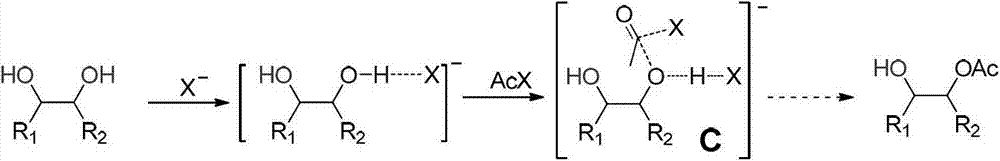
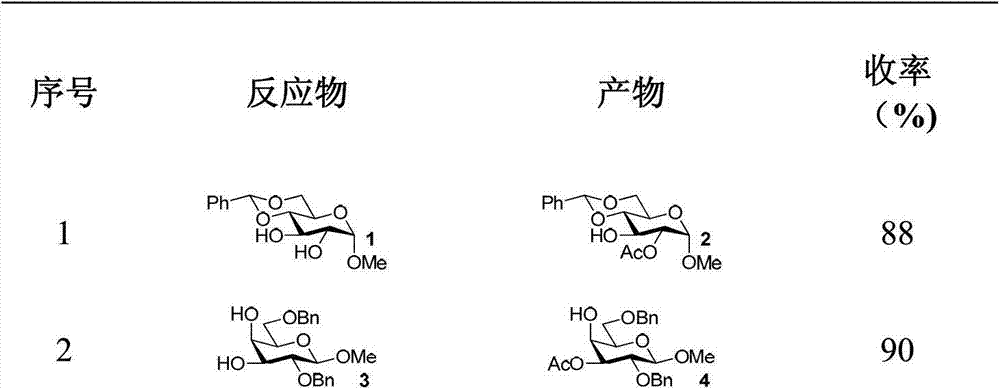
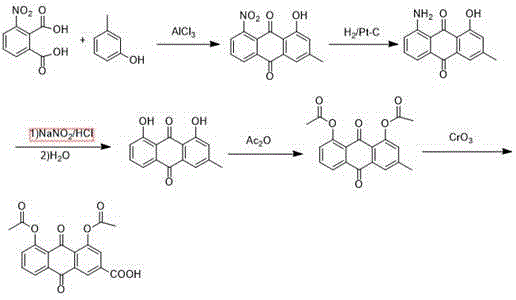
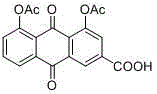
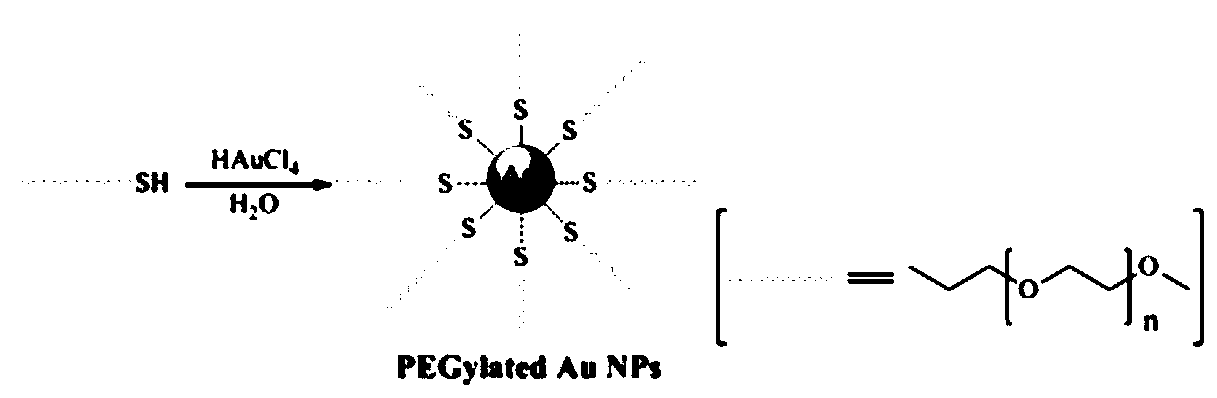
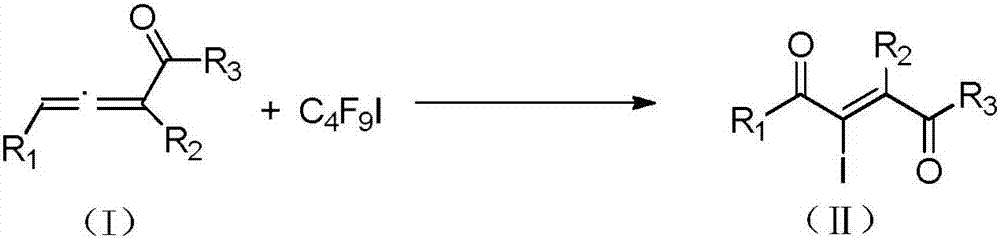Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31results about "Carboxylic acid esters preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Self-supporting nickel phosphide catalyst and preparation method and application thereof
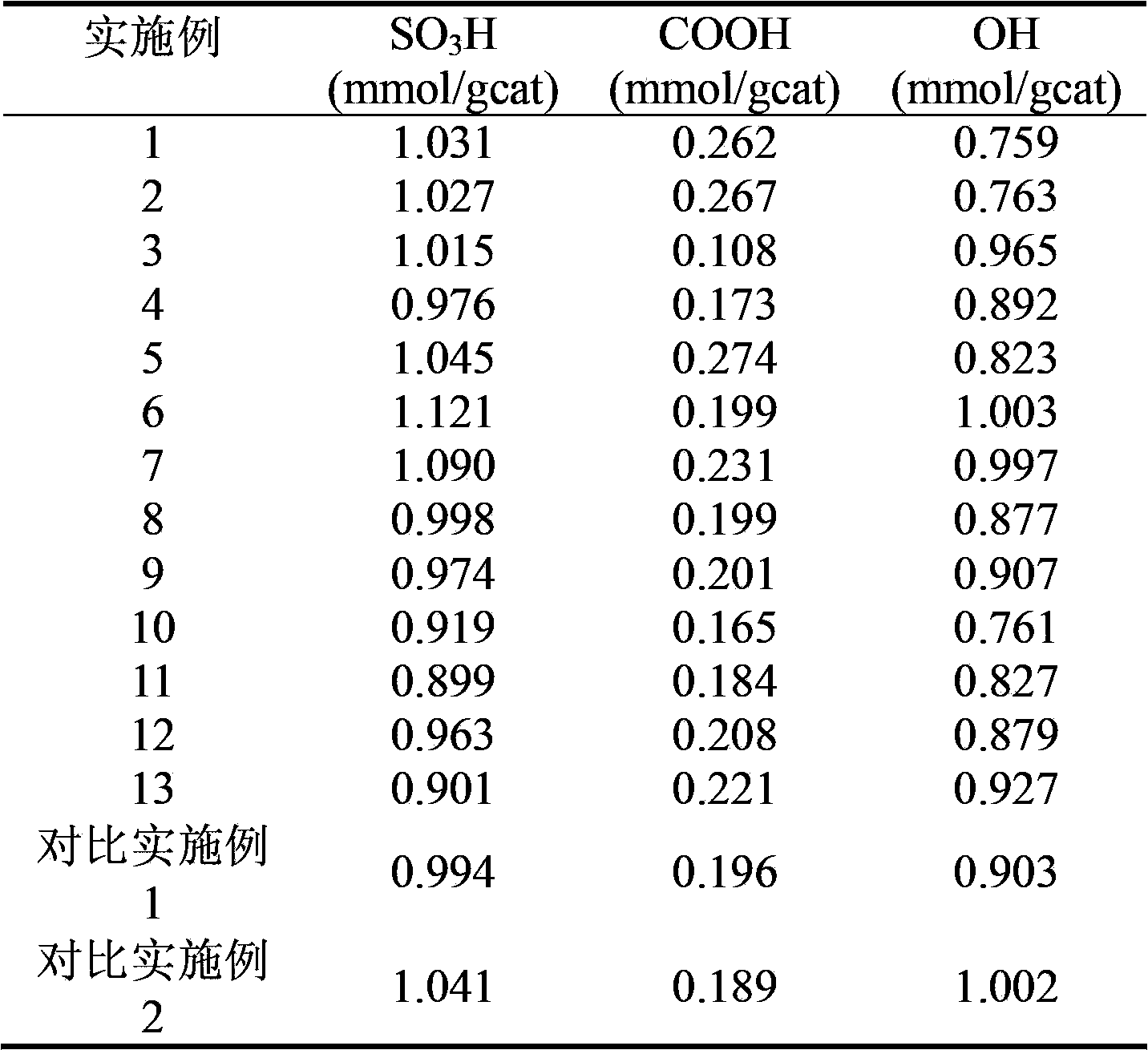
ActiveCN107694584AImprove thermal conductivityGood choiceOrganic compound preparationCarboxylic acid esters preparationOxalateNickel oxide hydroxide
The invention discloses a self-supporting nickel phosphide catalyst and a preparation method and application thereof. The self-supporting nickel phosphide catalyst is a nickel phosphide catalyst obtained by in-situ growing of a nickel oxalate or nickel hydroxide crystal layer on a framework matrix through a hydrothermal method and performing phosphating while secondary forming is not needed, and the nickel phosphide catalyst is composed of the framework matrix and a nickel-phosphorus compound, wherein the nickel-phosphorus compound is at least one of Ni3P, Ni12P5, Ni2P and Ni5P4, total mass ratio of the nickel-phosphorus compound is 0.1-50%, and the balance is the framework matrix. Experiments show that the self-supporting nickel phosphide catalyst is high in stability and thermal conductivity, easy to form and fill, high in flux and low in pressure drop, especially has the advantages of high low-temperature activity, high dimethyl oxalate conversion rate and high methyl glycolate selectivity and can be used as a reaction catalyst for hydrogenating dimethyl oxalate to prepare methyl glycolate.
Owner:EAST CHINA NORMAL UNIV
Method for synthesizing citric acid ester type compound
InactiveCN101830803AHigh catalytic activityRich sourcesOrganic compound preparationCarboxylic acid esters preparationChemical synthesisBenzene
The invention discloses a method for synthesizing a citric acid ester type compound, which belongs to the technical field of chemical synthesis. The method comprises the following steps of: using citric acid and fatty alcohol as main raw materials, and using benzene sulfonic acid or amino benzene sulfonic acid as a catalyst; and performing esterification and the purification processes of acetylation, neutralization, washing, drying, distillation and the like. The catalyst has rich sources, a low cost and high activity, can be separated from an esterification liquid easily after the neutralization, is coke-free during the distillation, has less corrosion to equipment, and is safe and environment-friendly; the water generated by the esterification is separated out by adopting a binary heterogeneous separation technique, and no water separating agent is additionally added; and acetyl citric acid ester is produced by adopting an esterification-acetylation continuous synthesis method, the flow is greatly simplified, and a synthesis process is shortened. The citric acid ester prepared by the method has the advantages of high quality, high purity, low degree of color and wide applicationrange.
Owner:NORTHWEST NORMAL UNIVERSITY
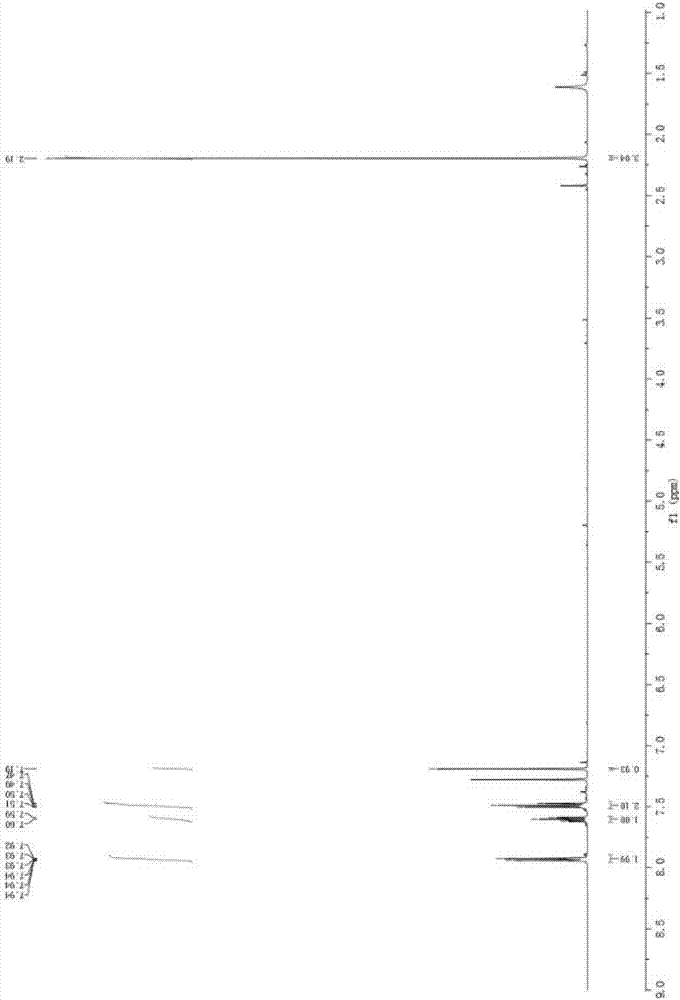
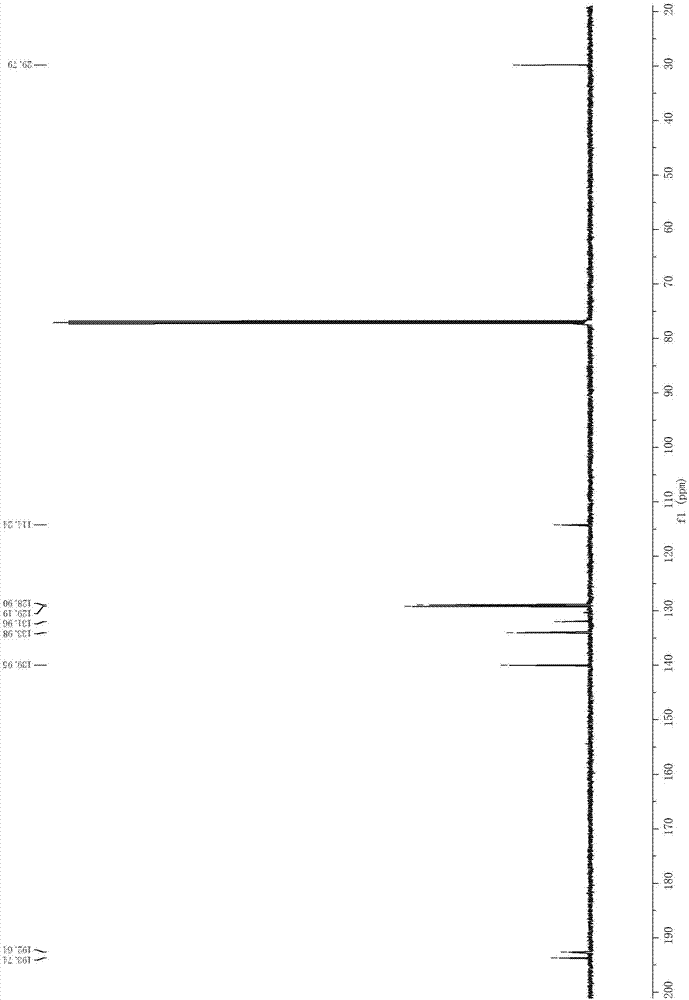
Method for preparing 2-iodine amyl -2-ene-1,4-diketone derivative by adopting visible light catalysis
ActiveCN107011145AMild reaction conditionsEasy to operateOrganic compound preparationCarboxylic acid esters preparationIodidePollution
Owner:ZHEJIANG UNIV OF TECH
Method for composite phosphotungstate catalyzed synthesis of citrate ester
InactiveCN106008207AAchieve reuseHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsEsterification reactionFatty alcohol
The invention relates to a method for composite phosphotungstate catalyzed synthesis of citrate ester. The citrate ester is synthesized through an esterification reaction of citric acid and fatty alcohol with composite phosphotungstate as a catalyst. The structure formula of the composite phosphotungstate adopted in the invention is (NH4)xM(3-x-y) / 4HyPW12O40, wherein M is Ti or Zr, x is 0.4-1, and y is 0.4-1. The method has the following advantages: the catalyst has low cost, is easy to prepare, has a high catalysis efficiency, can be simply separated from the above product, and has excellent performances when being reused.
Owner:SHAOYANG UNIV
Organic bottom antireflective coating composition for nanolithography
ActiveUS20150185614A1Strong Gap Filling CapabilityMinimizing contentOrganic compound preparationCarboxylic acid esters preparationNanolithographyAnti-reflective coating
Owner:ROHM & HAAS ELECTRONICS MATERIALS LLC
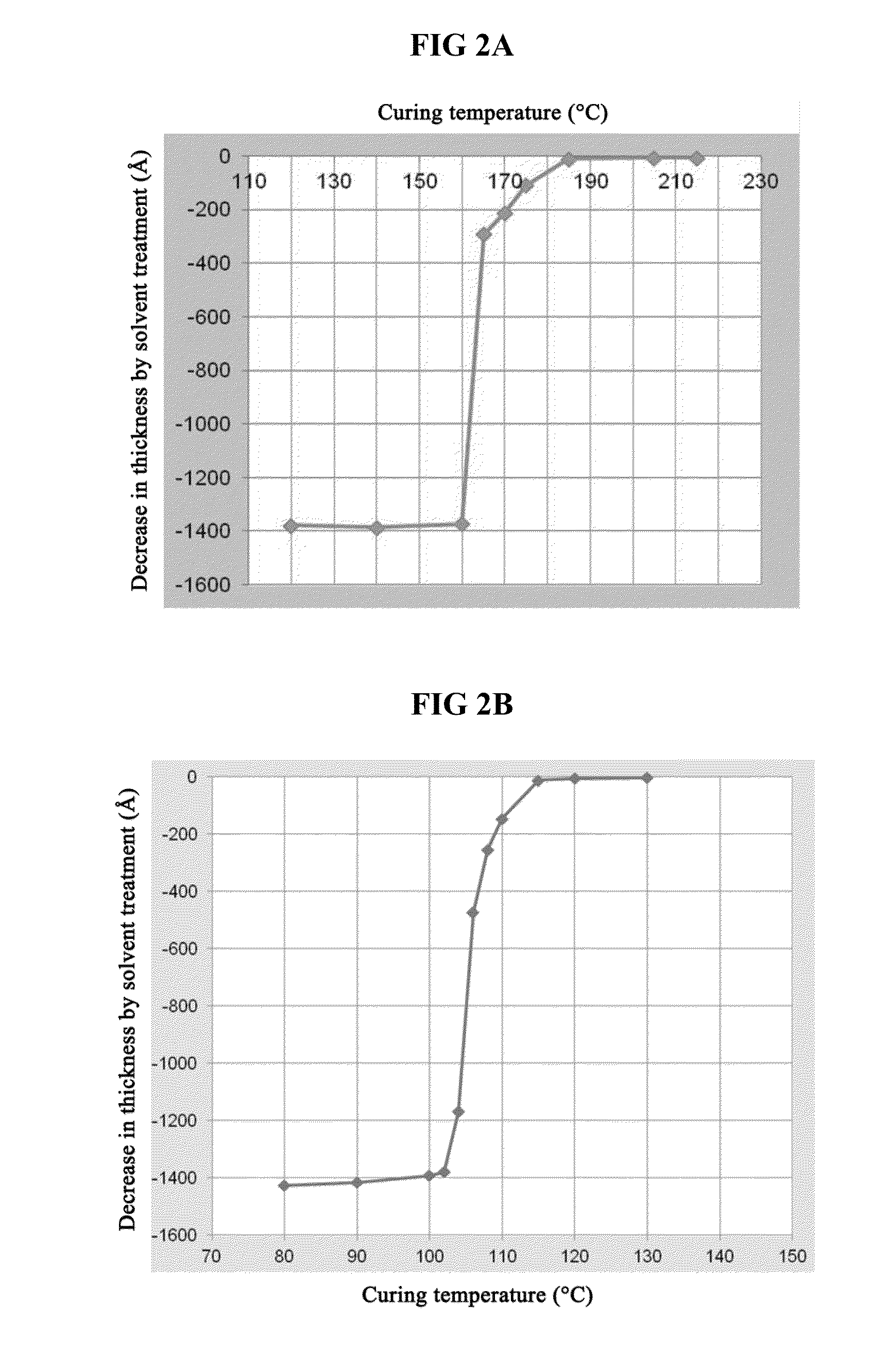
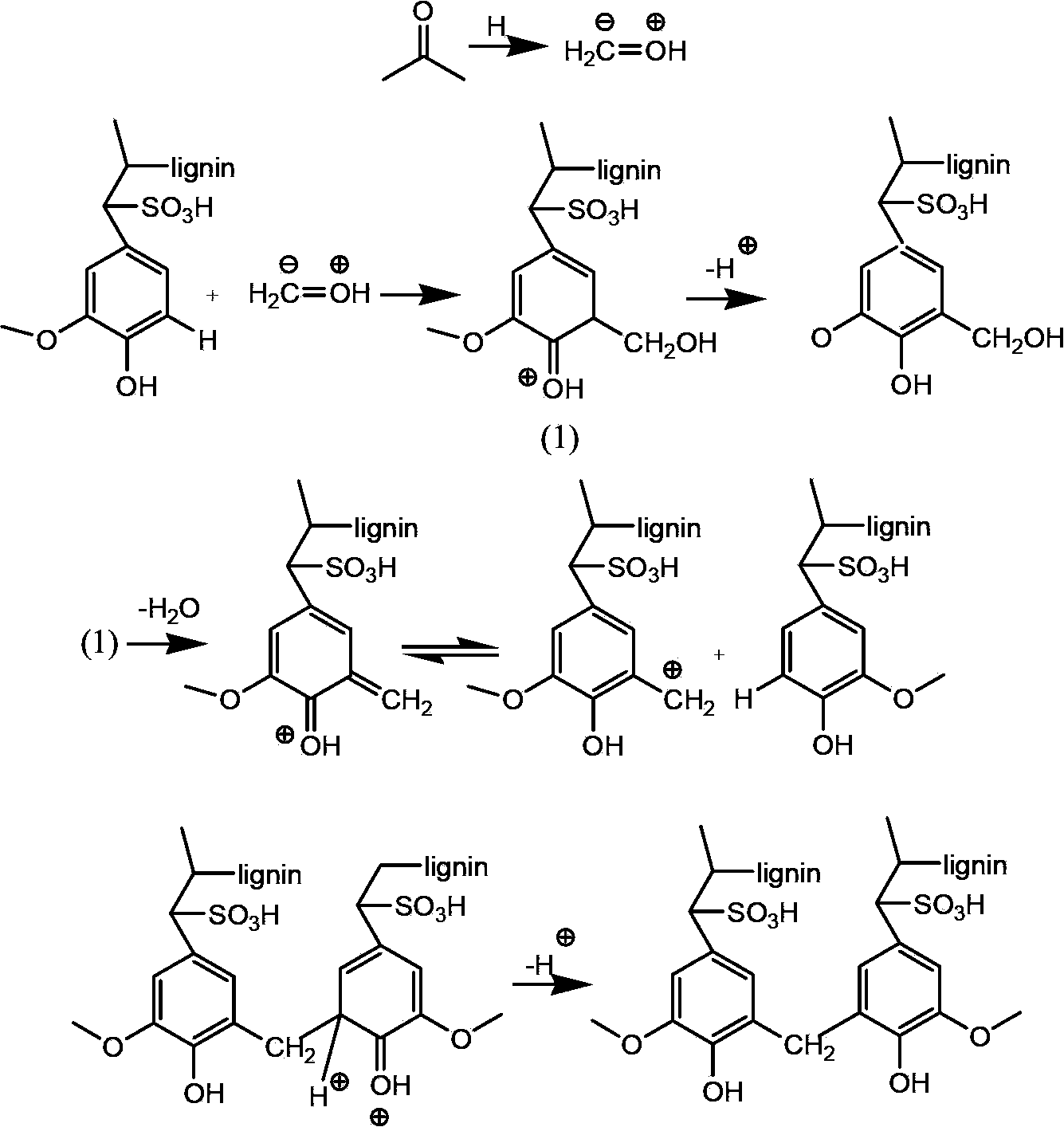
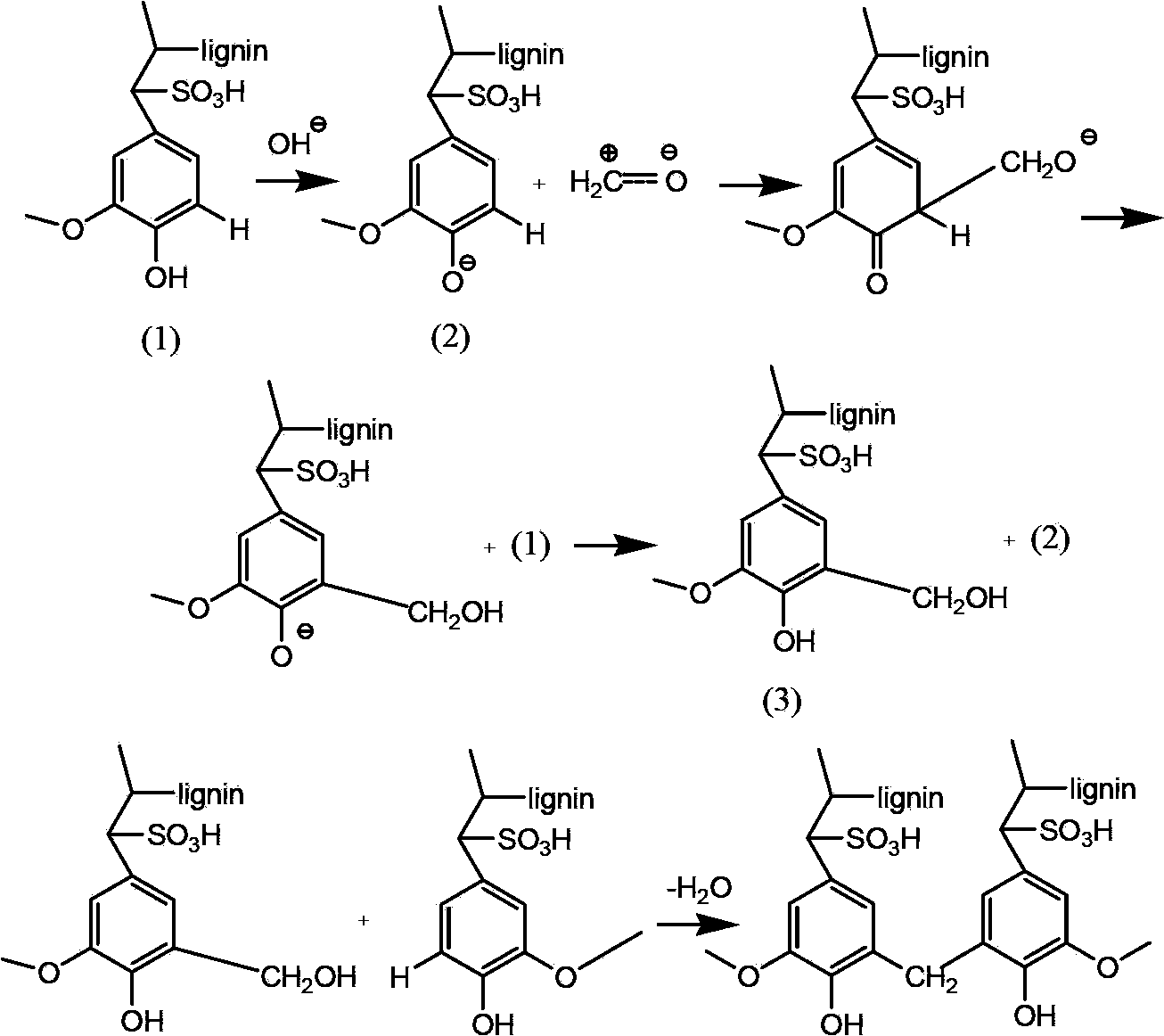
Porous biomass acidic solid material, and preparation and application thereof
InactiveCN103509194ARich sourcesLow costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsLignosulfonatesEsterification reaction
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing methyl formate and coproducing dimethyl ether
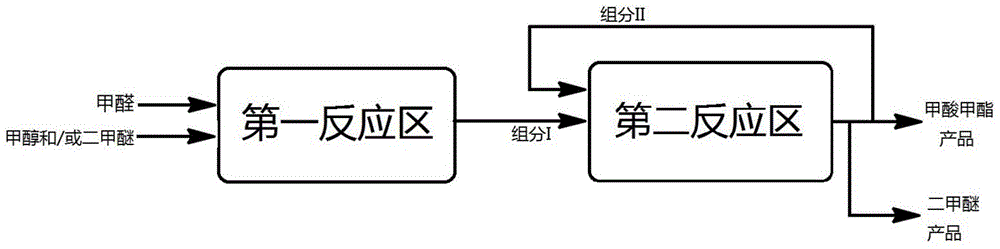
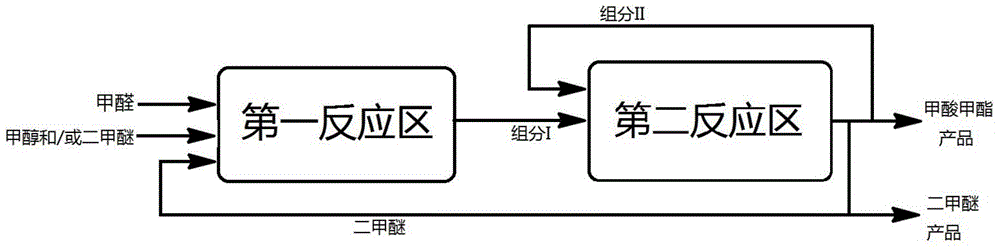
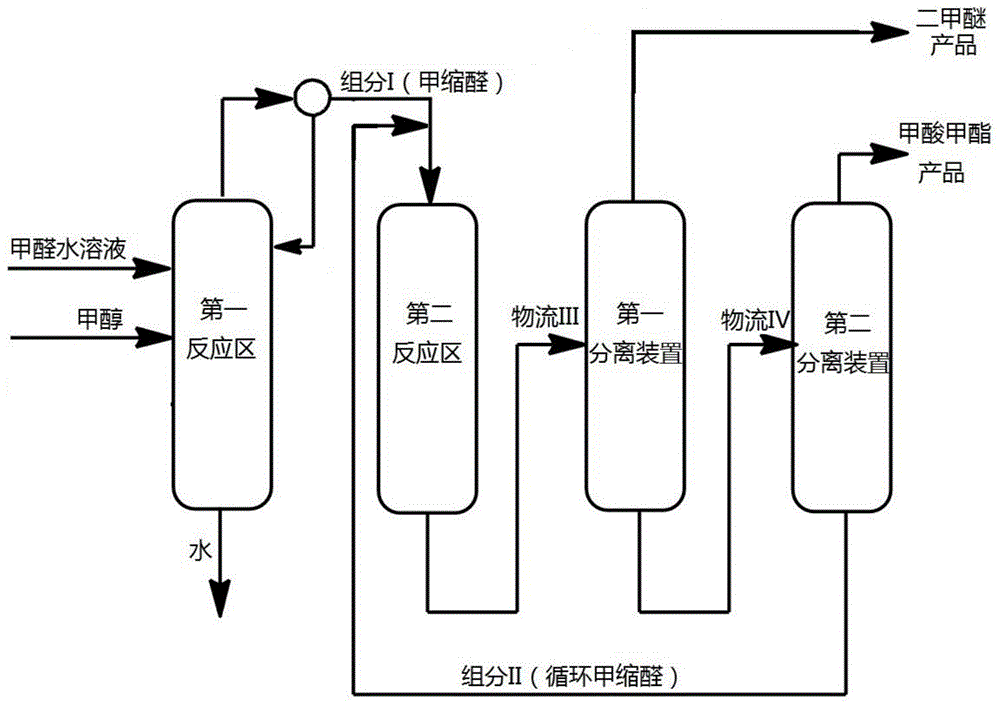
ActiveCN105669452AEasy to separateReduce energy consumptionOrganic compound preparationCarboxylic acid esters preparationGas phaseReaction zone
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
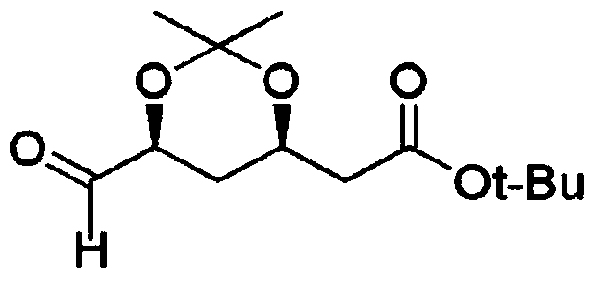
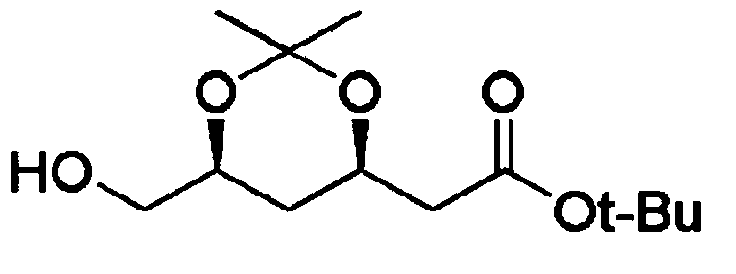
Method for preparing t-butyl 2-((4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxan-4-yl)acetate
InactiveCN103502234AOrganic active ingredientsGroup 4/14 element organic compoundsAcetic acidHMG-CoA reductase
Owner:WELL E&C
Process for producing polyol fatty acid ester
ActiveCN1683314AStable and reliable productionStable productionOrganic compound preparationCarboxylic acid esters preparationPolyethylene glycolStearic acid
Owner:WENGFU (GRP) CO LTD
Milnacipran hydrochloride intermediate as well as preparation method and application thereof
ActiveCN103613513AEconomical method of preparationEfficient preparation methodOrganic compound preparationCarboxylic acid esters preparationMedicinal chemistryMilnacipran hydrochloride
Owner:SHANGHAI SHYNDEC PHARMA CO LTD
3, 3-difluoro-1, 5-hexadiene compound as well as preparation method and application thereof
ActiveCN113024349AHigh selectivityEasy to operateSugar derivativesCarboxylic acid nitrile preparationMeth-Silanes
The invention discloses a 3, 3-difluoro-1, 5-hexadiene compound as well as a preparation method and an application thereof. The preparation method comprises the following steps: in an inert gas atmosphere, sequentially adding alpha-trifluoromethyl styrene, allyltrimethylsilane, tetrabutylammonium fluoride and a solvent into a reaction tube, and magnetically stirring uniformly to obtain a mixture; performing a magnetic stirring reaction on the mixture for 12 h under the condition of a 130 DEG C oil bath, cooling the reaction mixture to the room temperature, and obtaining the 3, 3-difluoro-1, 5-hexadiene compound from the reaction mixture through rapid column chromatography separation. The compound can be used for synthesizing a precursor compound containing a CF2 organic framework. The preparation method has the advantages of high selectivity, economy, environmental protection, simple operation, cheap and easily available raw materials, and is suitable for industrial application.
Owner:NANJING TECH UNIV
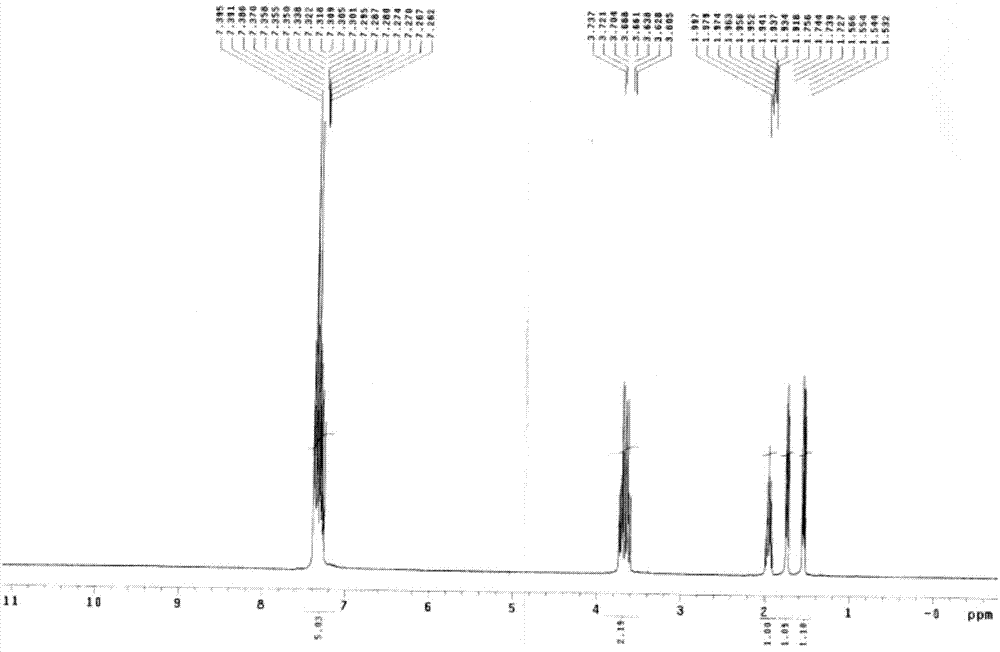
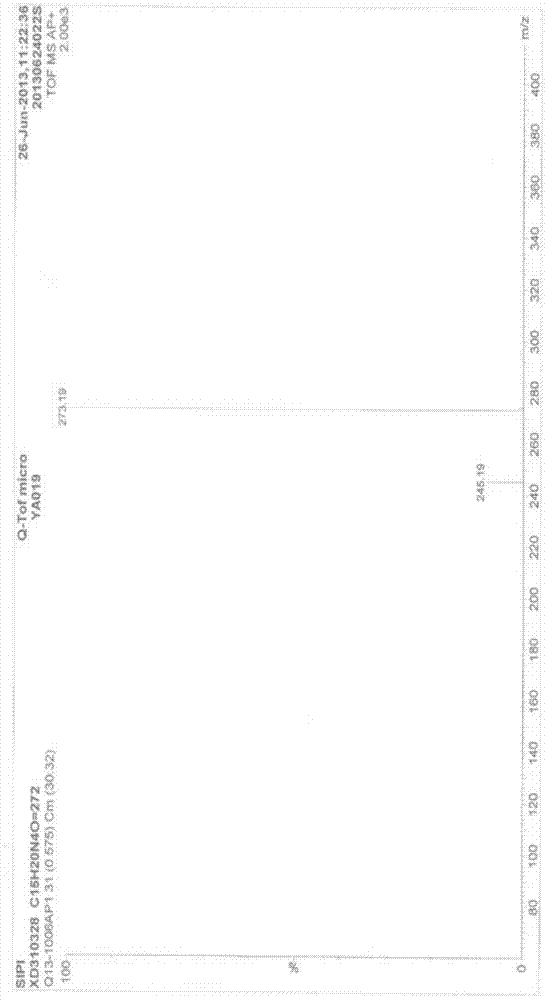
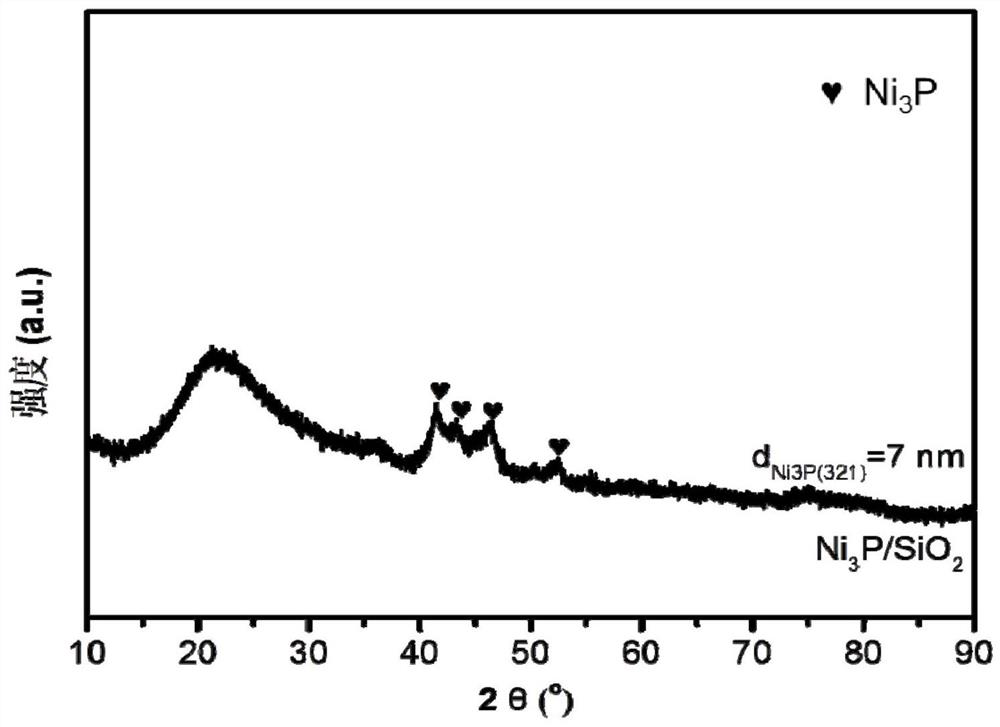
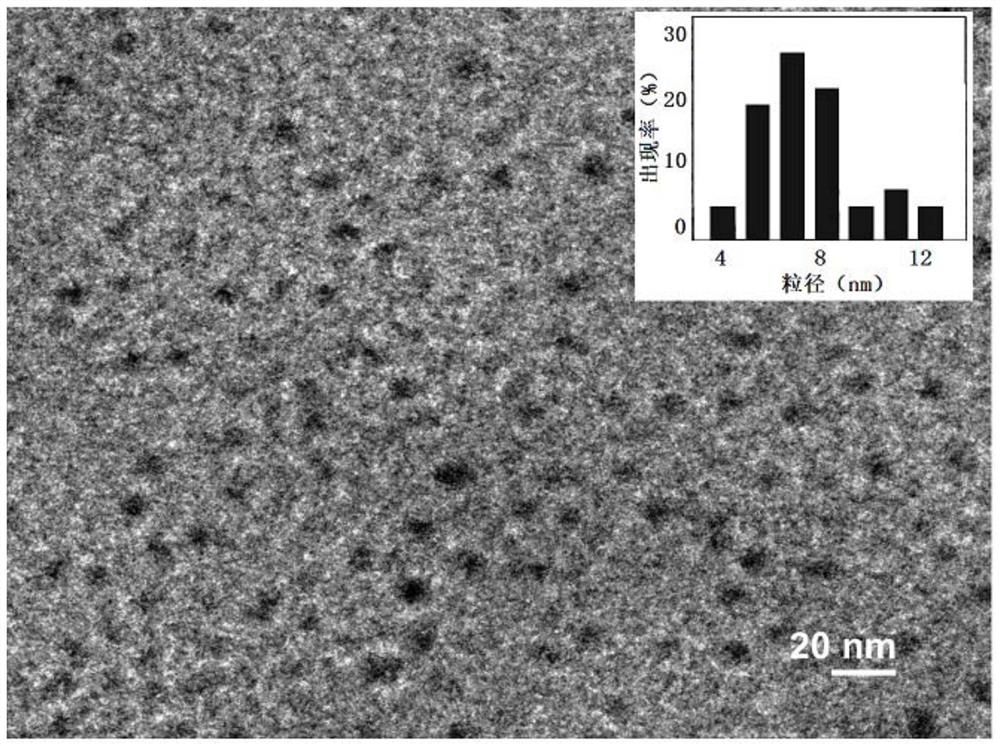
Ni3P/SiO2 catalyst as well as preparation method and application thereof
ActiveCN113145144AHigh selectivityAvoid signs of inactivationPhysical/chemical process catalystsOrganic compound preparationPtru catalystCombinatorial chemistry
Owner:EAST CHINA NORMAL UNIV
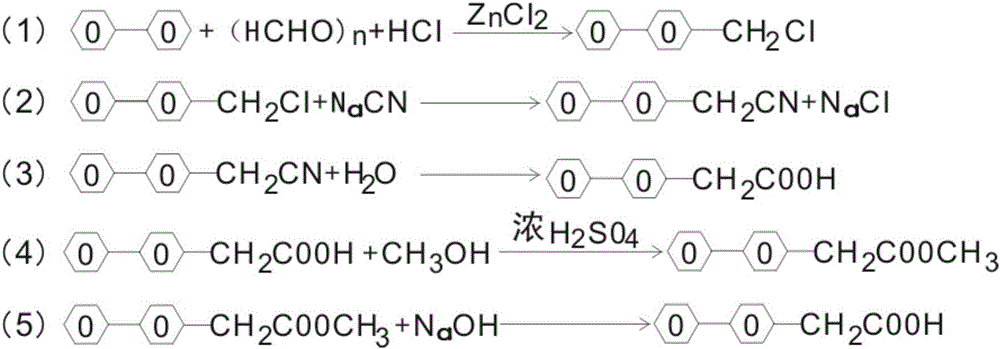
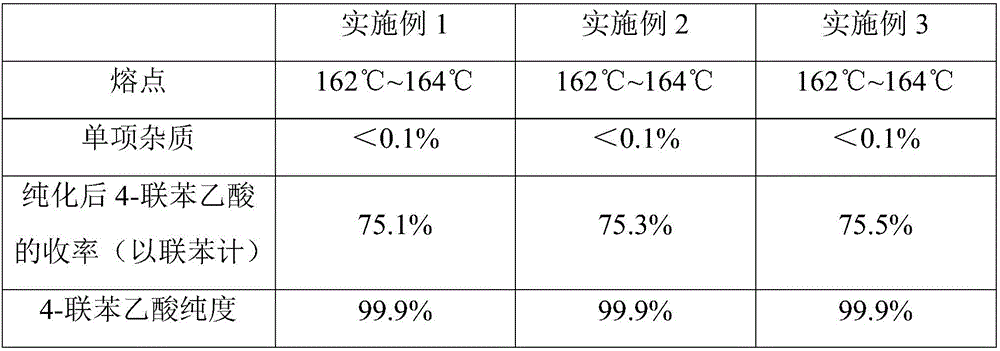
Preparation and purification methods of 4-biphenylacetic acid
ActiveCN106431911AOvercome the technical difficulty of further purification of 4-biphenylacetic acidIncrease valueOrganic compound preparationCarboxylic acid esters preparationPurification methodsBenzyl chloride
Owner:黄石市利福达医药化工有限公司
Preparation method of fatty alcohol sebacate
ActiveCN105237393AOutstanding advantagesHighlight positive effectsOrganic compound preparationCarboxylic acid esters preparationPhosphorous acidAlcohol
Owner:ZHEJIANG HUANGMA TECH
Preparation method of pyroxsulam intermediate
ActiveCN113292487AAvoid Polymerization Side ReactionsGuaranteed purityOrganic compound preparationCarboxylic acid esters preparationAcyl groupDouble bond
The invention discloses a preparation method of a pyroxsulam intermediate, which comprises the following steps: (1) carrying out condensation reaction on 4-alkoxy-1,1,1-trifluoro-3-buten-2-one and phosphonoacetic acid trialkyl ester in an alcohol solvent in the presence of sodium alcoholate to generate an intermediate 3-trifluoromethyl-5,5-dialkoxy pentenoic acid alkyl ester and an isomer of the intermediate 3-trifluoromethyl-5,5-dialkoxy pentenoic acid alkyl ester; and (2) carrying out cyclization reaction on the intermediate generated in the step (1) and ammonium acetate in the presence of a polymerization inhibition catalyst to generate 2-hydroxy-4-trifluoromethylpyridine. According to the method disclosed by the invention, hydroquinone and other polymerization inhibition catalysts are added in the cyclization process, so that polymerization side reaction of a condensation intermediate containing double bonds can be avoided, and the reaction yield and the product purity of the cyclization reaction are ensured. Meanwhile, the 4-butoxy-1,1,1-trifluoro-3-buten-2-one and the phosphonoacetic acid trimethyl ester are adopted as the condensation reaction raw materials, so that not only can higher condensation reaction yield be obtained, but also the cost is lower, and the safety is higher.
Owner:JIANGSU AGROCHEM LAB CO LTD
High-selectivity protection method of hydroxy
ActiveCN103193570AMild reaction conditionsGentle operationSugar derivativesOrganic compound preparationEnvironmental resistanceSimple Organic Compounds
Owner:HUBEI LAIFENG TENGSHEN FLAVOR CHEM +1
Preparation method of diacerein
InactiveCN105985242AWide variety of sourcesAvoid pollutionOrganic compound preparationCarboxylic acid esters preparation4-chlorobenzaldehydeChromium
Owner:SHAOYANG UNIV
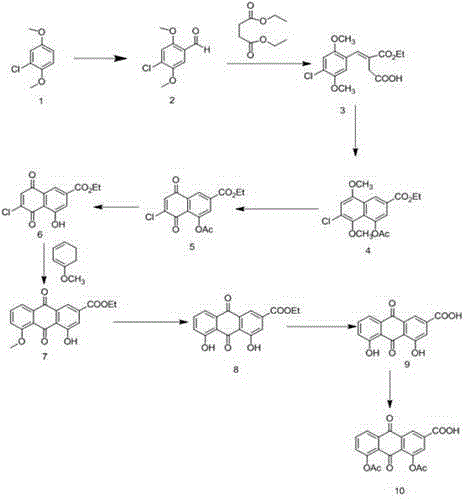
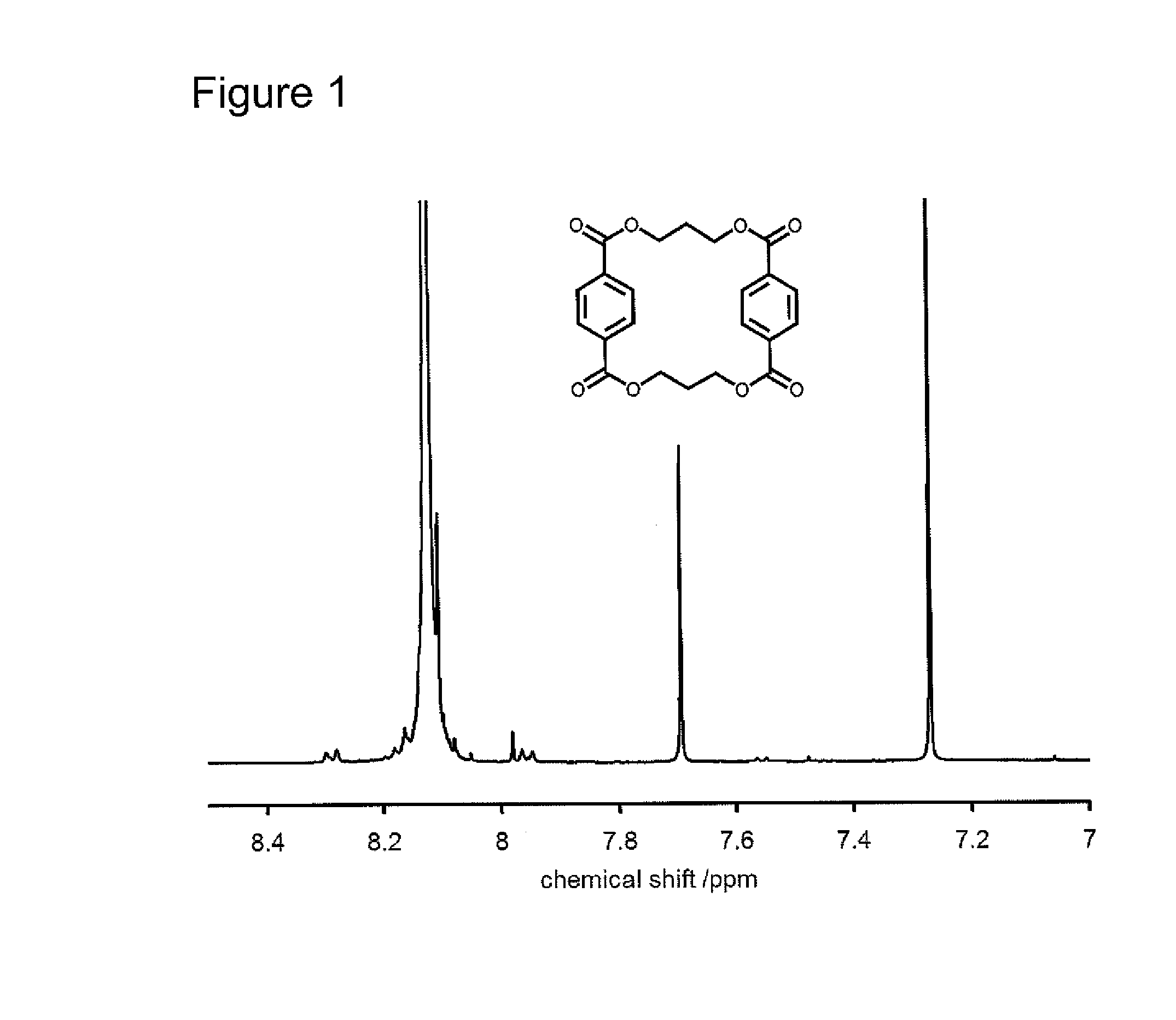
Process of making a poly(trimethylene terephthalate) resin having low cyclic dimer content, and compositions and articles therefrom
InactiveUS20100227960A1High viscosityOrganic compound preparationCarboxylic acid esters preparationNMR - Nuclear magnetic resonancePolyethylene terephthalate
Owner:EI DU PONT DE NEMOURS & CO
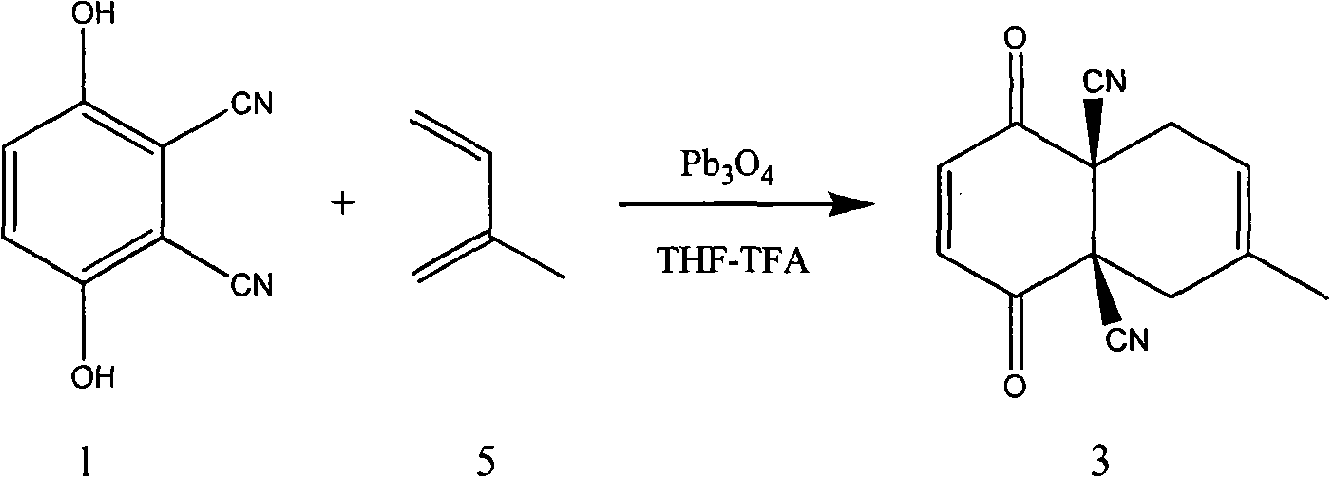
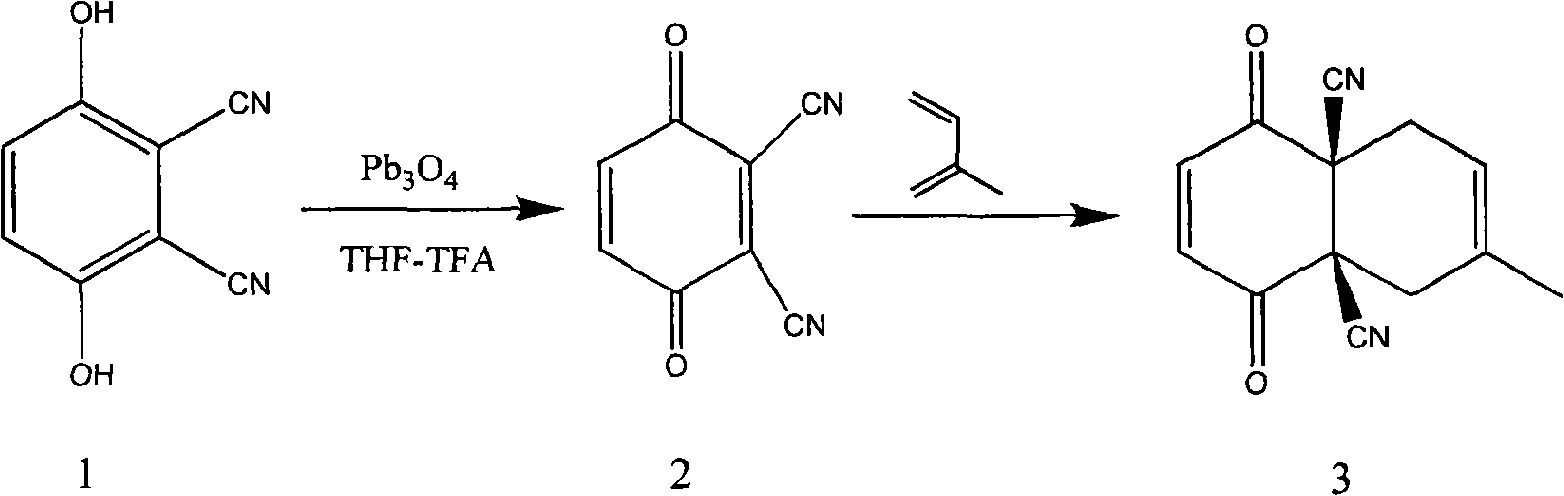
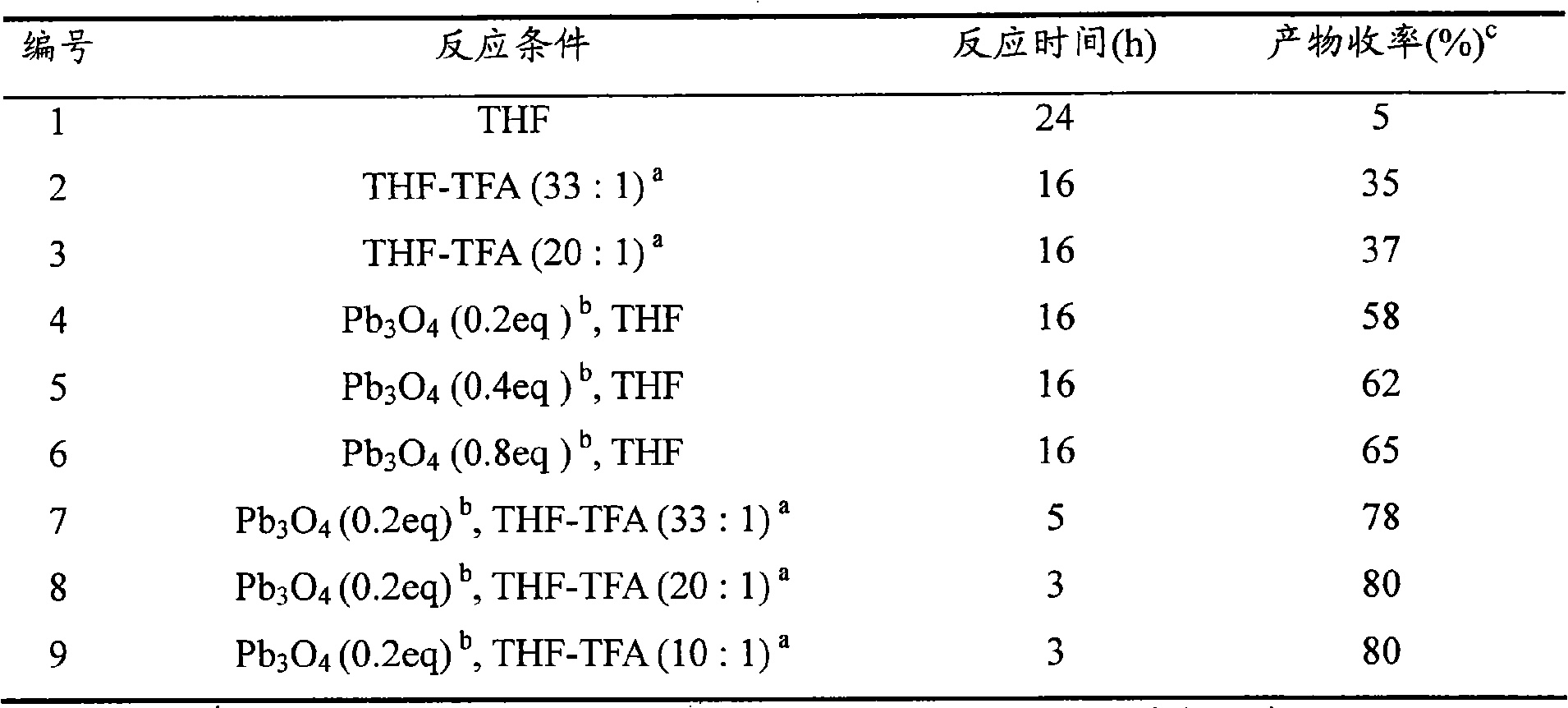
Synthetic method of carbocyclic compound
InactiveCN101570460AEasy to purifyHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventLead(II,IV) oxide
Owner:SOUTHWEST UNIVERSITY
Refining method of spice benzyl acetate
InactiveCN108130188AOrganic compound preparationCarboxylic acid esters preparationChemistryBenzaldehyde
Owner:QIANJIANG XINYIHONG ORGANIC CHEM
Process for preparation of methacrylic acid and methacrylic acid esters
InactiveCN103827071ASolvent extractionOrganic compound preparationSimple Organic CompoundsMethacrylate
The invention relates to a process for preparation of at least one of methacrylic acid and a methacrylic acid ester, comprising process steps: a1) gas phase oxidation of at least one C4 compound to obtain a reaction phase comprising methacrylic acid; a2) quenching of the reaction phase to obtain a crude aqueous phase comprising methacrylic acid; a3) extraction of at least a part of the methacrylic acid from the crude aqueous phase comprising methacrylic acid into an organic solvent to obtain a crude organic phase comprising methacrylic acid and a first aqueous phase, wherein the first aqueous phase comprises components: i. at least 65 wt.%, preferably in the range of from 65 wt.% to 99.9 wt.%, more preferably in the range of from 70 wt.% to 99.8 wt.% water, yet more preferably in the range of from 75 wt.% to 99 wt.%, more preferably in the range of from 76 wt.% to 98.5 wt.%, more preferably in the range of from 77 wt.% to 98 wt.%, even more preferably in the range of from 78 wt.% to 97.5 wt.%, even more preferably in the range of from 79 wt.% to 95 wt.%, yet more preferably in the range of from 80 wt.% to 90 wt.% water, based on the total weight of the first aqueous phase, and ii. not more than 35 wt.%, preferably in the range of from 0.1 wt.% to 35 wt.%, preferably in the range of from 0.2 wt.% to 30 wt.%, more preferably in the range of from 1 wt.% to 25 wt.%, yet more preferably in the range of from 1.5 wt.% to 24 wt.%, more preferably in the range of from 2 wt.% to 23 wt.%, even more preferably in the range of from 2.5 wt.% to 22 wt.%, even more preferably in the range of from 5 wt.% to 21 wt.%, yet more preferably in the range of from 10 wt.% to 20 wt.% of at least one organic compound, based on the total weight of the first aqueous phase, wherein the sum of the weight amounts of i. and ii. is 100 wt.%; a4) separation and optionally purification of at least a part of the methacrylic acid from the crude organic phase comprising methacrylic acid; a5) optionally, esterification of at least a part of the methacrylic acid obtained in step a4); b) separation of at least a part of the water comprised in the first aqueous phase obtained in step a3) from at least a part of at least one component ii. to obtain a second aqueous phase and an organic phase, wherein the organic phase comprises at least one component ii., and wherein the second aqueous phase is depleted in at least one component ii. compared to the first aqueous phase; c) optionally, separation of at least a part of at least one organic compound from the second aqueous phase obtained in process step b) to obtain a third aqueous phase; d) optionally, separation of at least a part of at least one component ii from the organic phase obtained in process step b), to a process for treatment of an aqueous phase comprising at least one organic compound, and a device for production of at least one of methacrylic acid and a methacrylic ester.
Owner:EVONIK ROEHM GMBH
Nanogold micelle catalyst as well as preparation method and application thereof
ActiveCN110898831AEfficient self-esterification reactionHigh yieldOrganic compound preparationCarboxylic acid esters preparationPtru catalystFatty alcohol
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Esterification product for preparing water reducing agent, preparation method of esterification product, high-workability polycarboxylic acid water reducing agent and preparation method of high-workability polycarboxylic acid water reducing agent
PendingCN114181086AThere is no problem of uneven distributionExcellent high temperature corrosion resistanceOrganic compound preparationCarboxylic acid esters preparationSuperplasticizerBackbone chain
Owner:KZJ NEW MATERIALS GROUP CO LTD +1
Reaction kettle for n-amyl acetate
InactiveCN104415719AMixed cooling effect is goodOrganic compound preparationCarboxylic acid esters preparationCooling effectAmyl acetate
The invention belongs to the field of chemical equipment, and in particular provides a reaction kettle for n-amyl acetate. The reaction kettle for n-amyl acetate comprises a reaction kettle body, wherein a material inlet and an observation window are formed in the reaction kettle body, a cooling clamping sleeve is arranged on the exterior of the reaction kettle body, is provided with a cooling liquid outlet and is internally provided with a cooling liquid feeding tube, the cooling liquid guiding tube comprises a cooling liquid inlet and a feeding hole, the feeding hole is formed in the bottom of the cooling clamping sleeve, and the cooling liquid inlet is formed in the top of the cooling clamping sleeve. The reaction kettle for n-amyl acetate has the advantages that the cooling liquid feeding tube is directly communicated with the bottom of the cooling clamping sleeve and separates cold water from hot water so that the hot water and the cold water are prevented from being mixed and the cooling effect is better.
Owner:JIANGSU NEW MATERIALS SCI & TECH
Catalyst and method for preparing alpha-acetoxyacetone through hydroformylation of vinyl acetate
PendingCN114588945ASynthesis fastImprove conversion rateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIsomerizationPtru catalyst
Owner:PETROCHINA CO LTD
Granular alkali metal alkoxides and alkaline earth metal alkoxides
InactiveUS20050033096A1Readily apparentOrganic compound preparationCarboxylic acid esters preparationAlkaline earth metalNuclear chemistry
Owner:DEGUSSA AG
Preparation method of soybean oil base polyhydric alcohol
InactiveCN108250073AReduce dosageFewer post-processing stepsOrganic compound preparationCarboxylic acid esters preparationSolventSOYBEAN SEED OIL
The invention relates to a preparation method of soybean oil base polyhydric alcohol, in particular to a method for preparing soybean oil base polyhydric alcohol by directly using soybean oil. The soybean oil diluted by using a solvent reacts with hydrogen peroxide with the weight percentage being 30% for 2-5 hours at the temperature of 60-70 DEG C under catalysis of a catalyst with the weight percentage being 3-5%; vacuum distillation is conducted after the reaction is ended, and the solvent is evaporated; then low alcohol is added in a reactor to be subjected to a reaction for 4-6 hours under the pressure of 0.3-0.4 MPa and at the temperature of 70-80 DEG C, then the reaction is stopped, and the soybean oil base polyhydric alcohol is obtained. The method saves the using amount of hydrogen peroxide, reduces the post-treatment steps, and simplifies the production procedures, and accordingly the product cost is declined directly.
Owner:义乌市诠铈新材料有限公司
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap