Nitrogen-containing multi-degree-of-functionality acrylate resin as well as preparation method and application thereof
An acrylate, multifunctional technology, applied in the field of photosensitive polymer materials, can solve the problems of isocyanates with irritating odor, increased film brittleness, skin irritation, etc., to achieve high reactivity, reduce stress shrinkage, and improve adhesion. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation method of nitrogen-containing polyfunctionality acrylate resin of the present invention is as follows: comprise the following steps:
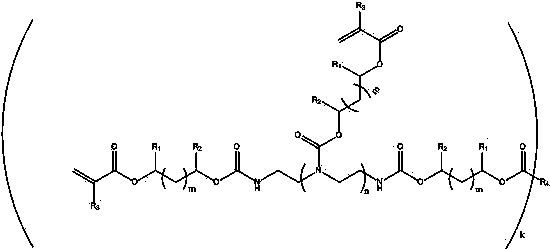
[0041] (1) Add the amine compound shown in the formula (II) into the reaction kettle, and then add the cyclocarbonate compound shown in the formula (III) under nitrogen atmosphere, wherein the cyclocarbonate compound and the nitrogen atom in the amine compound The molar ratio is 1:1; the temperature rises after the feeding is completed, and the reaction temperature is 60-120 o C, after the reaction finishes, lower the temperature, and the reaction generates an intermediate product shown in the following formula (IV):
[0042] (II)
[0043] Where n is an integer from 0 to 16, such as ethylenediamine, diethylenetriamine, triethylenetetramine, tetraethylenepentamine, pentaethylenehexamine, hexaethyleneheptamine, heptaethyleneoctamine, octaethylenenonamine, nine Ethylene Decylamine, Decaethylene Undecylamine, Undecylethylen
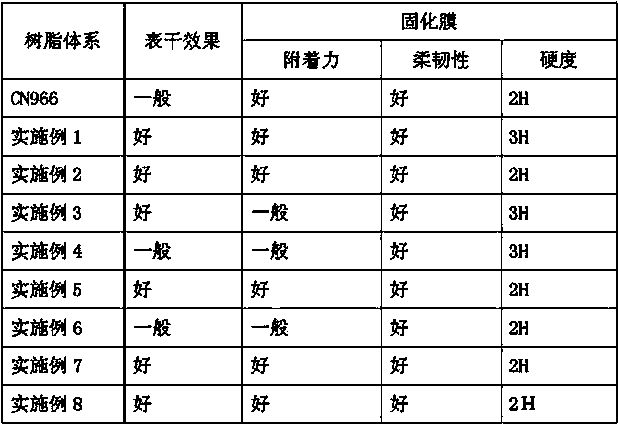
Embodiment 1
[0056] Add 100 parts of diethylenetriamine to a 5 L double-layer glass reactor equipped with a mechanical stirrer, thermometer, sampling port, gas inlet, constant pressure dropping funnel, water separator, vacuum joint, and vacuum jacket condensation column. After feeding nitrogen for 10 minutes, slowly add 300 parts of ethylene carbonate, while keeping the reaction kettle under nitrogen atmosphere and the temperature does not exceed 50 o c. After the addition was complete, the temperature of the reactor was slowly raised to 80 o c. Monitor the reaction by infrared, when 1805cm -1 After the characteristic absorption peak of cyclocarbonate disappeared completely, the reaction was continued for 0.5 hour to complete the reaction, and the temperature was lowered.
[0057] When the temperature of the reactor dropped to 40 o When C is below, add 5 parts of p-hydroxyanisole, 3 parts of p-toluenesulfonic acid, 500 parts of cyclohexane and 200 parts of acrylic acid into the reactor.
Embodiment 2
[0061] Add 100 parts of triethylenetetramine to a 5-L double-layer glass reactor equipped with a mechanical stirrer, thermometer, sampling port, gas inlet, constant pressure dropping funnel, water separator, vacuum joint, and vacuum jacket condensation column. After feeding nitrogen for 10 minutes, slowly add 400 parts of ethylene carbonate, while keeping the reaction kettle under nitrogen atmosphere and the temperature does not exceed 50 o c. After the addition was complete, the temperature of the reactor was slowly raised to 80 o c. The reaction was monitored by infrared, when 1805 cm -1 After the characteristic absorption peak of cyclocarbonate disappeared completely, the reaction was continued for 0.5 hour to complete the reaction, and the temperature was lowered.
[0062] When the temperature of the reactor dropped to 40 o When C is below, add 2 parts of p-hydroxyanisole, 5 parts of p-toluenesulfonic acid, 500 parts of cyclohexane and 300 parts of acrylic acid into the
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap