Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46results about How to "Mild reaction conditions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of submicron CuS (copper sulphide) classification ball
The invention discloses a preparation method of submicron CuS (copper sulphide) classification balls. The method comprises the following steps: adding polymer into a good solvent to dissolve and remove big gel particles; adding copper source solution to the good solvent and stirring; adding sulfur source solution and then stirring; reacting the reaction liquid under 100-1,000 KPa at 100-200 DEG C; naturally cooling to the room temperature to obtain black precipitate; and washing and drying the precipitate to obtain the classification balls. The classification balls has the advantages of cheap and readily available templates, environmental friendliness, safety without toxicity, renewability and high water solubility, the contents of raw materials are abundant in nature and the operation of the reaction system is simple; the size and structure of the prepared classification ball are adjustable: the diameter can be controlled by adjusting the molar weight of the added precursor, the template concentration, the reaction temperature and time and the like; the operation is simple; and the prepared classification balls have wide application value in the fields of catalyst, catalyst carrier, optical equipment, sensor, lithium-ion rechargeable battery cathode material, superconductor and the like.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI +1
Heptatridecafluorooctylpropyl polyhedral oligomeric silsesquioxane and functionalized derivates thereof
The invention provides a preparation method for heptatridecafluorooctylpropyl polyhedral oligomeric silsesquioxane and functionalized derivates thereof. The preparation method comprises the steps as follows: adding tridecafluorooctylpropyl trimethoxy silane into an organic solvent, adding de-ionized water and NaOH, heating, stirring, reacting under reflux condition, washing by a washing solvent, and drying to obtain trisilanol sodium salt of heptatridecafluorooctylpropyl polyhedral oligomeric silsesquioxane; and adding trisilanol sodium salt into an organic reagent, dropwise adding hydrochloric acid, triethylamine and a silane coupling agent, stirring a mixture at normal temperature for reaction, removing generated deposit, carrying out rotary evaporation, removing the solvent, obtaining white crystals, dissolving the crystals in methanol, filtering for collecting insoluble parts, and carrying out vacuum drying to obtain a T8-type monofunctional tridecafluorooctylpropyl POSS (polyhedral oligomeric silsesquioxane) monomer. The preparation method can obtain long branch chain type active fluorine-containing POSS, is simple and easy in process, low in cost, high in yield and higher in product purity, and is suitable for large-scale industrial production.
Owner:HOHAI UNIV
Method for catalysis synthesis of glycerol formal by gemini dication acidic ion liquid
InactiveCN101962377AHigh catalytic activityReduce dosageOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsReaction temperatureControllability
The invention discloses a method for catalysis synthesis of glycerol formal by gemini dication acidic ion liquid. In the method, gemini dication acidic ion liquid is used as catalyst, glycerol and formaldehyde solution are taken as reactant, cyclohexane is taken as water-carrying agent, and the raw material is subject to catalysis synthesis at the temperature of 40-120 DEG C to obtain glycerol formal. The method has moderate reaction condition, low catalyst corrosivity, high catalytic activity, high product selectivity, simple operation process and strong controllability and can be reused.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of beta-amido-alpha-amino-acid ester derivative
InactiveCN105130834AThe synthesis process is simpleMild reaction conditionsOrganic compound preparationAmino-carboxyl compound preparationColumn chromatographyOrganosolv
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Hydrophobic graphene conductive material and preparation method of composite film of hydrophobic graphene conductive material
PendingCN114348995AGood hydrophobic and conductive propertiesMild reaction conditionsGrapheneCeramic materialsComposite membrane
Owner:厦门捌斗新材料科技有限公司
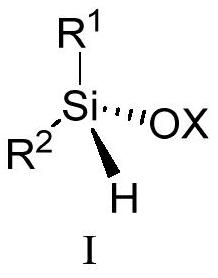
Silicon-center chiral silicon-oxygen compound and preparation method thereof
ActiveCN113150025AIncrease varietyGood areaSilicon organic compoundsBulk chemical productionOxygen compoundSilyl ether
The invention belongs to the field of chiral silicon synthesis, and discloses a silicon-center chiral silicon-oxygen compound. The compound has a structure represented by general formula I shown in the specification. In the formula I, X is Si(R<3>)n or a formula also shown in the specification, R<1> is selected from alkyl, cycloalkyl and aryl, R<2> is selected from alkyl, substituted phenyl and aryl, R<3> is selected from alkyl, phenyl and substituted phenyl, n is 3, the three R<3> are the same or different, R<4> is selected from hydrogen and (C1-C4) alkyl, m is selected from 0, 1, 2 and 3, and Y is selected from substituted phenyl, substituted pyrenyl, aryl, heteroaryl and cycloalkyl. The invention also discloses a preparation method of the compound. Various highly functionalized chiral siloxanes and silyl ethers are obtained with good chemical, regional and stereo control and high yield, the variety of silicon center chiral compounds is expanded, and the method has the advantages of high enantioselectivity, wide substrate application range, mild reaction conditions, atom economy and the like. In addition, the compound provided by the invention has a huge application prospect in chiral organic photoelectric materials.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Preparation method of nitrogen-doped TiO2 powder
ActiveCN107670681AThe synthesis process is simpleMild reaction conditionsWater/sewage treatment by irradiationWater treatment compoundsTetramethylammonium hydroxideCopper
Owner:LIAONING TECHNICAL UNIVERSITY
Device for preparing double-shell microcapsules
PendingCN108160014AEasy to manufactureEfficient manufacturingMicroballoon preparationMicrocapsule preparationSolubilityPeristaltic pump
A device for preparing double-shell microcapsules comprises a core material liquid inlet, a wall material liquid inlet, a vibrating system, a concentric nozzle, a collector, a stirrer, a peristaltic pump I, a second layer coating chamber, a rotatable screen, a coating liquid inlet, a peristaltic pump II, an atomizing nozzle, an air compressor, a temperature control system and the like. The deviceis simple; through control over the liquid inlet speeds of the core material liquid inlet and the wall material liquid inlet and the vibrating frequency of the vibrating system, the sizes of microcapsule cores are controlled, so that the microcapsules with uniform sizes are prepared and damage during microcapsule preparation is reduced; through hardening liquid filtration and hot air fluidization,rapid moulding of first-layer microcapsules is achieved; through control over the atomizing pressure and the coating liquid solubility, the shell thickness of second-layer coating is controlled. A preparation technology is simple, the reaction condition is mild, the control is easy, and the repeatability is relatively good; in addition, because of a sealing property of the device, influence of the environment on the microcapsules is greatly reduced, so that the double-shell microcapsules can be effectively prepared and the preparation time is greatly shortened.
Owner:NANCHANG UNIV
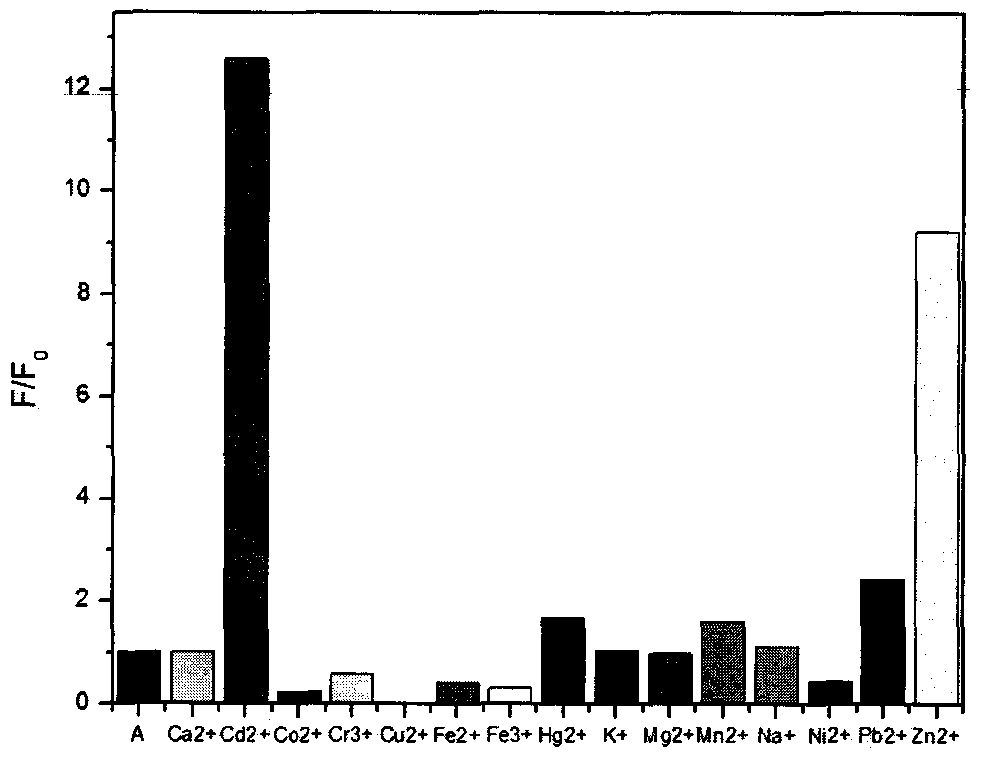
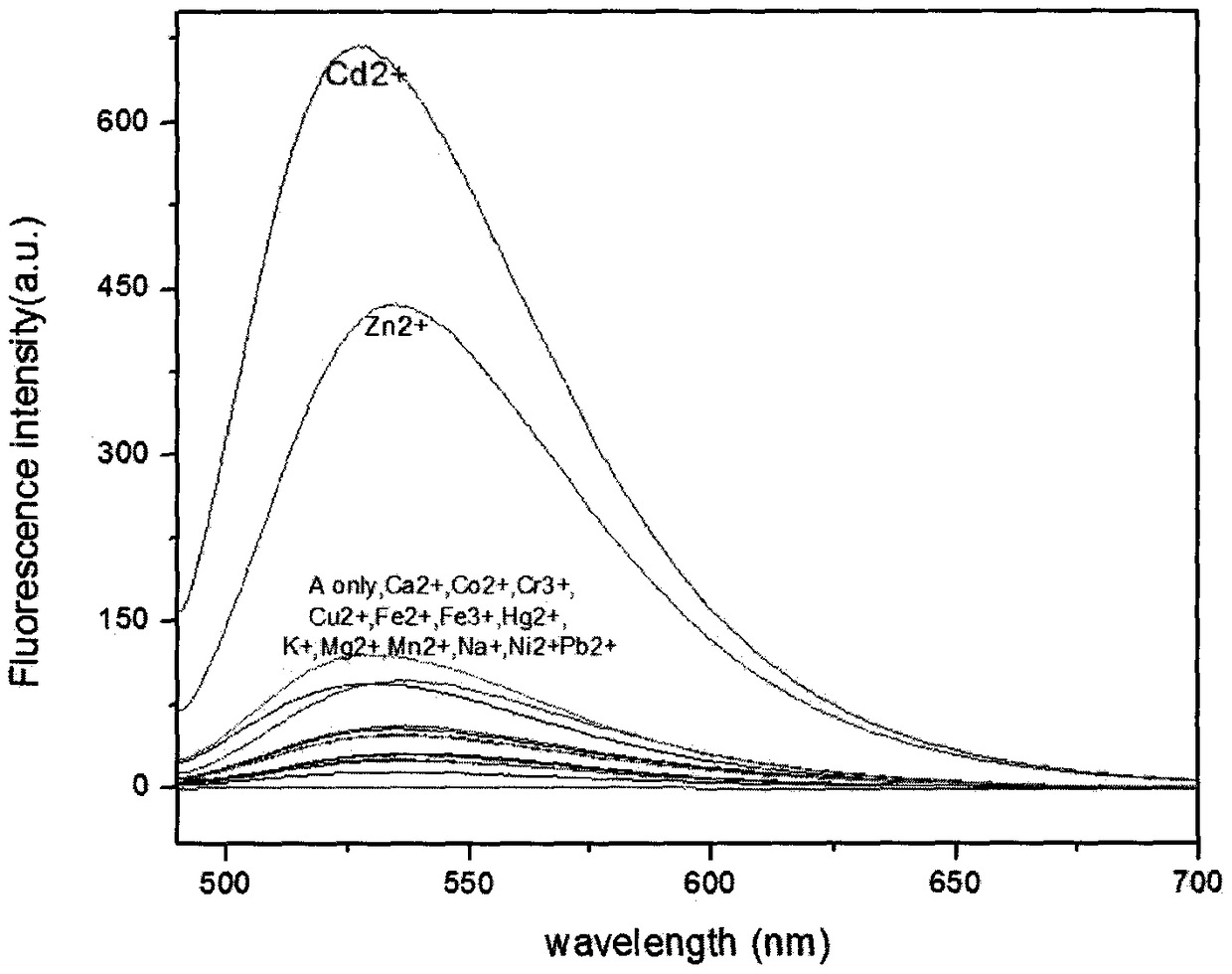
Cadmium-ion fluorescence probe based on pyrene exciplex, and preparation method and application thereof
ActiveCN106867516AEasy post-processingMild reaction conditionsFluorescence/phosphorescenceLuminescent compositions1-aminopyreneAcetonitrile
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of trans-cyclobutane o-dicarboxylic acid ester and derivative thereof
PendingCN110724058AMild reaction conditionsReduce process stepsCarboxylic acid esters preparationOrganic chemistry methodsBenzyl groupCombinatorial chemistry
Owner:JINLIN ASYMCHEM PHARM CO LTD
Phthalate type plasticizer hydrogenated catalyst preparation method thereof and application
InactiveCN106984310AEasy to prepareMild reaction conditionsMolecular sieve catalystsOrganic compound preparationSide productActivated carbon
Owner:CHANGZHOU UNIV
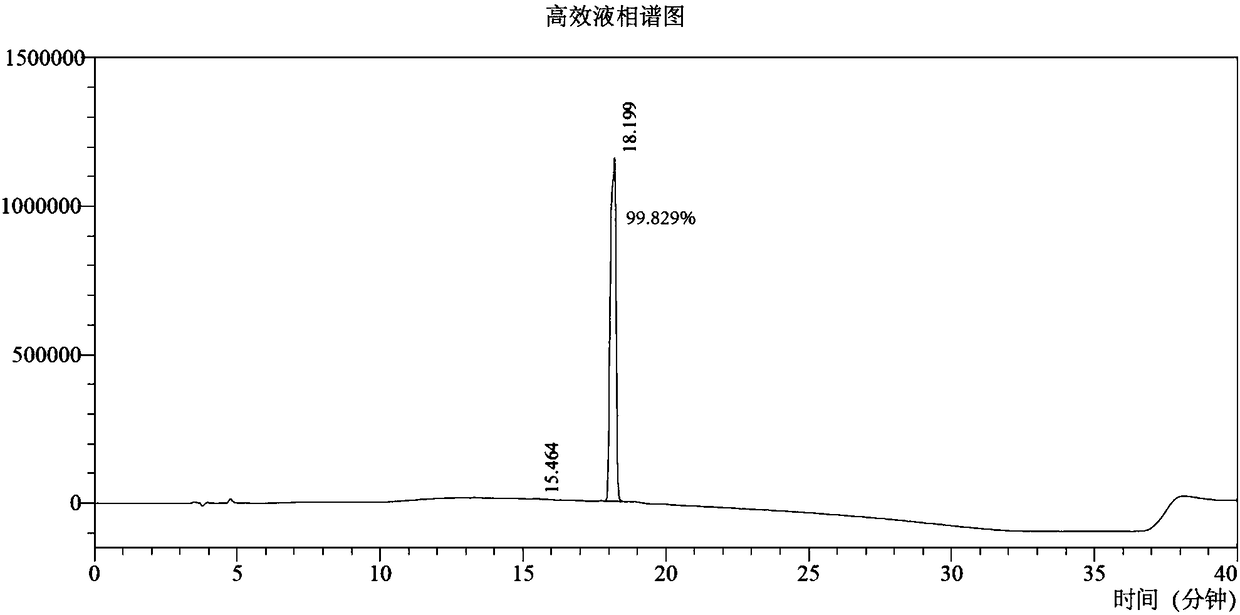
Amisulpride impurity and preparation method thereof
ActiveCN111269157AHigh yield and purityMild reaction conditionsOrganic chemistryOrganic compound preparationAmisulprideCombinatorial chemistry
Owner:NANJING HEALTHNICE MEDICAL TECH +2
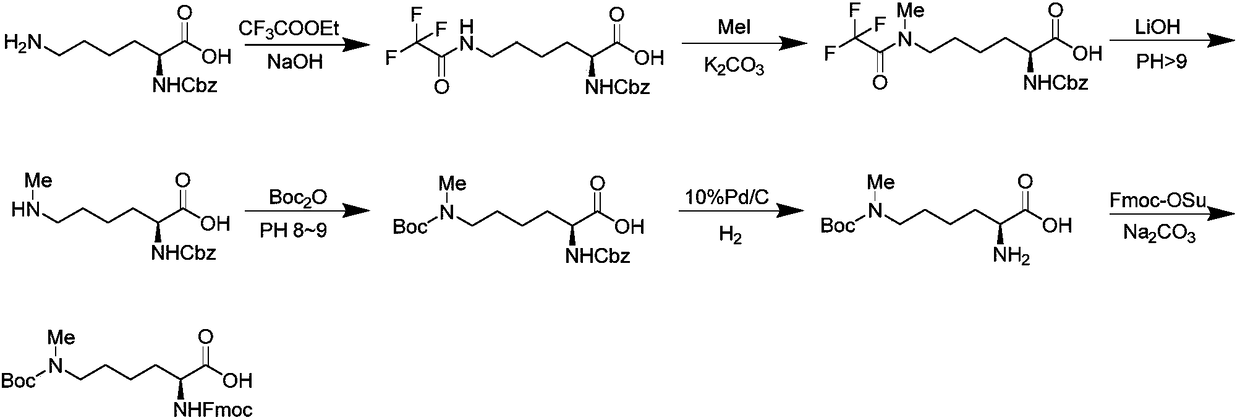
Preparation method of N epsilon-tert-butoxycarbonyl-N alpha-fluorenylmethoxycarbonyl-N epsilon-methyl-lysine
InactiveCN108383757AThe synthetic route is simpleMild reaction conditionsCarbamic acid derivatives preparationOrganic compound preparationRe crystallizationAlkyl transfer
Owner:JIANGSU GENSCRIPT BIOTECH CO LTD
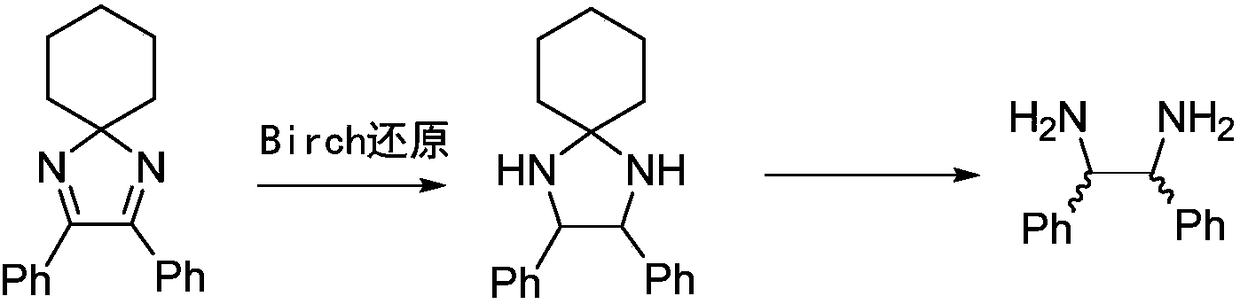
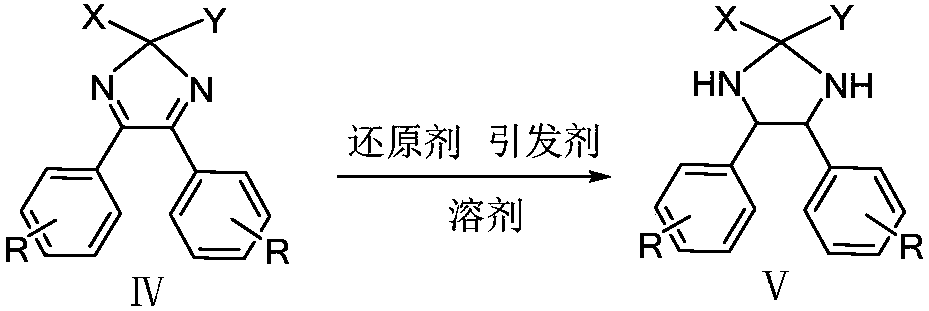
Method for preparing intermediate of chiral vicinal diamine
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI +1
Preparation method for 22E-alkene-3a,5-ring-5a-cholest-6-ketone
ActiveCN107474096AReduce manufacturing costWide variety of sourcesSteroidsOrganic synthesisFiltration
The invention belongs to the technical field of organic synthesis and relates to a preparation method for 22E-alkene-3a,5-ring-5a-cholest-6-ketone. The method comprises the following steps: placing 22E-alkene-3a,5-ring-5a-cholest-6-alcohol into an organic solvent, adding a catalyst under a certain temperature, performing oxidizing reaction under air or oxygen atmosphere and performing crystal suction filtration on the crude product, thereby obtaining an end product. According to the invention, any one of free radical catalyst, metal salt or free radical catalyst and metal salt is selected as the catalyst system of reaction; the 22E-alkene-3a,5-ring-5a-cholest-6-alcohol is directly oxidized into the 22E-alkene-3a,5-ring-5a-cholest-6-ketone under air or oxygen atmosphere; the yield thereof is above 90% and the content is above 98%; the raw materials are nontoxic or low-toxicity; the catalyst used in the reaction is low-cost and extensively sourced; the operation is safe, simple and convenient, the reaction condition is mild and the three wastes generated in the whole process are far less than those generated in the prior art; the production cost is low; no toxic three-waste emission exists; the total yield is high; the method is beneficial to industrialization.
Owner:JIANGSU QIANYUAN BIOTECHNOLOGY CO LTD
Low-silver-content fish scale gelatin-agar-Ag NPs composite film as well as preparation method and application thereof
PendingCN112430341AMild reaction conditionsEasy to operateAntifouling/underwater paintsPaints with biocidesChemistryBiology
Owner:JIMEI UNIV
Novel synthesis process of nitroimidazole pyran medicine for treating tuberculosis with wide drug resistance
PendingCN114249747AMild reaction conditionsOverall high yieldAntibacterial agentsOrganic chemistryAcetonitrilesBenzoyl bromide
Owner:苏州虞美景盛新药开发有限公司
Chiral phosphoric acid with 5,5'-bitetralone skeleton and preparation method thereof
InactiveCN105111228AHigh yieldMild reaction conditionsGroup 5/15 element organic compoundsSilicon organic compoundsChemical synthesisEnantio selectivity
Owner:NANJING UNIV
Process for full water phase synthesis of pharmaceutical intermediate dimethyl cyanoiminodithio-carbonate
Owner:SOUTHWEST UNIVERSITY
Deep desulfurization method for 380-780 nm visible light catalytic oxidation diesel oil
ActiveCN111471480AEasy to operateMild reaction conditionsHydrocarbon oils treatmentOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic oxidationDioxide titanium
Owner:YANTAI UNIV
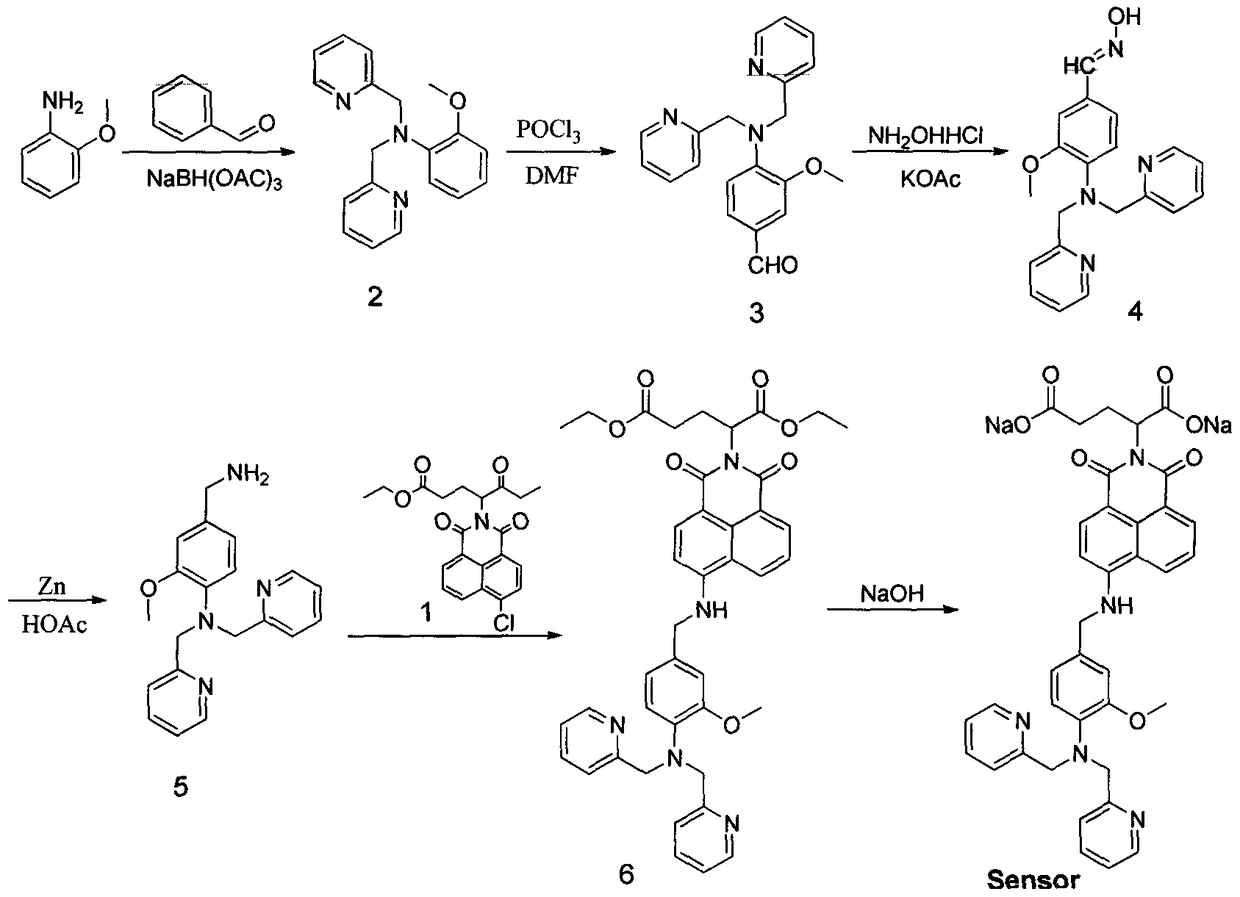
Organic compound for detecting content of metal ions in water environment and application of organic compound
InactiveCN108409719AImprove efficiencyMild reaction conditionsFluorescence/phosphorescenceGroup 3/13 element organic compoundsAnilineCadmium ion
Owner:TIANJIN AGRICULTURE COLLEGE
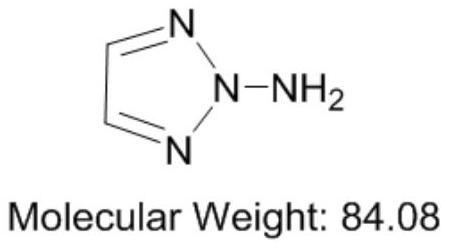
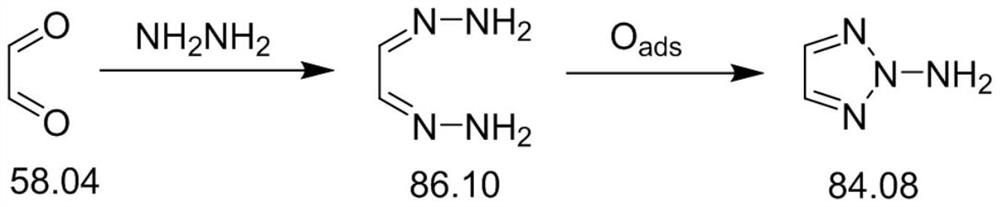
Synthesis method of 1-amino-1, 2, 3-triazole
PendingCN114525528AReduce pollutionEmission reductionElectrolysis componentsOrganic chemistryChemical energyTriazole
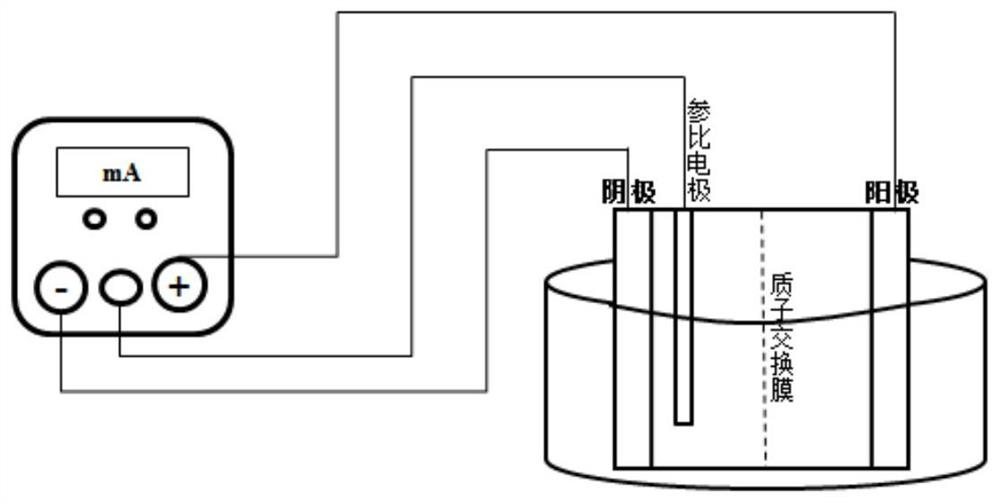
The invention discloses a synthesis method of 1-amino-1, 2, 3-triazole, which comprises the following steps: taking dihydrazone and a salt solvent as an electrolyte of an electrolytic anode, adding a metal oxide into the electrolyte as a catalyst, carrying out electrolytic reaction, and oxidizing dihydrazone into 1-amino-1, 2, 3-triazole. Active oxygen generated by anode electrolytic oxidation is used as an oxidizing agent, dihydrazone and a salt solvent with a certain concentration form an electrolyte of an electrolytic anode, a certain amount of transition metal oxide is added as a catalyst, electrolytic oxidation is performed under a certain current condition, electric energy is converted into chemical energy through electrolysis, and the oxidizing agent is generated; dihydrazone is oxidized into 1-amino-1, 2, 3-triazole, a target product can be well obtained through an electrolytic reaction, and meanwhile, danger and environmental pollution are not generated in the production process; according to the method, the 1-amino-1, 2, 3-triazole is prepared through the electrolytic reaction for the first time, and a new thought is provided for synthesis of the 1-amino-1, 2, 3-triazole.
Owner:CHENGDU ORGANOCHEM CO LTD
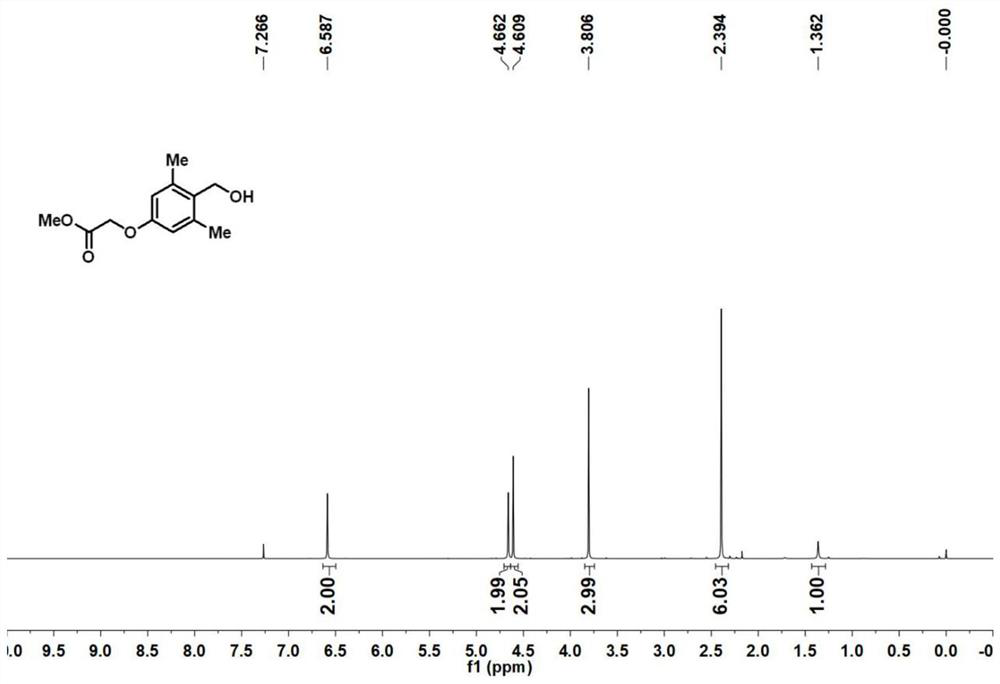
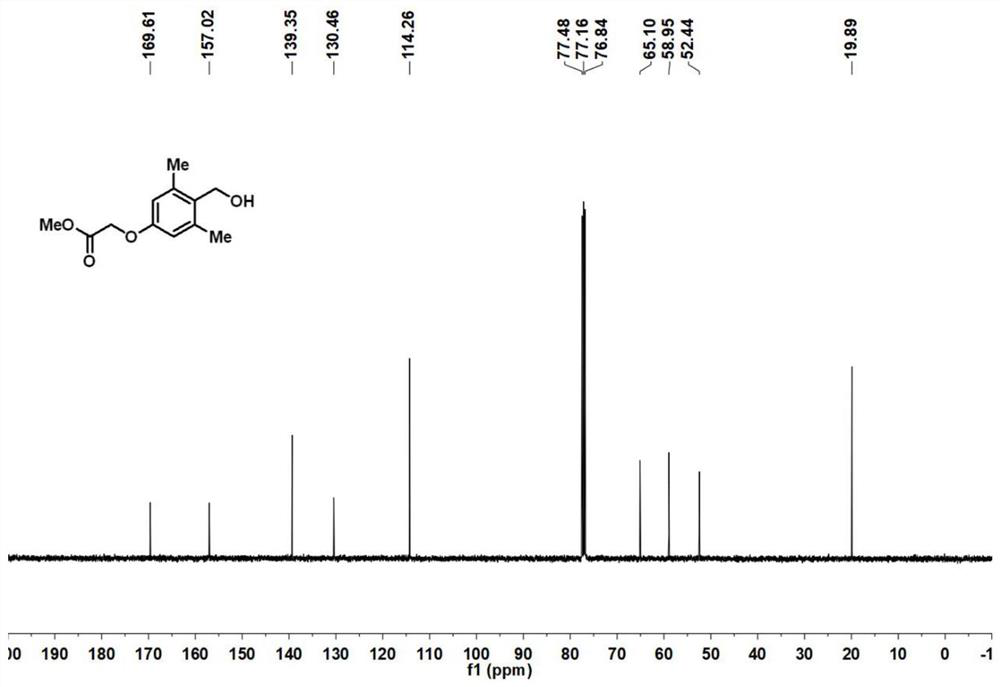
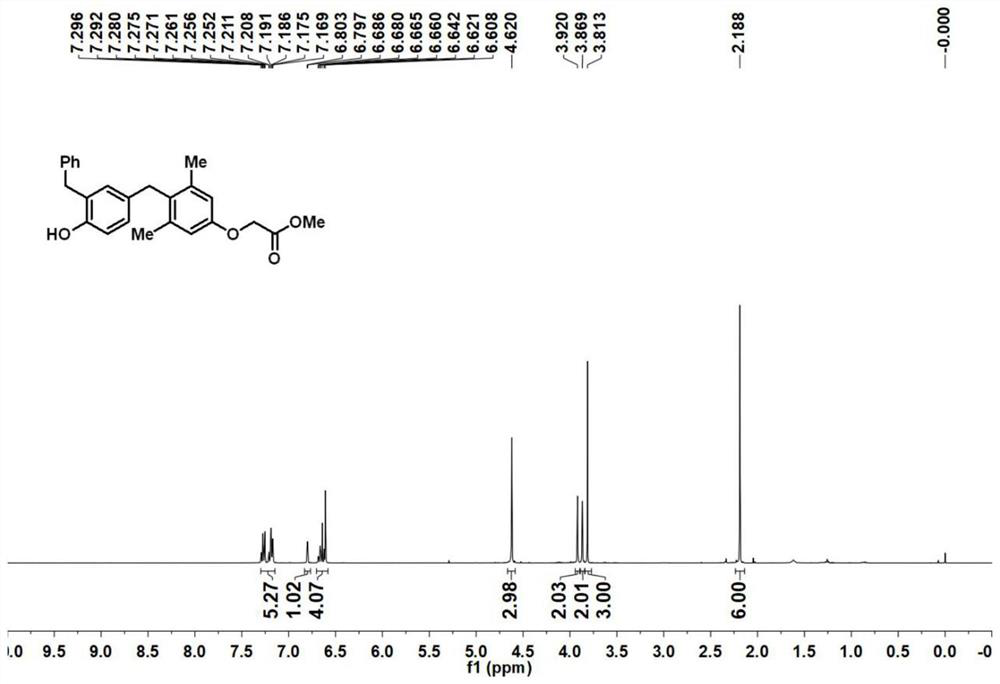
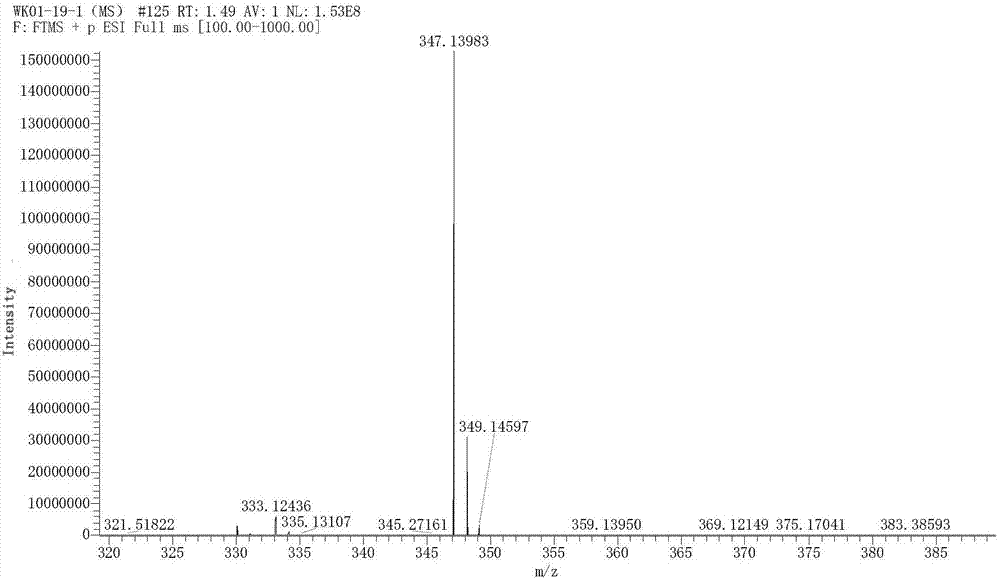
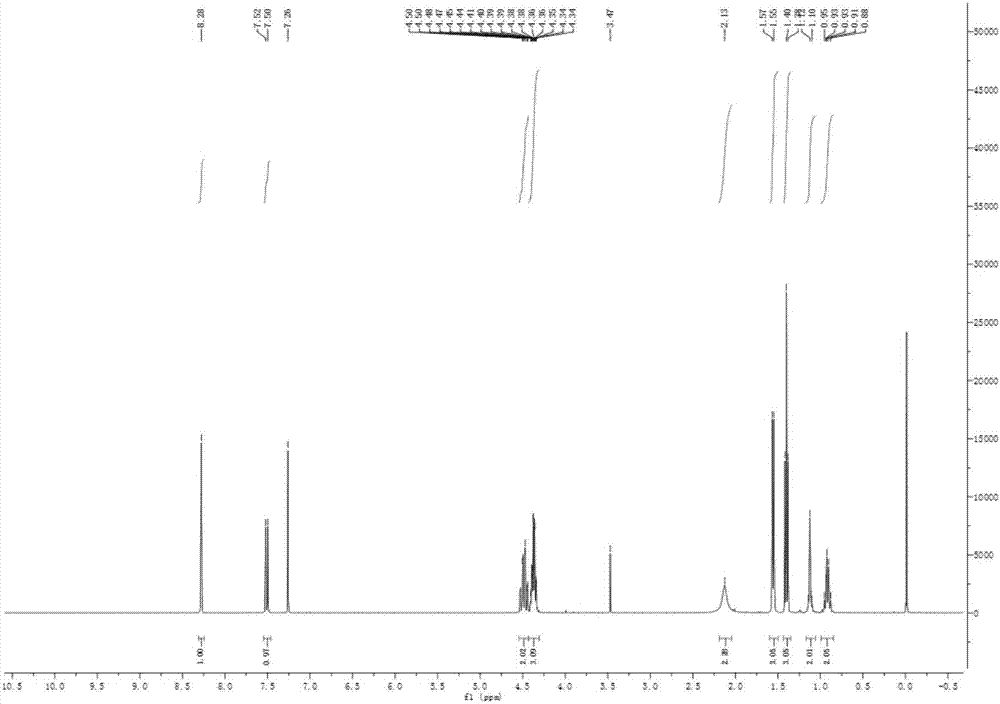
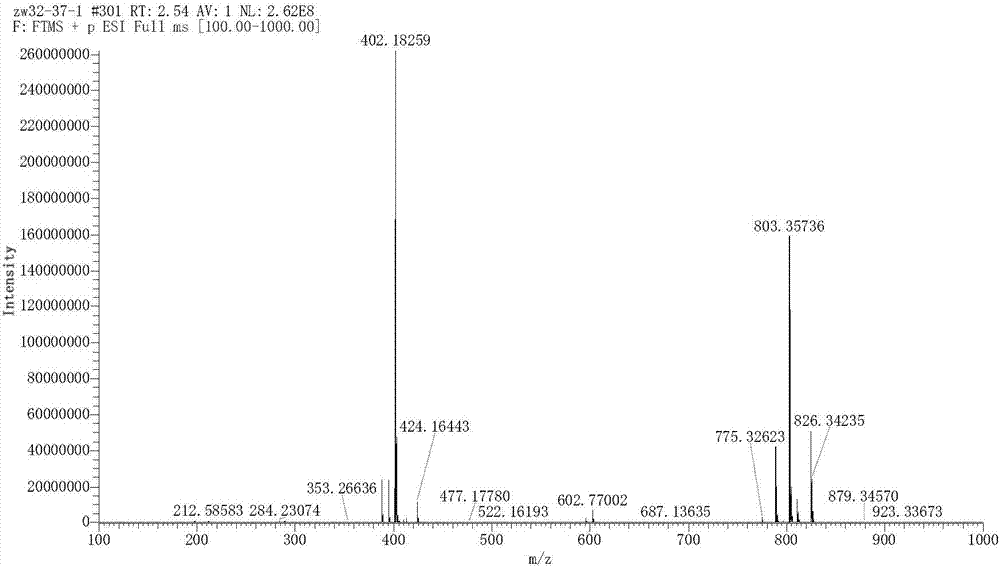
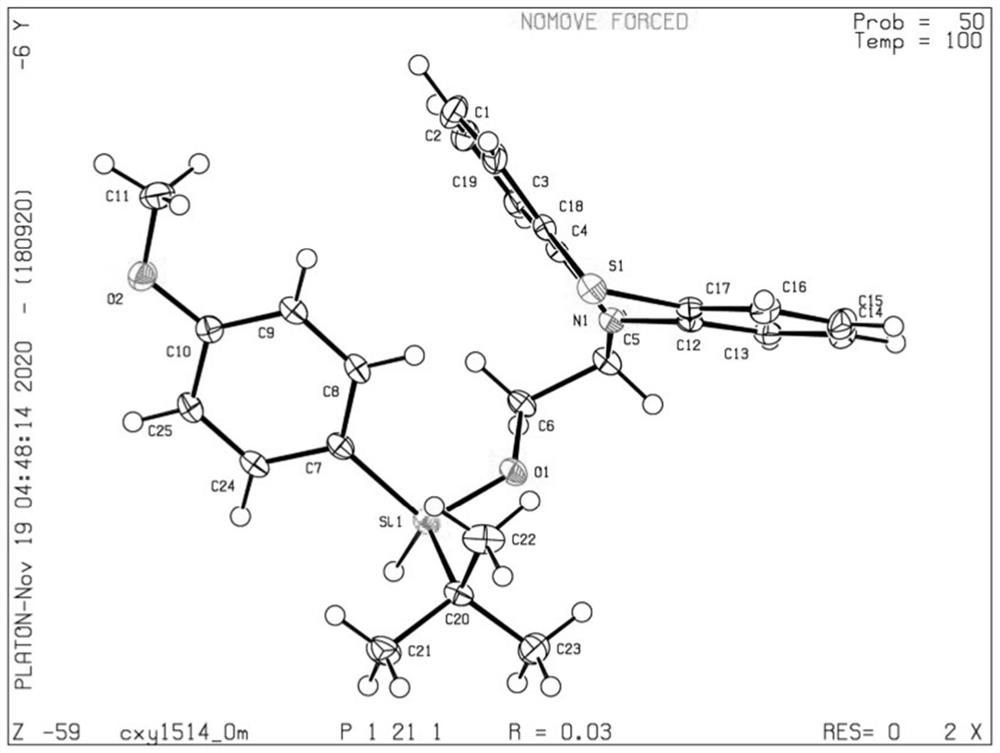
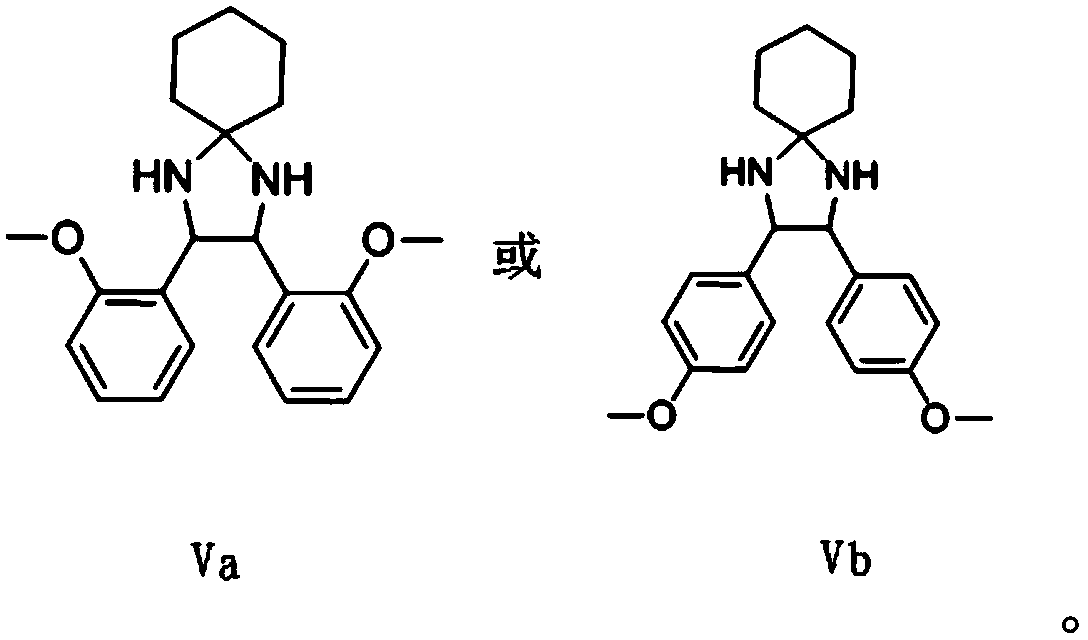
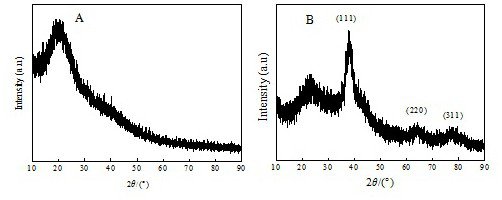
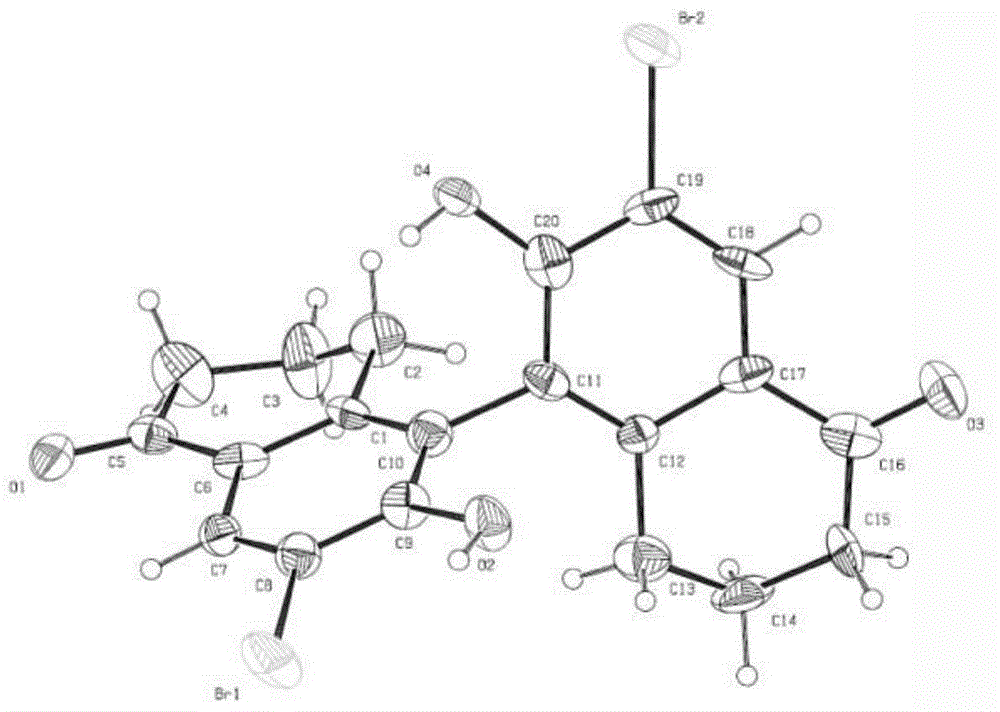
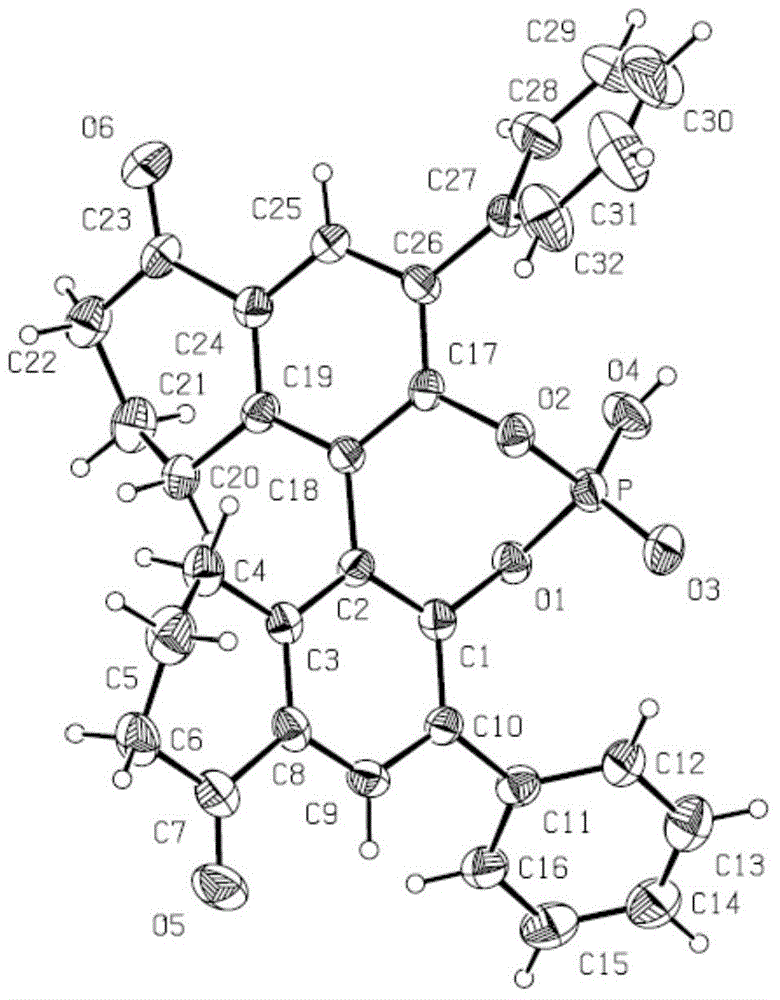
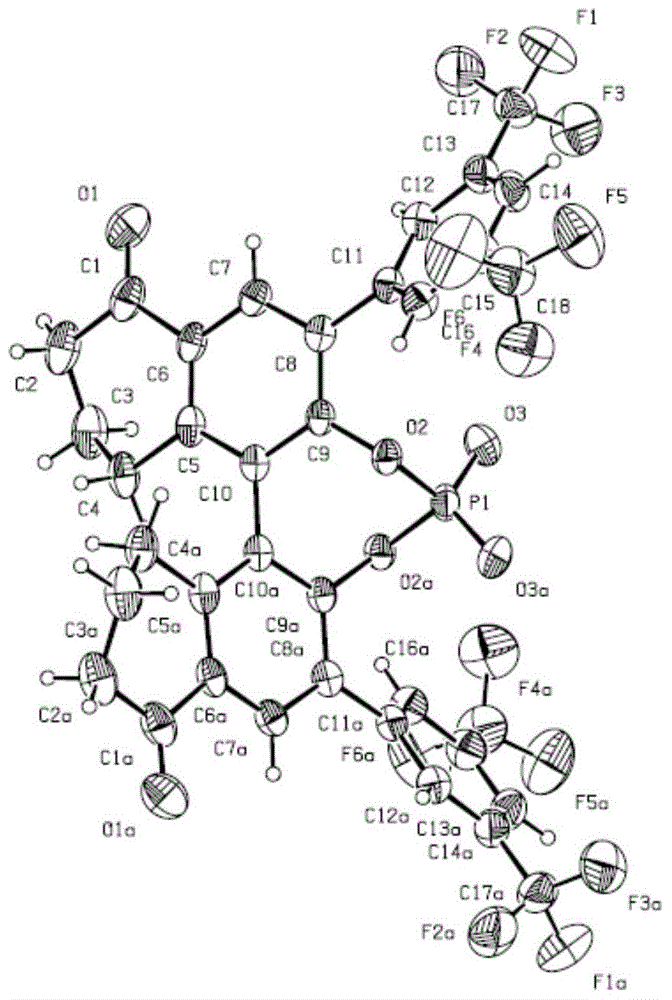
Preparation method of [1, 2, 4] oxadiazino indoline-3-ketone derivative
Owner:HUAQIAO UNIVERSITY
pH-sensitive modified chitosan transgene vector, preparation method and applications thereof
InactiveCN106279464AEasy to compressEfficient compressionOther foreign material introduction processesBackbone chainGene vector
The invention discloses a pH-sensitive modified chitosan transgene vector, a preparation method and applications thereof, wherein agmatine and dimethyl maleic anhydride are respectively grafted on the main chain of a chitosan macromolecule to form a biological macromolecule simultaneously having positive charge and negative charge, and the prepared material is used for gene transfection. According to the present invention, the preparation method is simple, the reaction condition is mild, the obtained vector can effectively compress DNA under the neutral condition to form the surface-charged nanometer complex so as to easily perform the adsorption of the complex antiserum protein, and under the acidic pH condition, the charge reversal occurs, and the surface charge of the nanometer complex is changed to the positive from the negative so as to easily achieve the cell endocytosis of the complex, such that the transfection efficiency is improved.
Owner:TIANJIN UNIV
Synthesis method of alpha-aminoacid derivative substituted by alpha-alkyl branch
InactiveCN105017043AEasy stepsMild reaction conditionsAmino-carboxyl compound preparationOrganic compound preparationAlkeneAlkyl
Owner:SOUTH CHINA UNIV OF TECH +1
Method for recycling utilization of epoxy chloropropane oil layer rectification kettle residues
ActiveCN111909009AMild reaction conditionsThe synthesis process is simpleEther preparation by compound dehydrationEther preparation by ester reactions1-ChloropropaneChemical engineering
Owner:JIANGSU RUIHENG NEW MATERIAL TECH CO LTD +3
Green synthesis method of drug active molecules GC-24 and furegrelate
ActiveCN111646889AMild reaction conditionsEasy to operateOrganic compound preparationCarboxylic acid esters preparationBENZYL ALCOHOL/WATEROxalomalic acid
Owner:LANZHOU UNIVERSITY +1
Compound for detecting copper ions or preparing drug for preventing and treating Alzheimer disease, preparation method of compound, and product
InactiveCN107488151AInhibition of crosslinkingMild reaction conditionsFluorescence/phosphorescenceMaterial analysis by observing effect on chemical indicatorAmyloid beta proteinsCopper
Owner:GUANGDONG UNIV OF TECH
Preparation method of quinolone carboxylic acid derivatives
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE +1
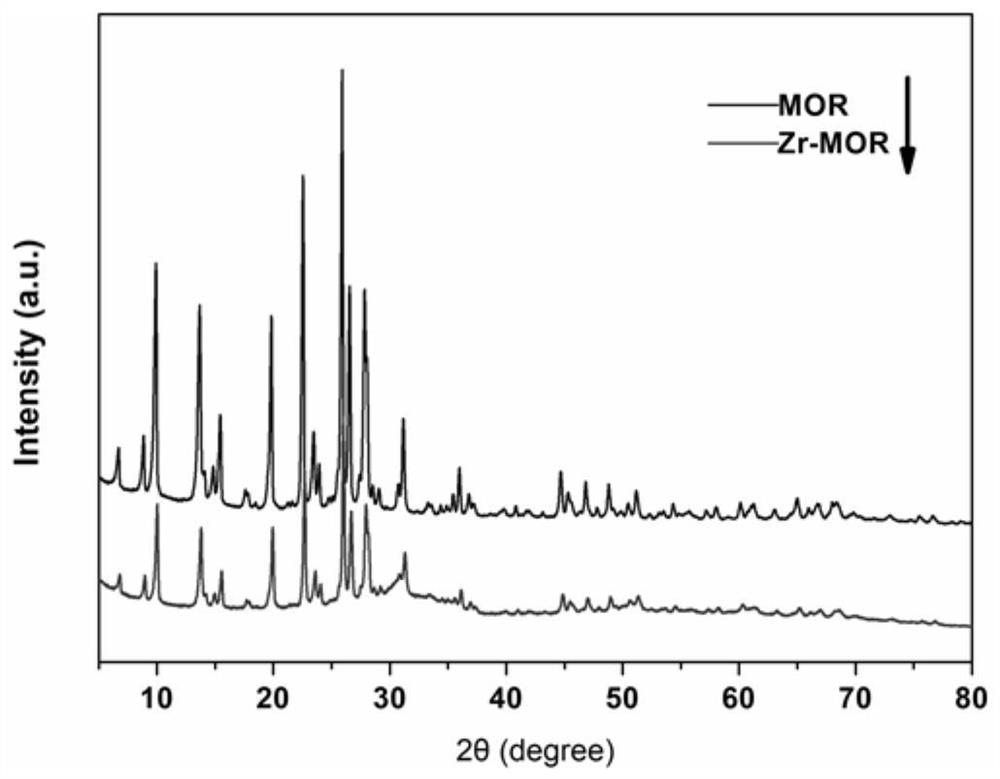
Zirconium-based alkylation catalyst and preparation method and application thereof
ActiveCN112973790AHigh catalytic activityImprove catalytic selectivityMolecular sieve catalystsMolecular sieve catalystHigh selectivityMaterials science
The invention discloses a zirconium-based alkylation catalyst and a preparation method and application thereof, and the preparation method of the catalyst comprises the following steps: (1) dispersing zirconium salt and a modifier in water, stirring, then adding a carrier, stirring under room-temperature magnetic force, evaporating to remove excessive moisture, and drying to obtain a compound modified precursor; and (2) grinding, calcining and annealing the compound modified precursor prepared in the step (1) to obtain the compound modified zirconium-based alkylation catalyst. The zirconium-based catalyst is high in activity, has relatively high selectivity on the target product 2-tert-amyl anthracene, is stable in catalytic performance, also has relatively excellent cyclic regeneration performance, and is suitable for popularization in industrial production.
Owner:ZHEJIANG UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap

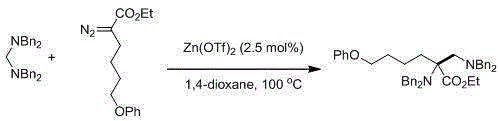
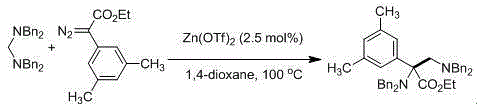




![Preparation method of [1, 2, 4] oxadiazino indoline-3-ketone derivative Preparation method of [1, 2, 4] oxadiazino indoline-3-ketone derivative](https://images-eureka.patsnap.com/patent_img_release/c33eb867-3f23-474f-afe4-2ce5f44ede31/HDA0003175968710000011.png)
![Preparation method of [1, 2, 4] oxadiazino indoline-3-ketone derivative Preparation method of [1, 2, 4] oxadiazino indoline-3-ketone derivative](https://images-eureka.patsnap.com/patent_img_release/c33eb867-3f23-474f-afe4-2ce5f44ede31/HDA0003175968710000012.png)
![Preparation method of [1, 2, 4] oxadiazino indoline-3-ketone derivative Preparation method of [1, 2, 4] oxadiazino indoline-3-ketone derivative](https://images-eureka.patsnap.com/patent_img_release/c33eb867-3f23-474f-afe4-2ce5f44ede31/BDA0003175968700000041.png)