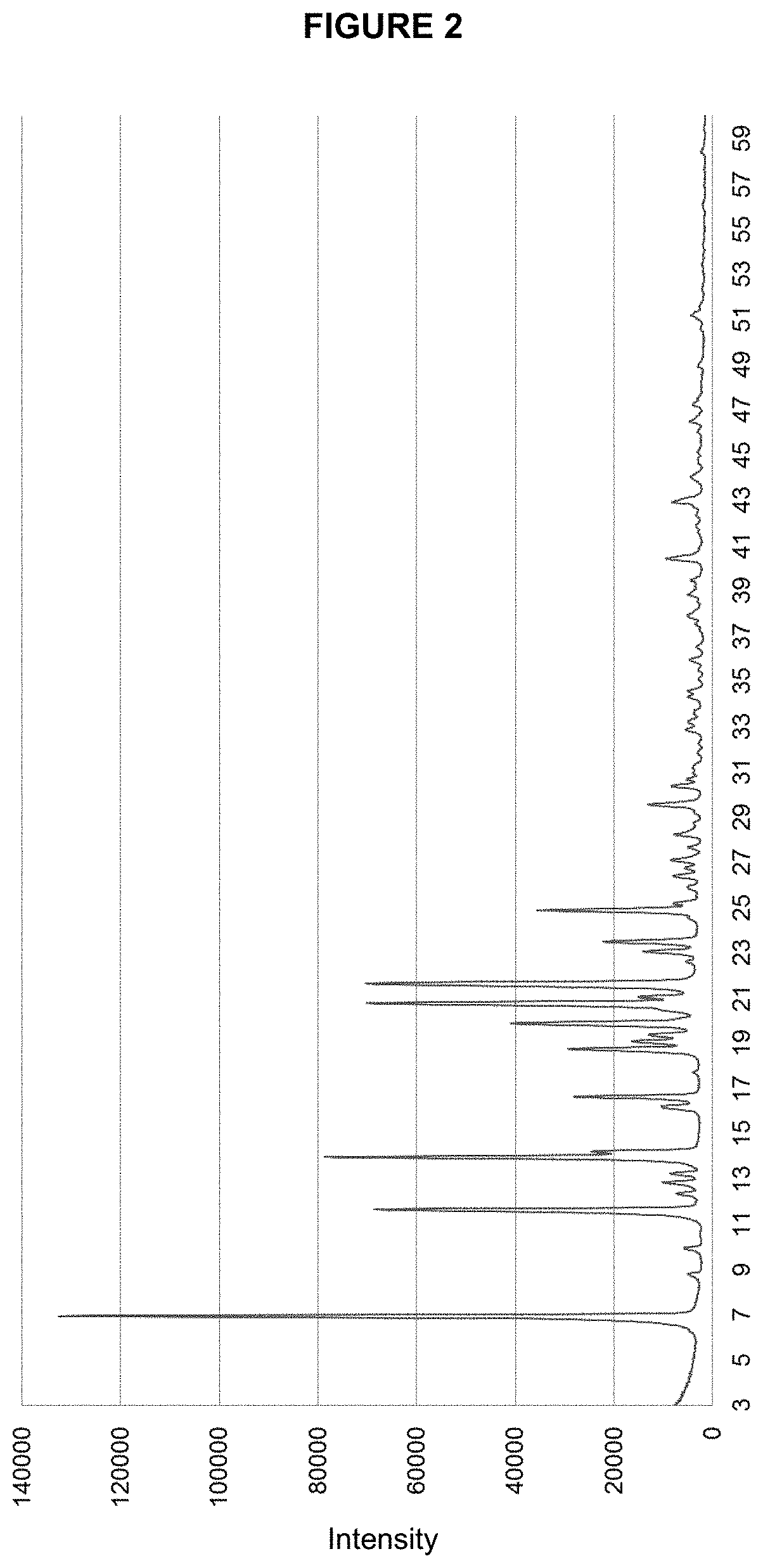
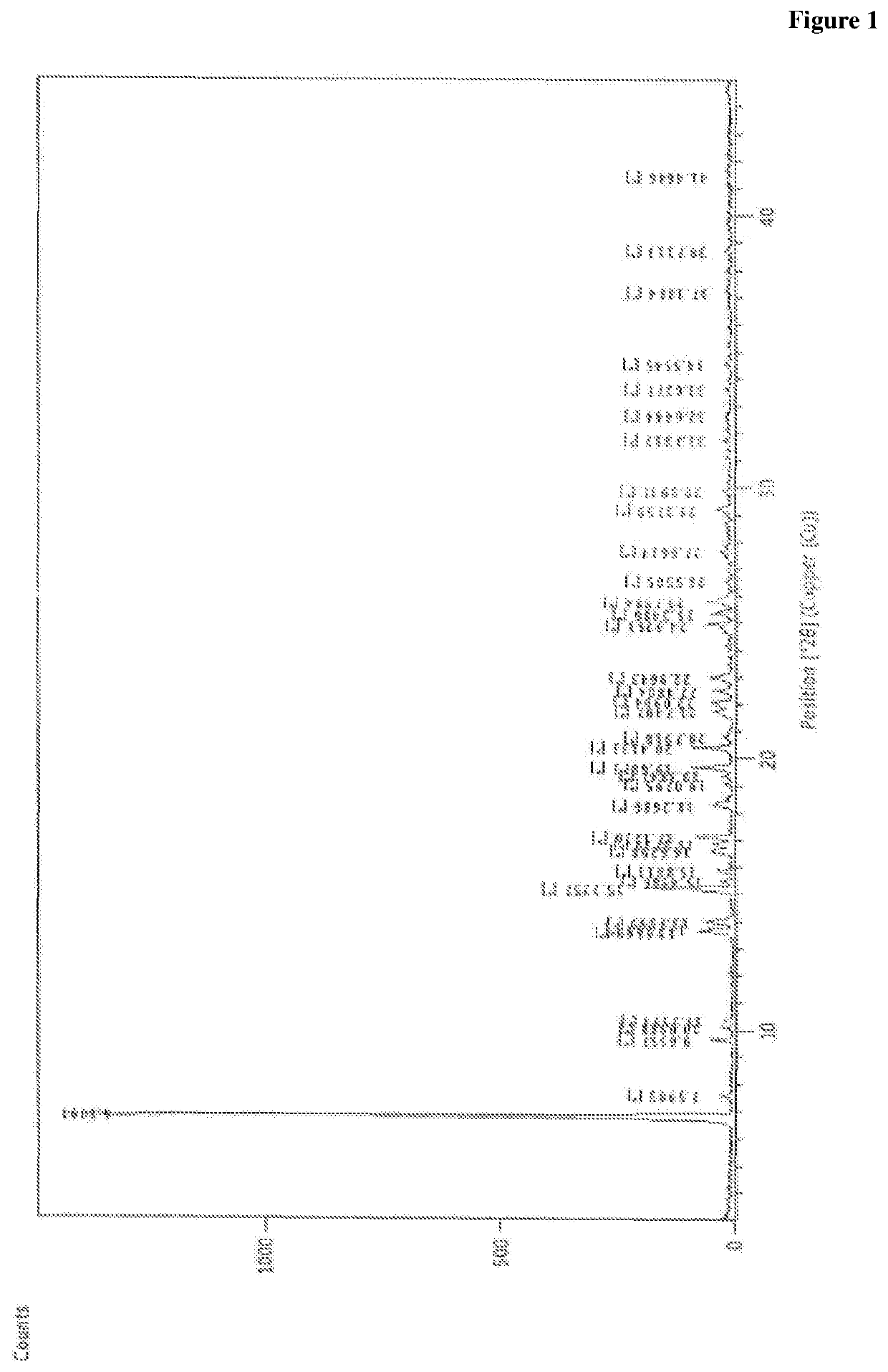
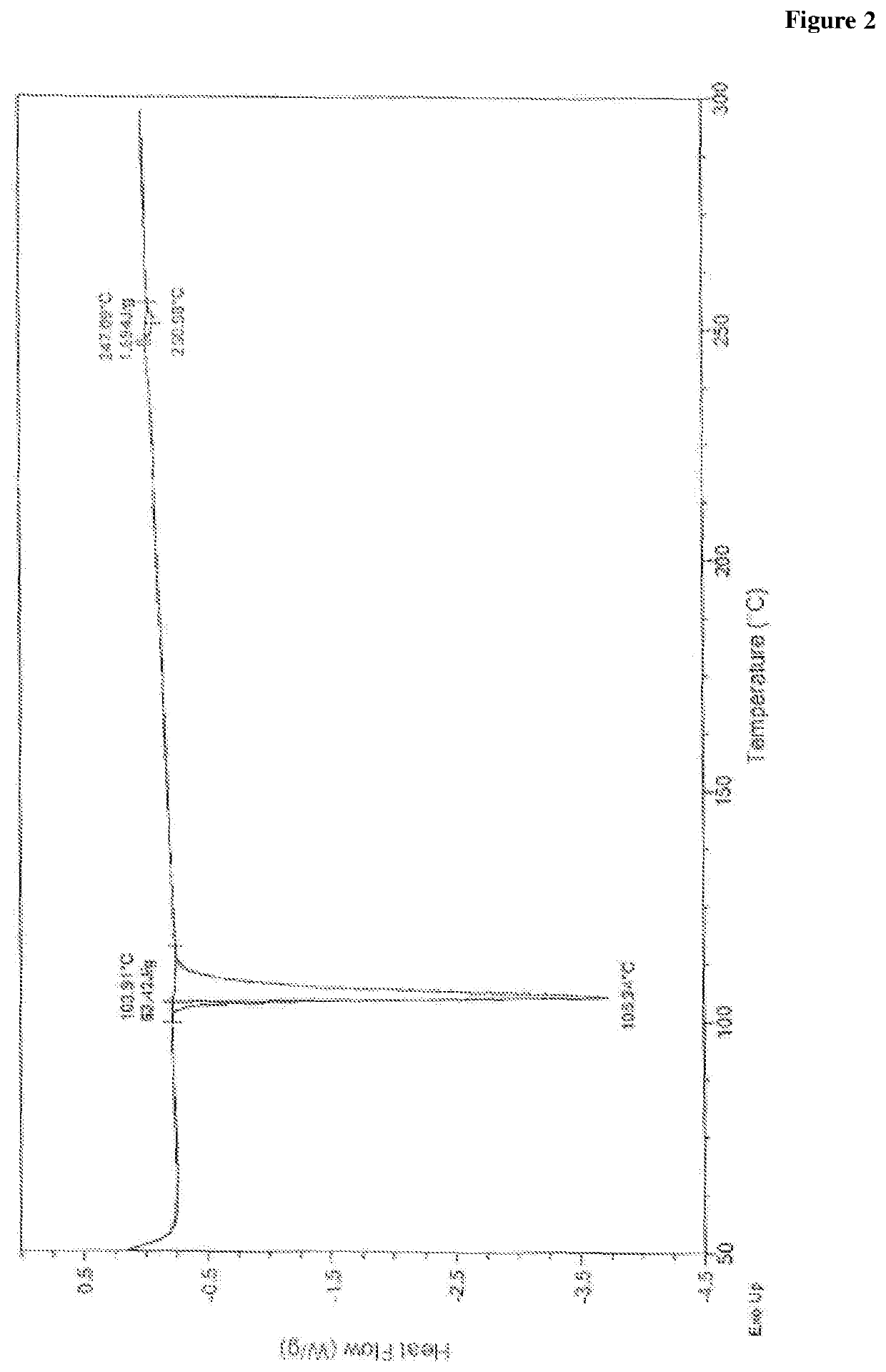
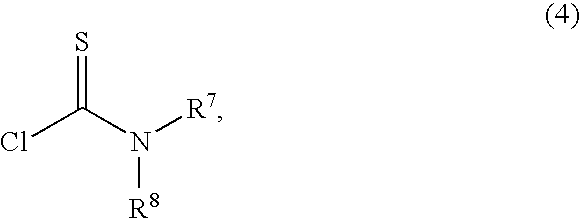Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42results about "Organic chemistry methods" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Crystal form G of ibrutinib and preparation method
InactiveCN105646499AImprove stabilityHigh purityOrganic active ingredientsOrganic chemistry methodsSolubilitySolvent
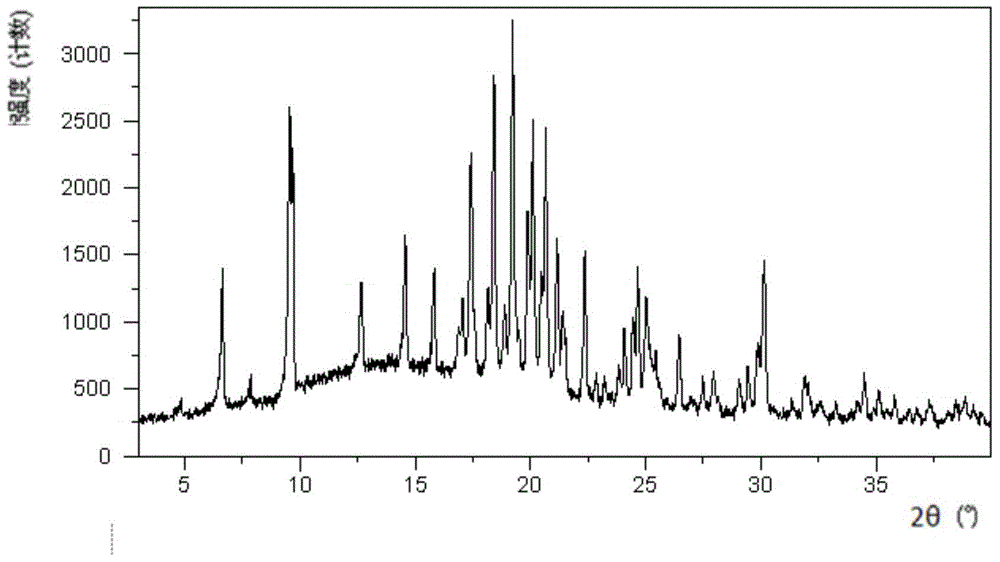
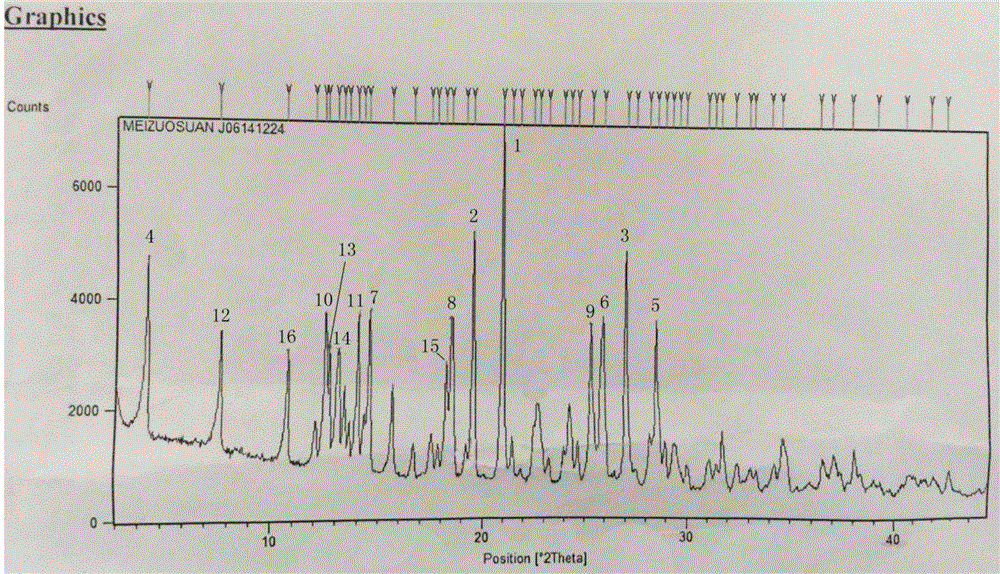
The invention discloses a crystal form G of ibrutinib. The crystal form G is characterized in that X-ray powder diffraction (X-RPD) which adopts Cu-Kalpha radiation and is represented with a 2theta angle has diffraction peaks in positions at angles of 5.0 degrees plus or minus 0.2 degrees, 7.3 degrees plus or minus 0.2 degrees, 10.1 degrees plus or minus 0.2 degrees, 12.0 degrees plus or minus 0.2 degrees, 13.2 degrees plus or minus 0.2 degrees, 17.1 degrees plus or minus 0.2 degrees, 19.5 degrees plus or minus 0.2 degrees, 20.8 degrees plus or minus 0.2 degrees, 22.3 degrees plus or minus 0.2 degrees, 24.3 degrees plus or minus 0.2 degrees, 27.4 degrees plus or minus 0.2 degrees and 31.2 degrees plus or minus 0.2 degrees. Related solvents in a preparation process of the crystal form G are cheap, the conditions are mild, the operation is simple, good controllability and reproducibility are realized, further, the prepared crystal form has great stability, the HPLC (high performance liquid chromatography) purity is higher than 99%, and the phenomenon of crystal transformation can be avoided; besides, the solubility is high, the dissolubility is good, and the bioavailability is high.
Owner:孙霖
Cariprazine tartrate, preparation method therefor and medical use thereof
ActiveCN105218484ANot easy to absorb moistureImprove solubilityNervous disorderOrganic chemistry methodsCariprazinePharmaceutical drug
Owner:ANHUI HEALSTAR PHARM CO LTD
Amorphous and a crystalline form of genz 112638 hemitartrate as inhibitor of glucosylceramide synthase
The hemitartrate salt of a compound represented by the following structural formula: (Formula I Hemitartrate), which may be used in pharmaceutical applications, are disclosed. Particular single crystalline forms of the Formula (I) Hemitartrate are characterized by a variety of properties and physical measurements. As well, methods of producing crystalline Formula (I) Hemitartrate, and using it to inhibit glucosylceramide synthase or lowering glycosphingolipid concentrations in subjects to treat a number of diseases, are also discussed. Pharmaceutical compositions are also described.
Owner:GENZYME CORP
Lead-free hybrid two-dimensional double perovskite material and preparation method thereof
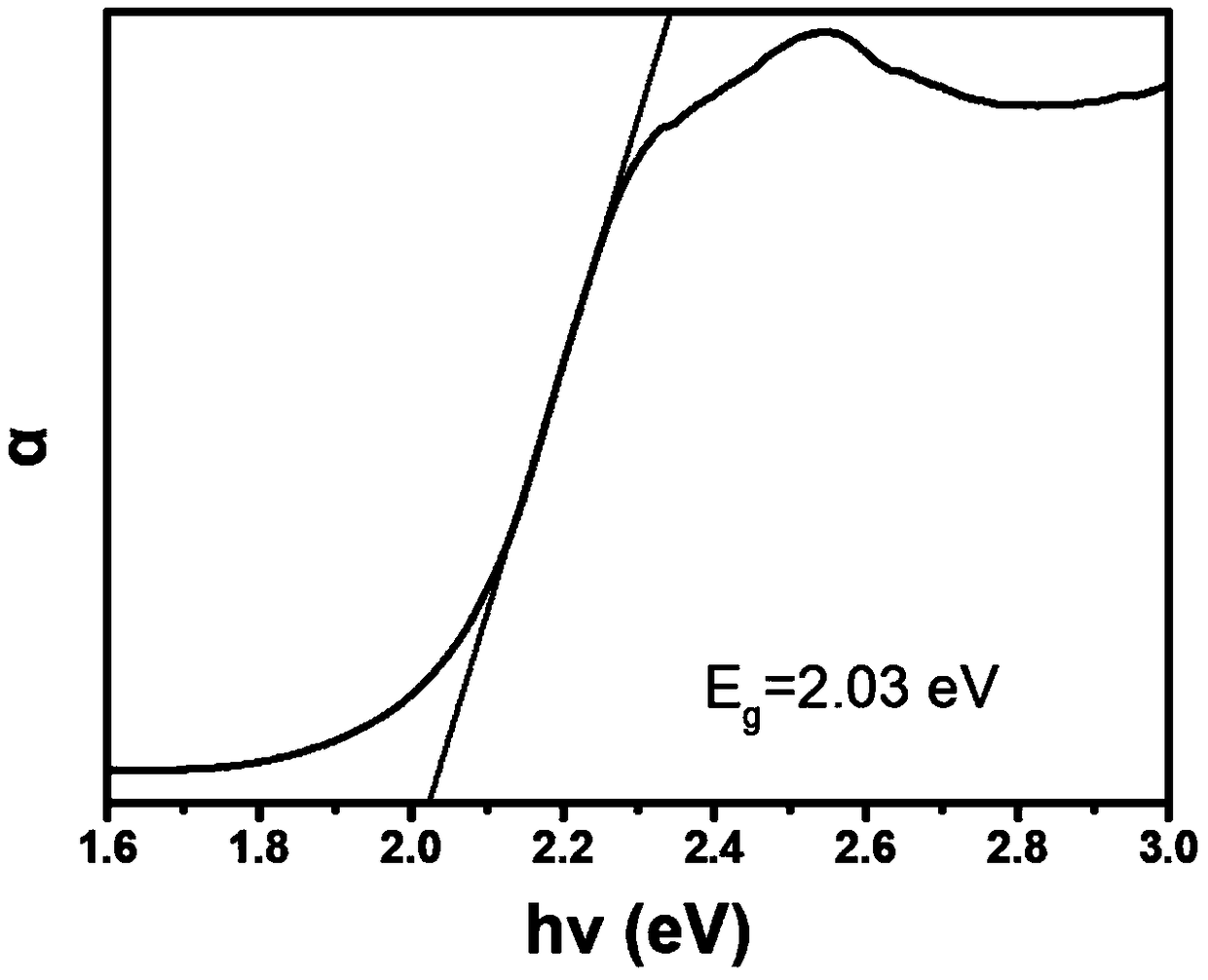
InactiveCN109369725AGood chemical stabilitySuitable and tunable optical absorption bandgapOrganic chemistry methodsBismuth organic compoundsPhoto stabilityOpto electronic
Owner:XI AN JIAOTONG UNIV
Method for preparing LCZ696
ActiveCN105330609AReduce generationPrecipitation state is goodOrganic compound preparationOrganic chemistry methodsSodium acetateReaction temperature
The invention discloses a method for preparing LCZ696. Please see the synthesis route in the specification. When sodium acetate is used as alkali in the compound III preparation process, the conversion rate is high, hydrolysis impurities are few, system stability is good, and the reaction time is greatly shortened; in the compound I preparation process, acetone and normal heptanes with the mass ratio between 5 to 1and 10 to 1 serve as cocrystallization solvent, the reaction temperature of 35-45 DEG C is adopted, a sodium hydroxide solution is dropwise added into a reaction system at a certain speed at the temperature of 35-45 DEG C, generation of hydrolysis products can be greatly reduced, the solid precipitation state is good, purity is high, aminolysis impurities and hydrolysis impurities can be effectively controlled, and the LCZ696 can directly serve as crude drug to be used for preparations.
Owner:NANJING CHIA TAI TIANQING PHARMA +1
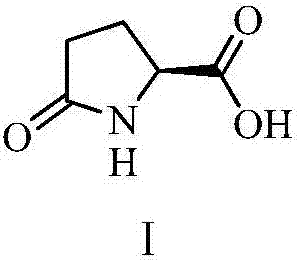
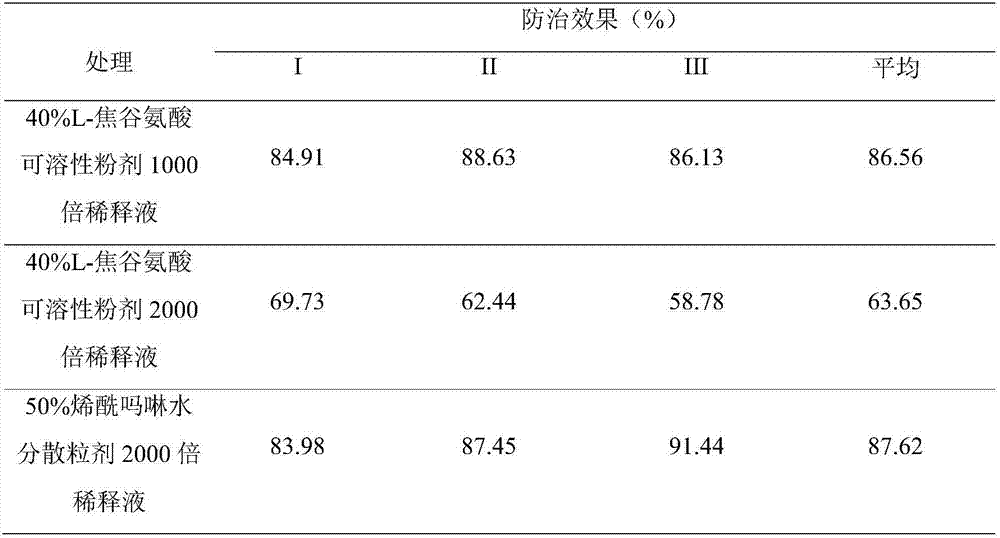
Agricultural amino acid composition
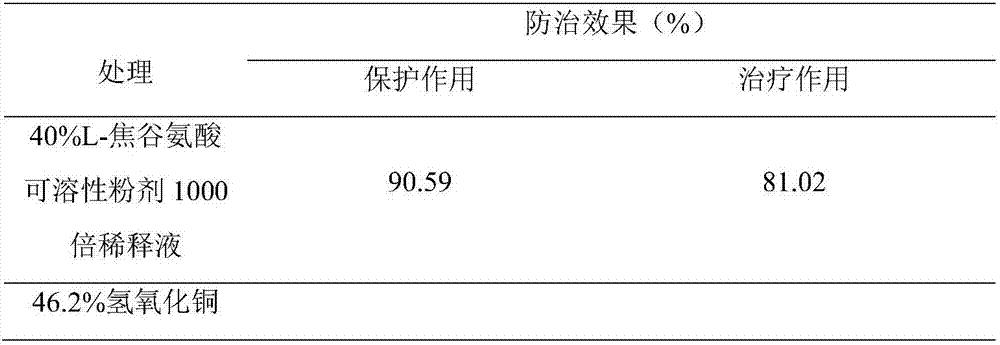
ActiveCN107467029AAvoid negative effectsAvoid compoundingBiocidePlant growth regulatorsActive componentL-Pyroglutamic Acid
Owner:NORTHWEST A & F UNIV
Preparation method of D-p-hydroxyphenylglycine methyl ester
PendingCN111153821AGuaranteed yieldGuaranteed quality indicatorsOrganic compound preparationOrganic chemistry methodsMethanolEster sulfate
Owner:SHANXI WEIQIDA PHARMA IND
Method for manufacturing high-purity sorbitol syrups from sucrose and uses thereof
ActiveUS20130225874A1Oxygen-containing compound preparationPreparation by isomerisationFructoseSucrose
Owner:ROQUETTE FRERES SA
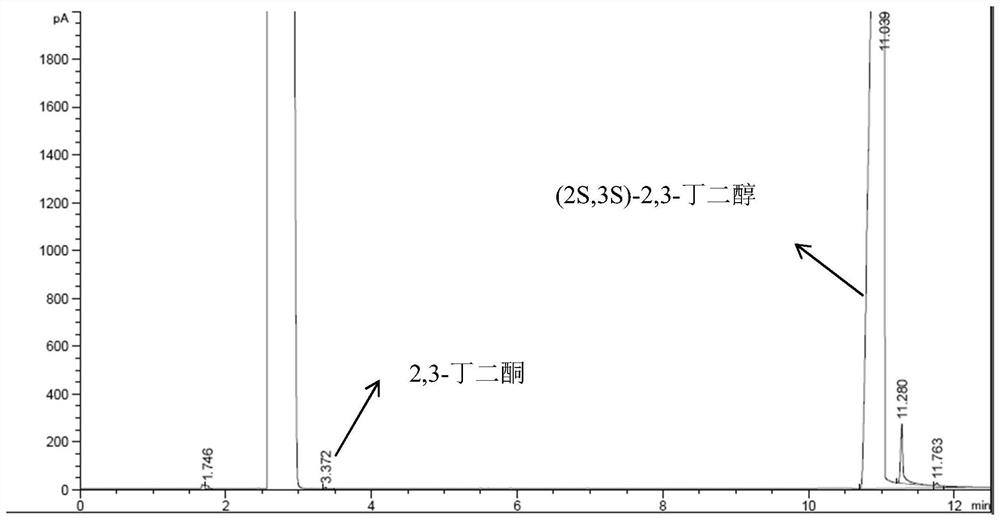
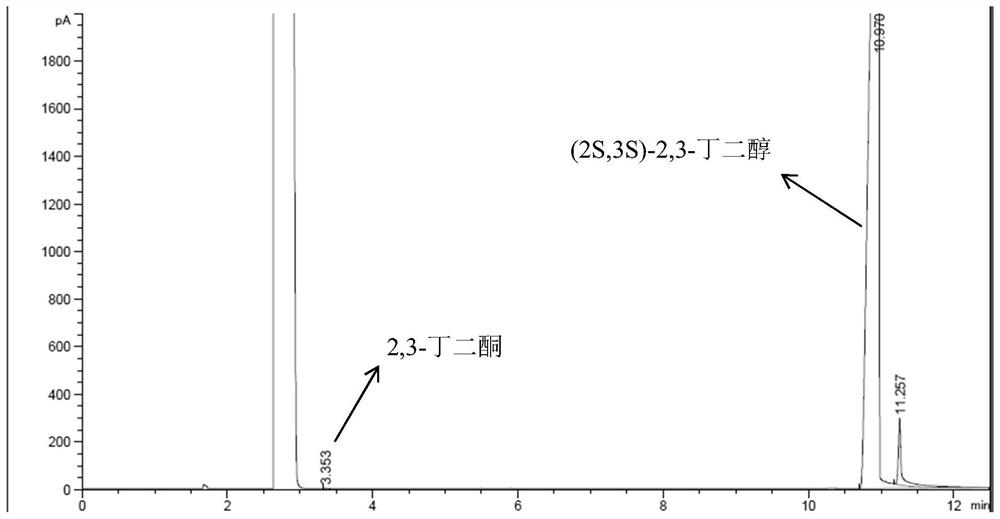
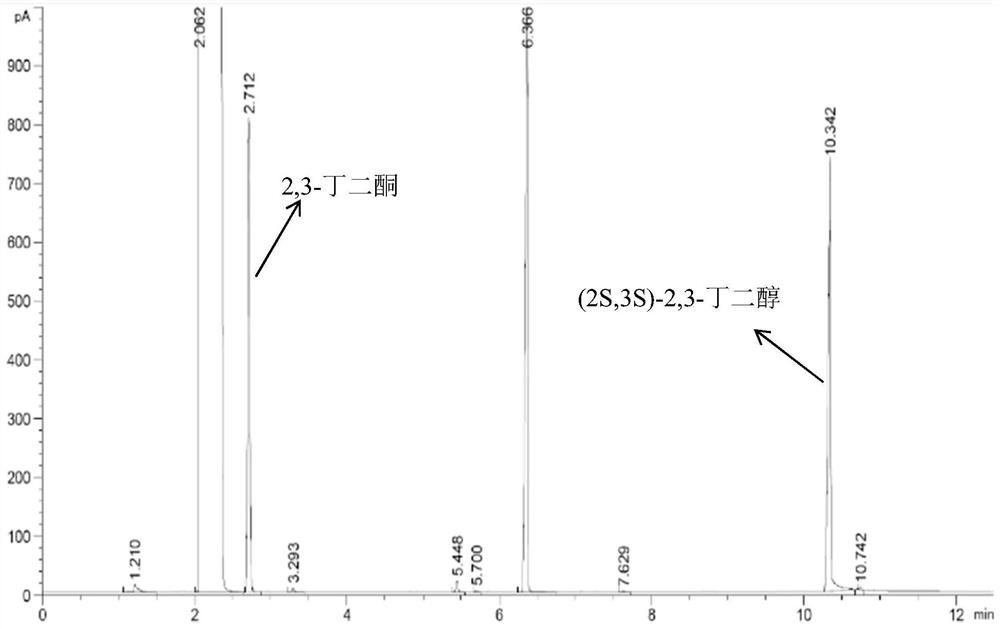
Preparation method of (2S,3S)-2,3-butanediol
Owner:ENZYMASTER NINGBO BIO ENG CO LTD
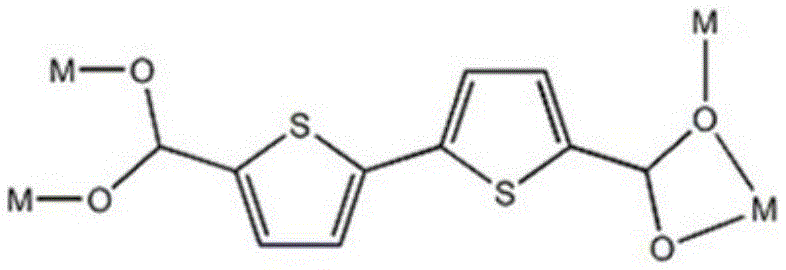
Metal organic complex containing thiophene functional group and preparing method and application of metal organic complex
InactiveCN105541883AOrganic chemistry methodsFluorescence/phosphorescenceDimethyl formamideBulk crystal
Owner:LIAONING UNIVERSITY
Salt of 5-fluorouracil and metformin as well as preparation method and crystal structure of salt
Owner:OCEAN UNIV OF CHINA
Heterocyclic spiro compounds and methods of use thereof for the treatment of cancer
Owner:ARAXES PHARMA LLC
Crystal form E and crystal form F of canagliflozin and preparation method thereof
ActiveCN104974146AGood physical and chemical stabilityFine and evenly dispersedOrganic chemistry methodsSolubilityDissolution
Owner:CRYSTAL PHARMATECH CO LTD
Crystalline state Lesinurad intermediate and preparation method thereof
The invention provides a crystalline state Lesinurad intermediate and a preparation method thereof. The invention discloses a crystalline state Lesinurad intermediate (II) and a preparation method thereof. The result of differential scanning calorimetry on the crystalline state Lesinurad intermediate (II) shows that peaks appear in a range of 98 to 102 DEG C. The result of powder X-ray diffraction shows that characteristic peaks appear at (2[theta]): 8.8+ / -0.2 degrees, 10.5+ / -0.2 degrees, 10.6+ / -0.2 degrees, 15.6+ / -0.2 degrees, 22.1+ / -0.2 degrees, and 22.9+ / -0.2 degrees. The solid of crystalline state Lesinurad intermediate (II) is obtained for the first time, the purity is high, the operation of the crystallization method is simple, column chromatography purification is not needed, the method is suitable for industrial production; the obtained crystalline state solid has stable properties, thus storage, transportation, and feeding operation become convenient, and the purity of final product Lesinurad prepared from the intermediate (II) is high.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Tadalafil crystallization method
Owner:WATERSTONE PHARMA WUHAN
Porphyrin covalently linked molybdenum disulfide nonlinear nano hybrid material as well as preparation and application of porphyrin covalently linked molybdenum disulfide nonlinear nano hybrid material
ActiveCN113735884ALarge control spaceEnhanced Saturated AbsorptionOrganic chemistry methodsGroup 2/12 organic compounds without C-metal linkagesNano hybridButyl lithium
The invention relates to a porphyrin covalently linked molybdenum disulfide nonlinear nano hybrid material as well as preparation and application of the porphyrin covalently linked molybdenum disulfide nonlinear nano hybrid material, zinc porphyrin is axially coordinated to the surface of layered molybdenum disulfide, and the nonlinear performance of an organic-inorganic hybrid material is enhanced through energy transmission between porphyrin and molybdenum disulfide. The preparation method comprises the following steps: stripping molybdenum disulfide into few layers through butyl lithium, covalently connecting p-aminopyridine to the surface of layered molybdenum disulfide through diazonium salt reaction, and connecting porphyrin with metal zinc coordinated at the center to the surface of p-aminopyridine modified molybdenum disulfide through axial coordination to obtain the porphyrin-molybdenum disulfide hybrid material. The covalent linkage enhances energy transmission between molybdenum disulfide and porphyrin, so that the hybrid material shows enhanced nonlinear optical performance under nanosecond 532 nm.
Owner:TONGJI UNIV
Novel crystal form of nilotinib
Owner:WEIHAI YUNRUI INFORMATION TECH CO LTD
Chemical compound, crystal form, and preparation methods and applications of chemical compound and crystal form
Owner:CHENGDU FIRST PHARMACEDTICAL CO LTD
Method for separating all-trans fatty acid with antitumor activity from antarctic krill oil
ActiveCN106117044AStrong lethalityOrganic active ingredientsFatty acids production/refiningAdditive ingredientStructural composition
Owner:DALIAN UNIV OF TECH
Crystallization method for preparing high-purity monodisperse I crystal form atorvastatin calcium in single kettle
The invention relates to a crystallization method for preparing high-purity monodisperse I crystal form atorvastatin calcium in a single kettle. The method is as follows: adding a mixed solvent consisting of a good solvent and an antisolvent of atorvastatin calcium into a crystallizer, adding crystalline powder of crystal form I atorvastatin calcium into the crystallizer, keeping the temperature of the solution at normal temperature, dispersing crystal particles by using ultrasound, and then raising the temperature to the temperature of the dissolution process; adding two solutions of the anti-solvent and the good solvent for dissolving the atorvastatin calcium into seed crystal suspension at the same time, and keeping the solvent composition in the seed crystal suspension in the process basically unchanged; adding the antisolvent into the crystallizer continuously, and then keeping temperature and suspending; reducing the temperature of the solution to normal temperature, and then filtering the solution, washing by pure water and drying to obtain crystal the form I crystalline atorvastatin calcium powder. The crystal particles of the powder are long rod-shaped, and the longitudinal dimension of the powder is not more than 30mu m, and the cross-sectional dimension is not more than 5mu m. The production cycle is short, the cost of the solvent is low, the operation is simple, andthe method is suitable for industrial production.
Owner:TIANJIN UNIV +1
Zuclomiphene Salts and Crystalline Forms Thereof
ActiveUS20210147338A1High purityHigh yieldOrganic compound preparationOrganic chemistry methodsZuclomipheneHydrogen phosphate
Owner:APOTEX INC
Quinolizine aza-bis-aromatic ring axial chiral compound and synthesis method thereof
ActiveCN113493454AImprove compatibilityMild reaction conditionsOrganic chemistry methodsMethylpyridiniumIsopropyl
Owner:NANJING UNIV OF SCI & TECH
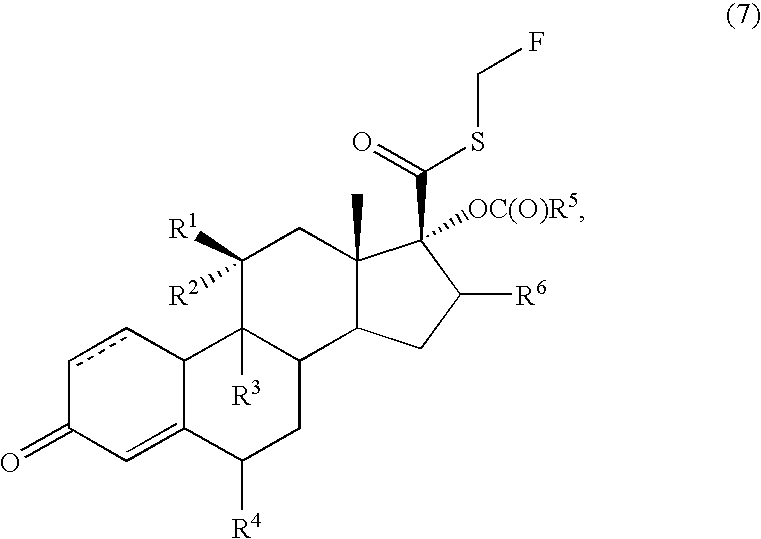
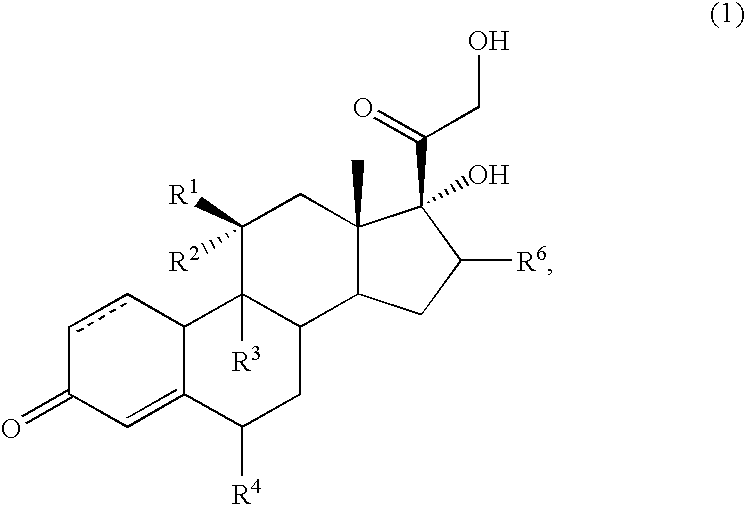
Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods
InactiveUS7214807B2Large scaleOrganic active ingredientsOrganic chemistry methodsAndrostaneAndrostanes
Owner:ABBVIE INC
Cefmetazole crystal-form compound and preparation method thereof
ActiveCN105541871AImprove quality stabilityGood physical propertiesOrganic active ingredientsAntibacterial agentsState of artCefmetazole
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Stable isotope labeled sulfadoxine and synthesis method thereof
InactiveCN110003120AAtom utilization is highSimple processOrganic chemistry methodsStable Isotope LabelingSynthesis methods
The invention discloses stable isotope labeled sulfadoxine and a synthesis method thereof. The synthesis method comprises following steps: S1, sulfanilamide and sodium hydroxide are subjected to a reaction, and sulfanilamide sodium salt is prepared; S2, sulfanilamide sodium salt and 4,6-dichloro-5-methoxypyrimidine are subjected to a condensation reaction, and 4-sulfanilamide-5-methoxy-6-chloropyrimidine is prepared; S3, 4-sulfanilamide-5-methoxy-6-chloropyrimidine and stable isotope labeled methanol are subjected to an etherification reaction under the alkaline condition, and stable isotope labeled sulfadoxine is prepared. The synthesized stable isotope is high in atom utilization rate, synthesis steps are simple, and a product is easy to separate and purify and meets the requirement of astandard reagent for quantitatively detecting sulfadoxine; stable isotope labeled sulfadoxine has high use value and good economic efficiency.
Owner:SHANGHAI ANPEL SCI INSTR
Synthesis method of guanine-based pearlescent pigment
ActiveCN111039943AImprove stabilityGood solubility resistanceOrganic chemistry methodsPhysical chemistryPotassium hydroxide
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Purification of Cannabinoids From Crude Cannabis Oil
Owner:TABA IP LLC
Novel process for preparation of empagliflozin or its co-crystals, solvates and their polymorphs thereof
Owner:LAURUS LABS
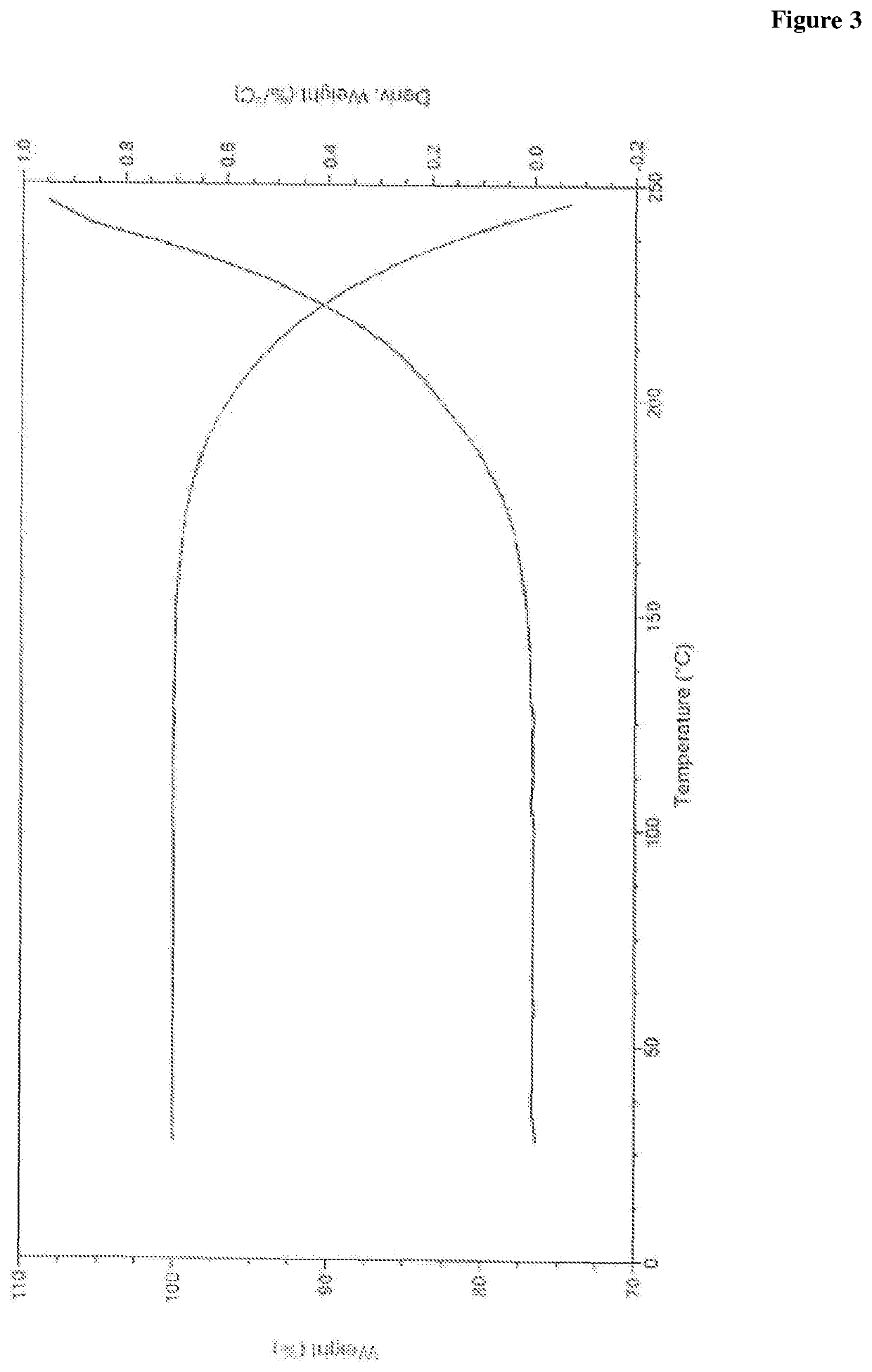
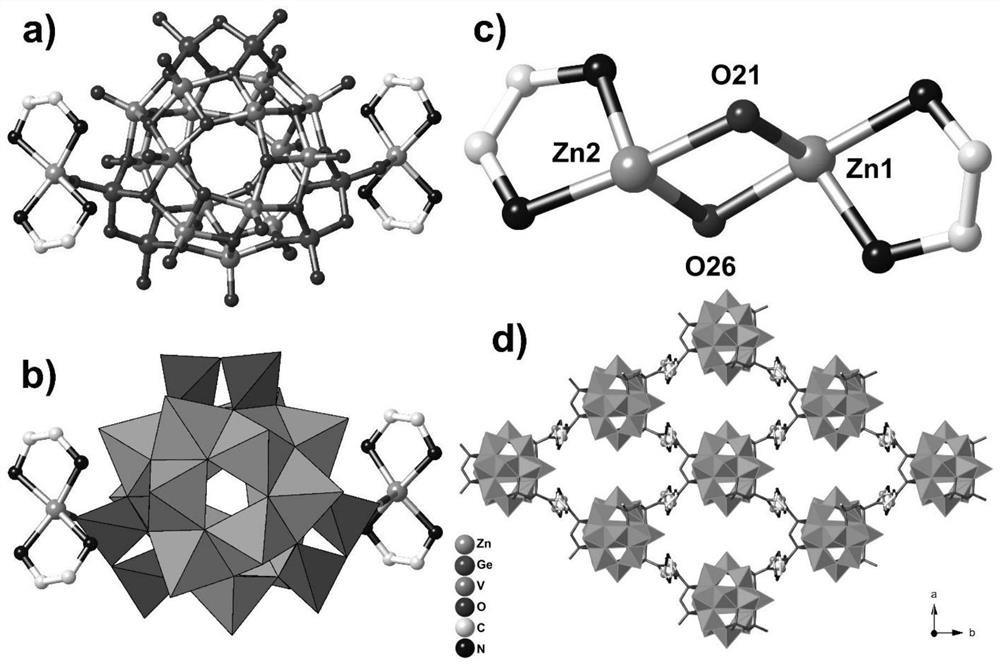
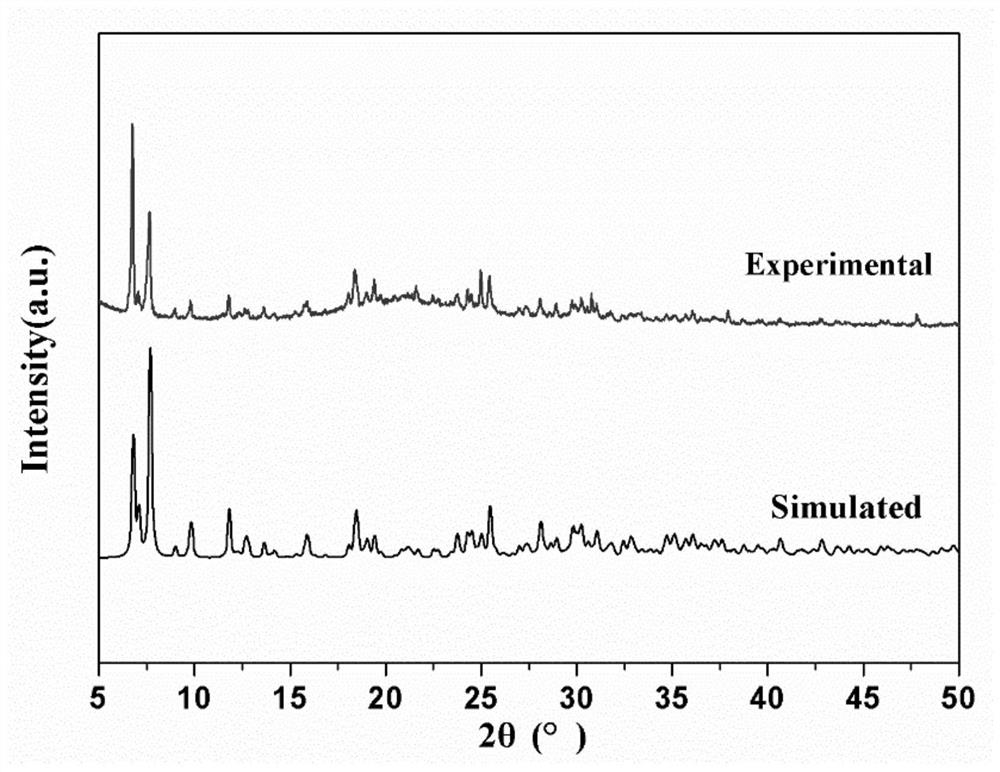
Two-dimensional layered germanium-vanadium-oxygen cluster compound as well as synthesis method and application thereof
ActiveCN113402567AHas magnetic application valueImprove bindingOrganic chemistry methodsGroup 5/15 organic compounds without C-metal linkagesEthylenediaminesTerephthalic acid
Owner:NINGDE NORMAL UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap