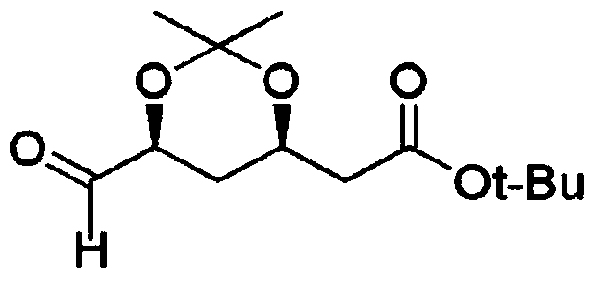
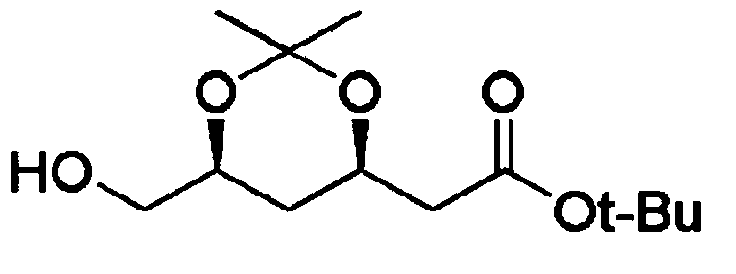
Method for preparing t-butyl 2-((4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxan-4-yl)acetate
A kind of technology of tert-butyl acetate and dioxane, applied in 2-((4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxane-4-yl ) The field of manufacture of tert-butyl acetate, can solve problems such as difficult storage, difficult temperature control, long refining process, and achieve the effect of suppressing rapid heat generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Embodiment 1: Utilize PIPO-TEMPO to manufacture the compound of chemical formula 1
[0110] 2-(4R,6S)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-yl) tert-butyl acetate (335g, 1.3mol ) was dissolved in toluene solvent (6.7L), cooled at -15°C, and then sodium bicarbonate (NaHCO 3 , 487g, 5.8mol). Pay attention to heating, and add sodium bromide (NaBr, 131g, 1.3mol) at the same time, then, keep the temperature below -10°C, slowly add sodium hypochlorite (NaClO, 798ml, 1.42mol).
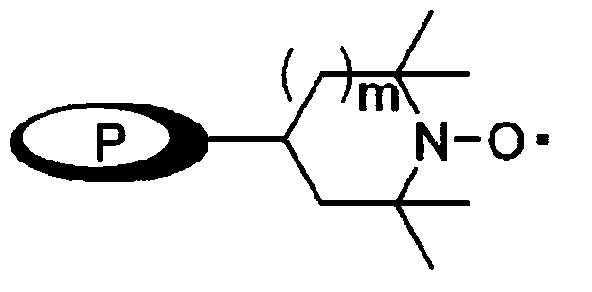
[0111] Keep the temperature below -5°C and slowly add the compound PIPO-TEMPO (R1=1,1,3,3-tetramethylbutyl) (0.001 equivalent, 0.838 g, 0.0013mol) was dissolved in toluene solvent (300ml), and then stirred for 30 minutes while keeping the temperature below -5°C. Add Na after the reaction 2 S 2 o 3 (1.5L), stirred at room temperature for 20 minutes to complete the reaction, and then separated the layers to recover the organic layer. The aqueous layer was washed twice with toluene solvent (2L),
Embodiment 2
[0116] Embodiment 2: utilize PHDM-TEMPO to manufacture the compound of chemical formula 1
[0117] 2-(4R,6S)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-yl) tert-butyl acetate (10g, 0.04mol ) was dissolved in toluene solvent (200ml), cooled at -15°C, and then added sodium bicarbonate (NaHCO 3 , 14.5g, 0.17mol). Pay attention to exothermic, add sodium bromide (NaBr, 3.9g, 0.04mol) at the same time, then, keep the temperature below -10 ℃, slowly add sodium hypochlorite (NaClO, 24ml, 0.04mol).
[0118] Keep the temperature below -5°C, slowly add dropwise the compound PHDM-TEMPO (0.001 equivalent, 0.023g, 0.04mmol) of the chemical formula 5 in the toluene solvent (10ml) to the mixed solution, and then keep Stir for 30 minutes at a temperature below -5°C. Add Na after the reaction 2 S 2 o 3 (50ml), stirred at normal temperature for 20 minutes to end the reaction. Layer separation was then performed to recover the organic layer. The aqueous layer was washed twice with toluene so
Embodiment 3
[0119] Embodiment 3: Utilize silica-TEMPO to manufacture the compound of chemical formula 1
[0120] 2-(4R,6S)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-yl) tert-butyl acetate (2g, 0.008mol ) was dissolved in toluene solvent (80ml), cooled at -15°C, and then added sodium bicarbonate (NaHCO 3 , 3.0g, 0.035mol). Pay attention to exothermic, add sodium bromide (NaBr, 0.78g, 0.008mol) at the same time, then, keep the temperature below -10 ℃, slowly add sodium hypochlorite (NaClO, 6.3ml, 0.012mol).
[0121] Then, keeping the temperature below -5°C, slowly add dropwise the compound of the chemical formula 6, silica-TEMPO (0.04 equivalent, 0.615g, 0.3mmol) dissolved in toluene solvent (40ml) into the mixed solution , and then stirred for 2.5 hours while keeping the temperature below -5°C, and put Na 2 S 2 o 3 (50ml), stirred at normal temperature for 20 minutes to end the reaction, and then separated the layers to recover the organic layer. The aqueous layer was washed twice with
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap