High-purity sodium p-styrenesulfonate with excellent hue, method for producing the same, poly (sodium p-styrenesulfonate) with excellent hue using the same, and dispersant and synthetic starch for clothing finishing using the poly ( sodium p-styrenesulfonate)
a sodium pstyrenesulfonate, high-purity technology, applied in the direction of organic chemistry, chemistry apparatus and processes, fibre treatment, etc., can solve the problems of not being satisfactory, styrenesulfonate having a median diameter of less than 25 m, handling properties, etc., to improve the fluidity, improve the hue, and excellent hue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of High-Purity Sodium p-Styrenesulfonate and Sodium PSS, and Evaluation Example 1 as Synthetic Starch for Clothing Ironing Agent
[0190]
[0191]In a polypropylene beaker, 600 ml of a cation exchange resin (manufactured by Organo Corporation, Amberlite RB120 (one regenerated with hydrochloric acid)) and 400 g of a 73 wt % aqueous p-β-bromoethylbenzenesulfonic acid solution were collected, and slowly stirred using a Teflon (tetrafluoroethylene) stirring blade at ordinary temperature for 5 hours. Thereafter, the ion exchange resin was separated by filtration through a glass filter, and the concentration of a filtrate was adjusted with a rotary evaporator to obtain 350 g of a 70 wt % aqueous p-β-bromoethylbenzenesulfonic acid solution. The iron content in the aqueous solution was 0.34 μg / g.
[0192]Incidentally, an intermediate product obtained during a production process of sodium p-styrenesulfonate was used as p-β-bromoethylbenzenesulfonic acid.
[0193]A stainless steel reactor havin
example 2
Production of High-Purity Sodium p-Styrenesulfonate and Sodium. PSS, and Evaluation Example 2 as Synthetic Starch for Clothing Ironing Agent
[0201]
[0202]A stainless steel reactor having a jacket and equipped with a stirrer was charged with 1,000 g of high-purity sodium p-styrenesulfonate obtained in Example 1, 1 g of sodium nitrite, 20 g of sodium hydroxide and 950 g of pure water, followed by stirring at 60° C. for 1 hour under a nitrogen atmosphere. Then, after cooling to room temperature over 3 hours, solid-liquid separation was performed with a centrifuge to obtain 899 g of a wet cake of high-purity sodium p-styrenesulfonate.
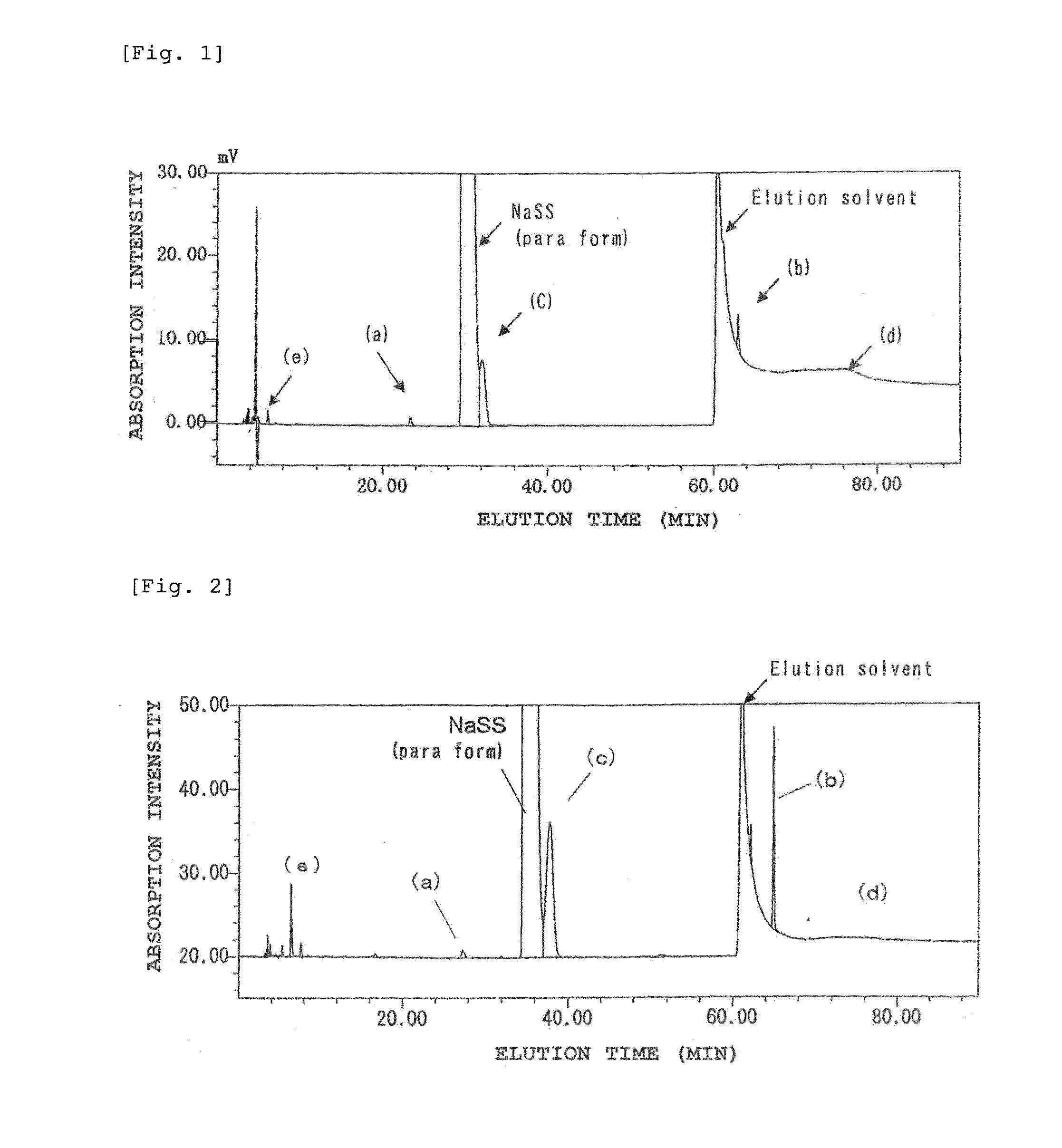
[0203]The above-mentioned high-purity sodium p-styrene-sulfonate had a purity of 89.1 wt %, a water content of 8.2 wt %, an iron content of 0.58 μg / g and a sodium bromide content of 0.20 wt %. The contents of organic impurities such as isomers were (a) 0.05%, (b) 0.00%, (c) 1.34%, (d) 0.01% and (e) 0.01%.
[0204]The above-mentioned sodium p-styrenesulfonate had
example 3
Production of Sodium PSS and Chloroprene Rubber and Evaluation Example 1
[0210]
[0211]A 1-liter glass flask equipped with a reflux condenser tube, a nitrogen introducing pipe and a paddle type stirrer was charged with 84.00 g of pure water, followed by heating in an oil bath at 85° C. under a nitrogen atmosphere. An aqueous sodium p-styrenesulfonate solution (in which 193.00 g of sodium p-styrenesulfonate obtained in Example 1 and 8.56 g of thioglycerol were dissolved in 700.00 g of pure water) separately prepared was added dropwise thereto over 73 minutes, and an aqueous initiator solution (in which 5.10 g of 2,2′-azobis(2-amidinopropane) dihydrochloride was dissolved in 104.00 g of pure water) was added dropwise thereto over 130 minutes to perform polymerization. After 3 hours from the initiation of polymerization, the oil bath temperature was increased to 90° C., and polymerization was further continued for 3 hours to obtain an aqueous poly(sodium p-styrenesulfonate) solution.
[0212]Th
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap