Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
7 results about "Side reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A side reaction is a chemical reaction that occurs at the same time as the actual main reaction, but to a lesser extent. It leads to the formation of by-product, so that the yield of main product is reduced: A+B->[k₁]P₁ A+C->[k₂]P₂ P₁ is the main product if k₁> k₂. The by-product P₂ is generally undesirable and must be separated from the actual main product (usually in a costly process).
Wound repair composition and preparation method and application thereof
ActiveCN102526121APromote growthRelieve painOrganic active ingredientsBacteria material medical ingredientsTissue repairBiocompatibility Testing
The invention provides a wound repair composition. A formula of the wound repair composition comprises the following components in percentage by weight: 10-50 percent of chitosan mucilage, 10-50 percent of an extracting solution of a staphylococcus aureus metabolic product and 0-80 percent of medicament auxiliary materials, wherein the chitosan mucilage is a solution consisting of chitosan and an aqueous solution of an acid; the volume concentration of the acid in the aqueous solution of the acid is 0.8-1.2 percent; and the mass concentration of the chitosan in the chitosan mucilage is 1-3 percent. The invention further provides a preparation method of the wound repair composition and an application thereof as a medicament for treating skin or mucosal injure. The wound repair composition provided by the invention has the advantages of capabilities of promoting tissue repair, promoting wound healing and promoting the growth of epidermal cells, good infection inhibiting effect, high biocompatibility, realization of flat healed wounds, freeness from adverse side reactions, relieving in patient pain, convenience for nursing and using, easiness for storing, and the like.
Owner:SHANGHAI RUNZHI BIOTECH
Preparation method for trelagliptin and salt thereof
InactiveCN105985316AReduce generationSimple separation and purification methodOrganic chemistryAcetic acidOrganic solvent
The invention discloses a preparation method for trelagliptin and a salt thereof. The preparation method comprises the following steps: a, subjecting a compound 3, Pd(OAc)2, ligand, K3PO4, 3-tertbutyloxycarbonyl-aminopiperidine, and an organic solvent to a reaction with stirring under the protection of nitrogen so as to obtain a reaction solution; b, carrying out separation and purification so as to obtain a compound 6; c, subjecting the compound 6, ethyl acetate and an ethyl acetate solution of HCl to a reaction with stirring and then carrying out separation and purification so as to obtain a solid; and d, dissolving the solid obtained in the step c in water, adjusting a pH value to 8 to 9 and then carrying out separation and purification so as to obtain a compound 4, i.e., trelagliptin. The preparation method provided by the invention reduces side reactions and production of impurity compounds; the method is simple and convenient in separating and purifying trelagliptin and has the advantages of short production period, high yield, high purity, low cost, etc.; and the yield of trelagliptin in the invention can reach 95% or above, and the preparation method is suitable for industrial production.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
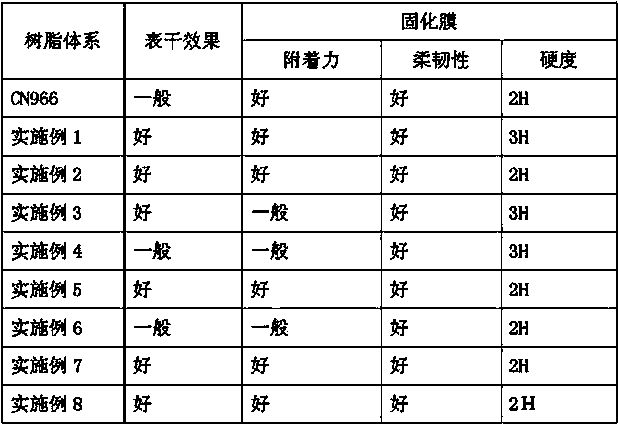
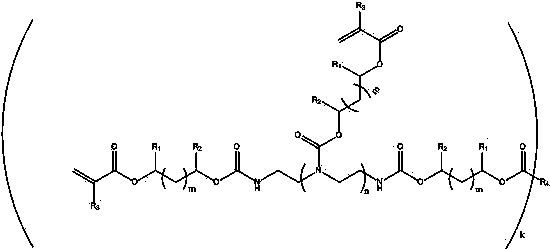
Nitrogen-containing multi-degree-of-functionality acrylate resin as well as preparation method and application thereof
ActiveCN104558600AImprove flexibilityHigh photopolymerization reactivityCarbamic acid derivatives preparationOrganic compound preparationPolymer scienceMeth-
Owner:LUCKY HUAGUANG GRAPHICS
Preparation method of pyroxsulam intermediate
ActiveCN113292487AAvoid Polymerization Side ReactionsGuaranteed purityOrganic compound preparationCarboxylic acid esters preparationAcyl groupDouble bond
The invention discloses a preparation method of a pyroxsulam intermediate, which comprises the following steps: (1) carrying out condensation reaction on 4-alkoxy-1,1,1-trifluoro-3-buten-2-one and phosphonoacetic acid trialkyl ester in an alcohol solvent in the presence of sodium alcoholate to generate an intermediate 3-trifluoromethyl-5,5-dialkoxy pentenoic acid alkyl ester and an isomer of the intermediate 3-trifluoromethyl-5,5-dialkoxy pentenoic acid alkyl ester; and (2) carrying out cyclization reaction on the intermediate generated in the step (1) and ammonium acetate in the presence of a polymerization inhibition catalyst to generate 2-hydroxy-4-trifluoromethylpyridine. According to the method disclosed by the invention, hydroquinone and other polymerization inhibition catalysts are added in the cyclization process, so that polymerization side reaction of a condensation intermediate containing double bonds can be avoided, and the reaction yield and the product purity of the cyclization reaction are ensured. Meanwhile, the 4-butoxy-1,1,1-trifluoro-3-buten-2-one and the phosphonoacetic acid trimethyl ester are adopted as the condensation reaction raw materials, so that not only can higher condensation reaction yield be obtained, but also the cost is lower, and the safety is higher.
Owner:JIANGSU AGROCHEM LAB CO LTD
Catalyst-based chlorinated neopentane preparation technology
InactiveCN103265399ASolve the problem of inability to produce high content of chloropentaneSimple processPreparation by halogen halide additionPhosphorous acidReaction temperature
Owner:ZHANGJIAGANG CITY ZHENFANG CHEM
Synthesis method of olmesartan intermediate
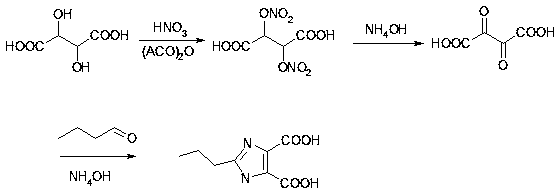
ActiveCN111170946AHigh yieldBasic raw materials are cheapOrganic chemistrySide reactionDe esterification
The invention relates to the field of drug intermediate synthesis, in particular to a synthesis method of an olmesartan intermediate, which is characterized in that tartaric acid is used as a raw material, and the olmesartan intermediate is prepared by cyclization reaction, esterification reaction and methylation reaction. According to the synthesis method of the olmesartan intermediate, the totalyield can reach 60% or above, the yield is high, and economic benefits are high; the synthesis route is simple, basic raw materials are cheaper, side reactions are few, and purification is easy; andthe whole steps are easy to control, the method is more suitable for industrial application from raw materials to production, selection of ingredients and determination of the addition amount, and theproduction cost is low.
Owner:ZHEJIANG RUIQING PHARM C0 LTD
Semi-synthesis method of aristololactam
The invention discloses a semi-synthesis method of aristololactam. Aristolochic acid (AAs) is use as a raw material, and the nitro thereof is reduced into an imino group to obtain corresponding aristololactam (ALs); and after separation and purification, the obtained product can be used as a traditional Chinese medicine control product. The semi-synthesis method of aristololactam disclosed by the invention has the advantages of simple process, few side reactions, high yield and high product purity.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Popular searches
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap