Crystal form E and crystal form F of canagliflozin and preparation method thereof
A crystal form and crystallization technology, applied in the field of hypoglycemic drugs, crystal form F and its preparation, and crystal form E of canagliflozin, which can solve the problem of poor crystallinity, poor fluidity and unstable baseline of crystal form C and other problems, to achieve good physical and chemical stability, improve drug solubility and dissolution rate, and control the process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1: Preparation of the crystal form E of canagliflozin
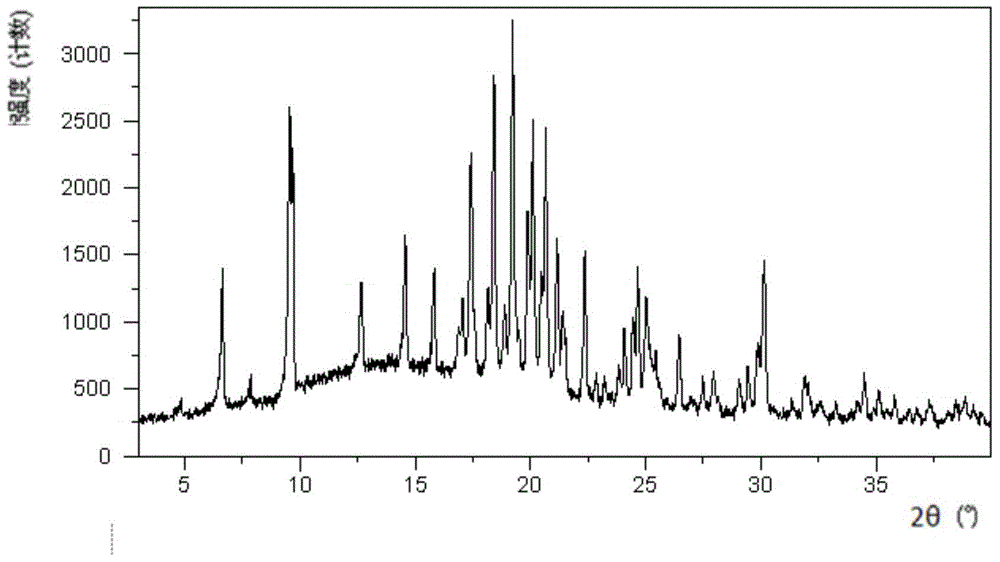
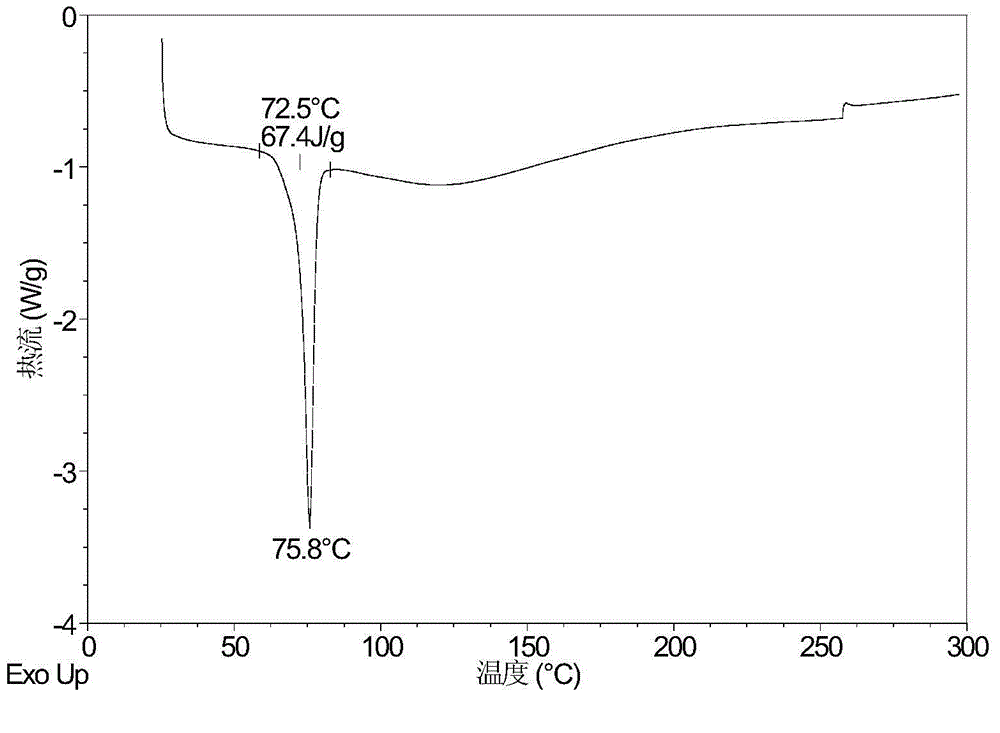
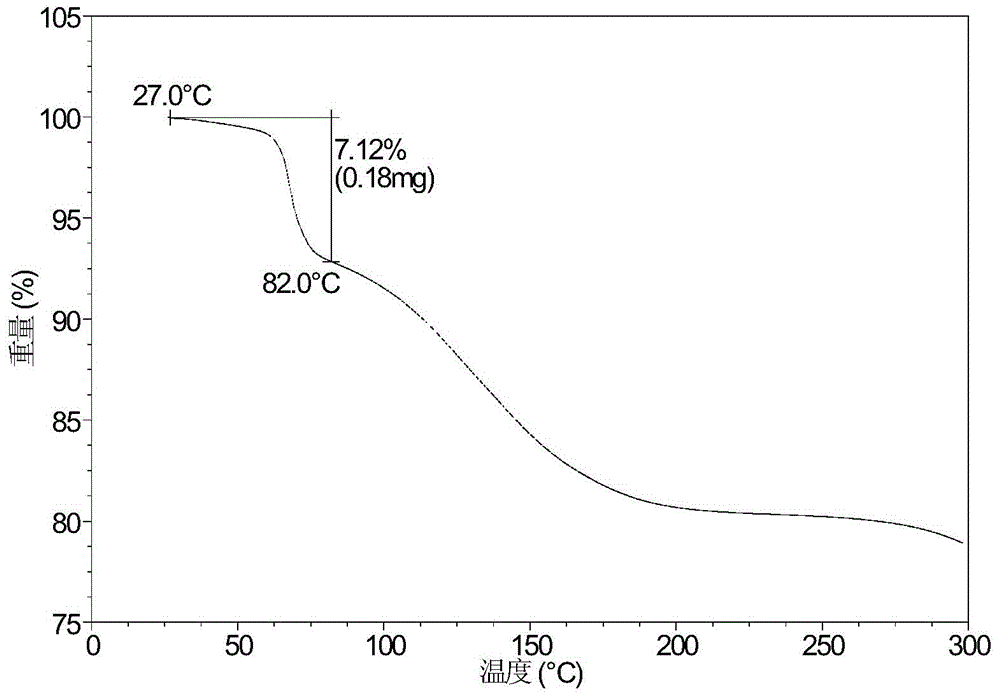
[0090] Dissolve 499.5 mg of canagliflozin powder in 13.25 mL of acetic acid: n-heptane = 1:3 (v:v) mixed system, and then quickly place it at -20°C and stir for 20 hours. The obtained solid is crystal Type E. Its XRPD pattern is shown in figure 1 , whose DSC diagram is shown in figure 2 , whose TGA diagram is shown in image 3 .
[0091] The corresponding values of 2theta value and intensity of crystal form E in this embodiment are shown in Table 1:
[0092] Table 1 Corresponding value of 2theta value and intensity of crystal form E
[0093] 2theta
[0094] 38.89
Embodiment 2
[0095] Embodiment 2: Preparation of crystal form E of canagliflozin
[0096] Dissolve 19.6 mg of canagliflozin powder in 0.20 mL of acetic acid: n-heptane = 1:1 (v:v) mixed solvent, then quickly place it at -20°C and stir for 20 hours, and the obtained solid is crystal Type E.
[0097] The corresponding values of 2theta value and relative intensity of crystal form E in this embodiment are shown in Table 2:
[0098] Table 2 Corresponding value of 2theta value and relative intensity of crystal form E
[0099] 2theta
Embodiment 3
[0100] Embodiment 3: Preparation of crystal form E of canagliflozin
[0101] Dissolve 21.1 mg of canagliflozin powder in 0.40 mL of acetic acid solvent, then add 0.50 mL of n-heptane and stir at room temperature for 5 days, and the obtained solid is Form E.
[0102] The 2theta value and intensity corresponding value of crystal form E in this embodiment are shown in Table 3:
[0103] Table 3 Corresponding values of 2theta value and intensity of crystal form E
[0104] 2theta
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap