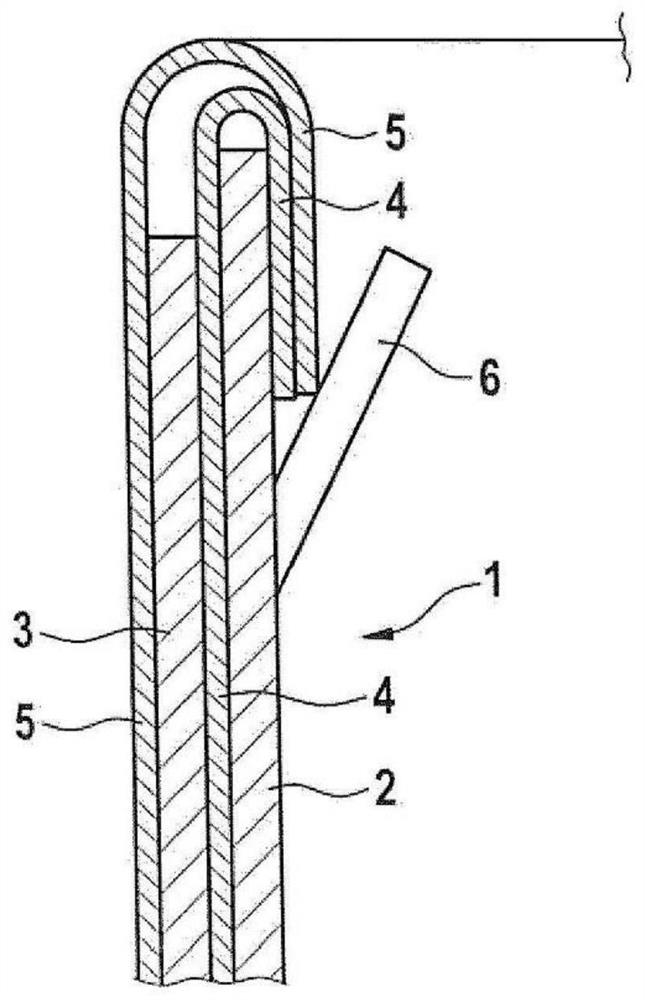
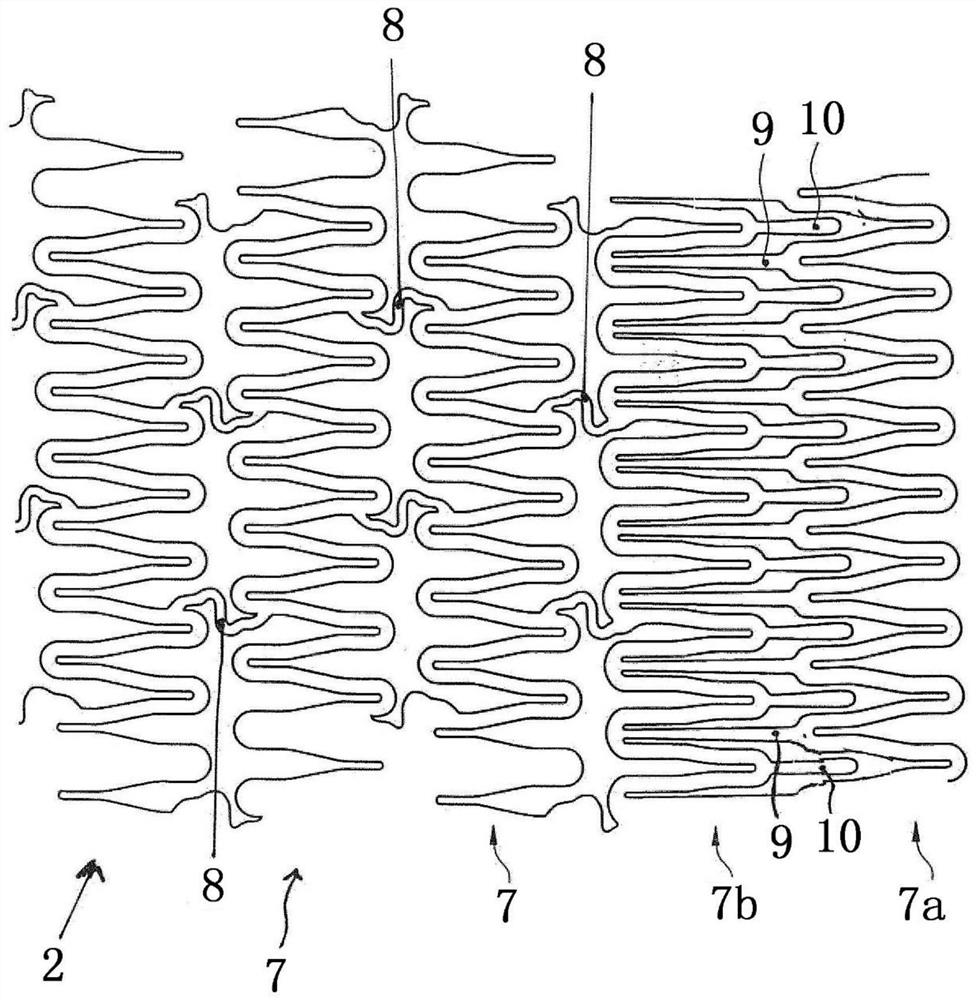
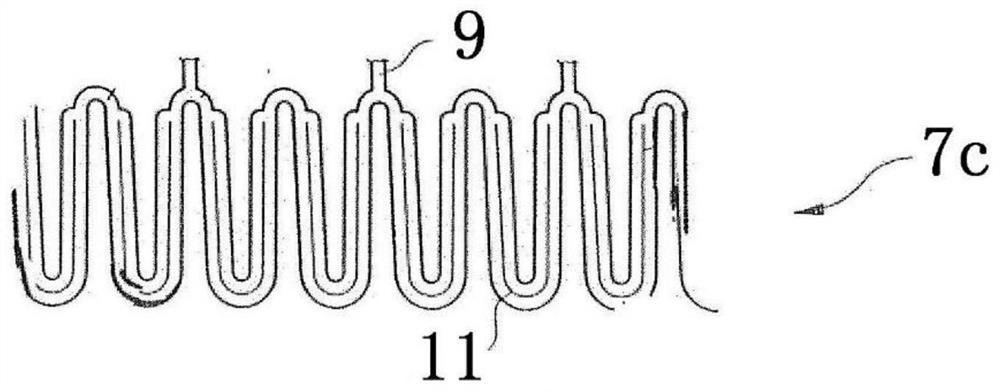
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34results about "Stents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
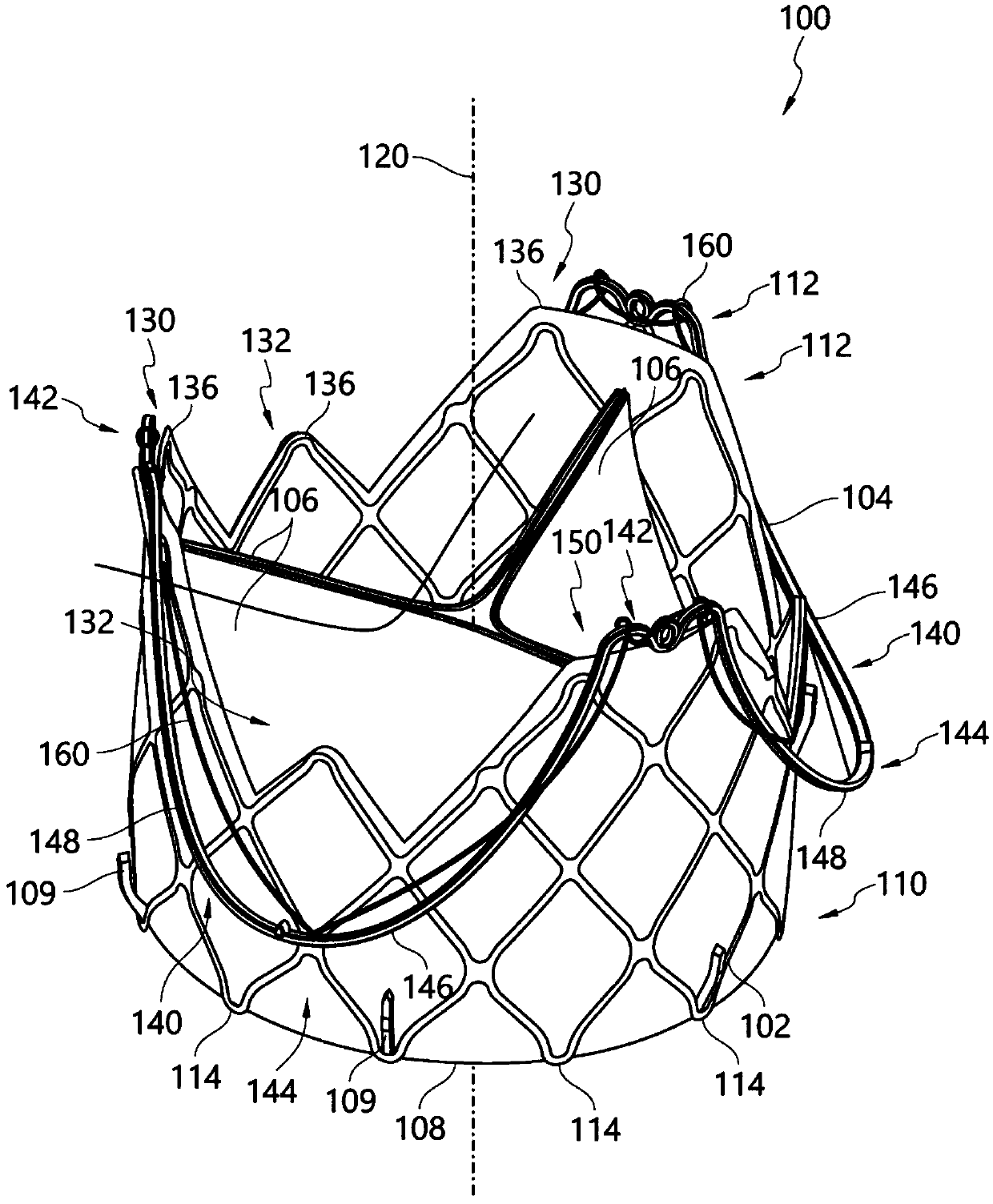
Quick-connect prosthetic heart valve and methods
Owner:EDWARDS LIFESCIENCES CORP
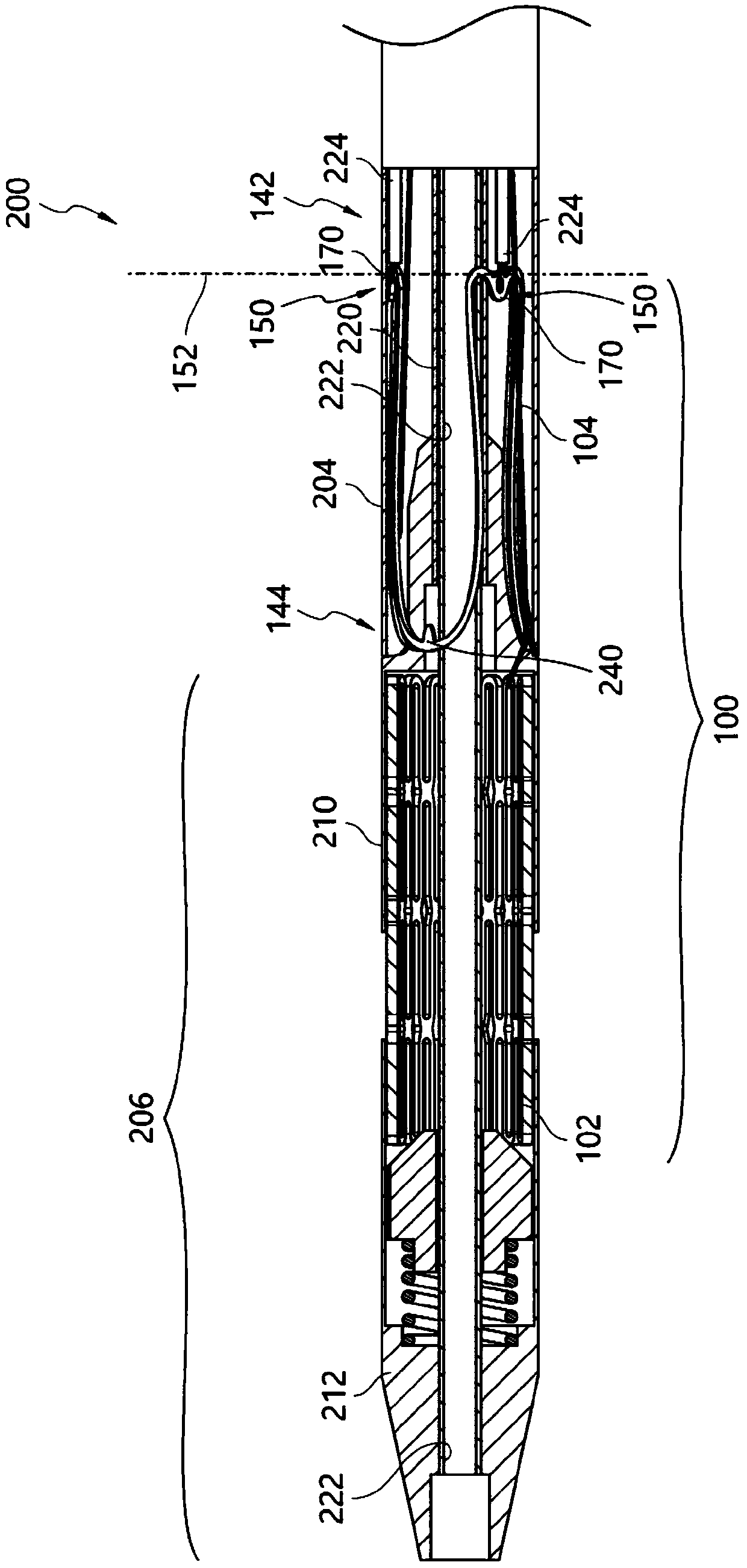
Stent delivery device and method
Owner:BOSTON SCI SCIMED INC
Devices, systems, and methods for reshaping a heart valve annulus, including the use of magnetic tools
Owner:VENTURE LENDING & LEASING IV
Eluting, implantable medical device
Owner:COOK INC
Stent
Owner:BIOTRONIK MESS UND THERAPIEGERAETE GMBH & CO
Medical devices having biodegradable polymeric regions
Owner:BOSTON SCI SCIMED INC
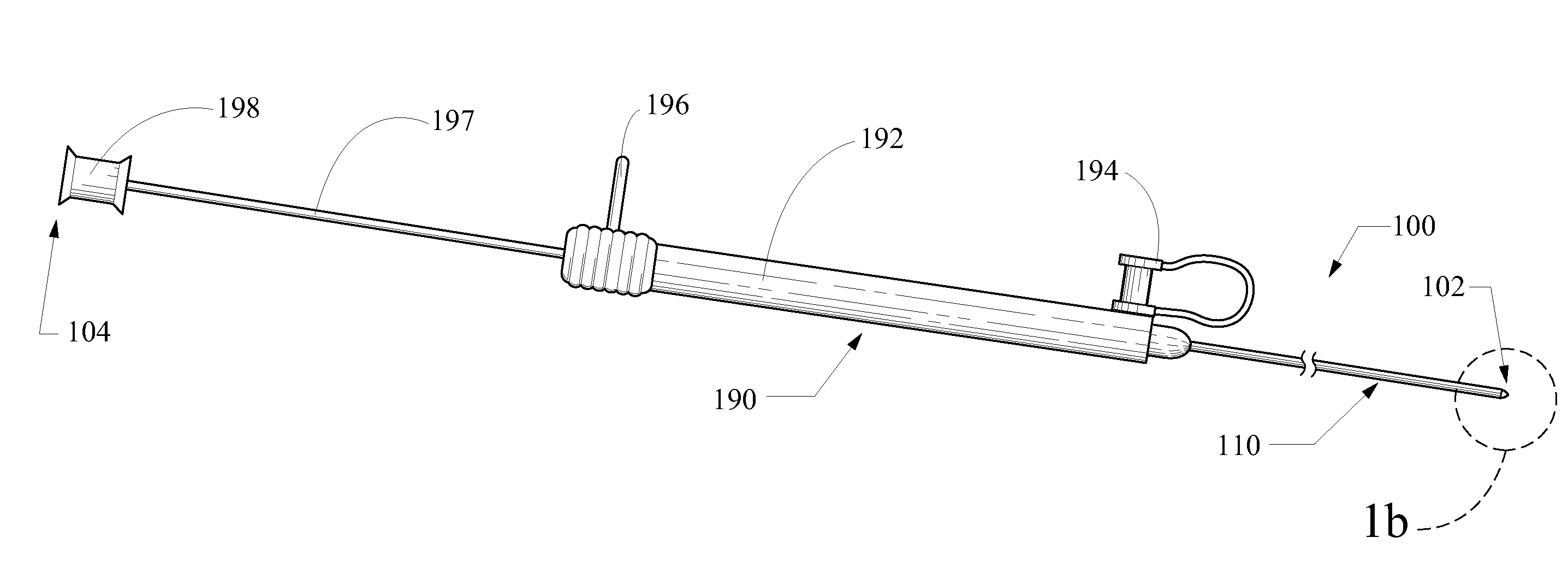
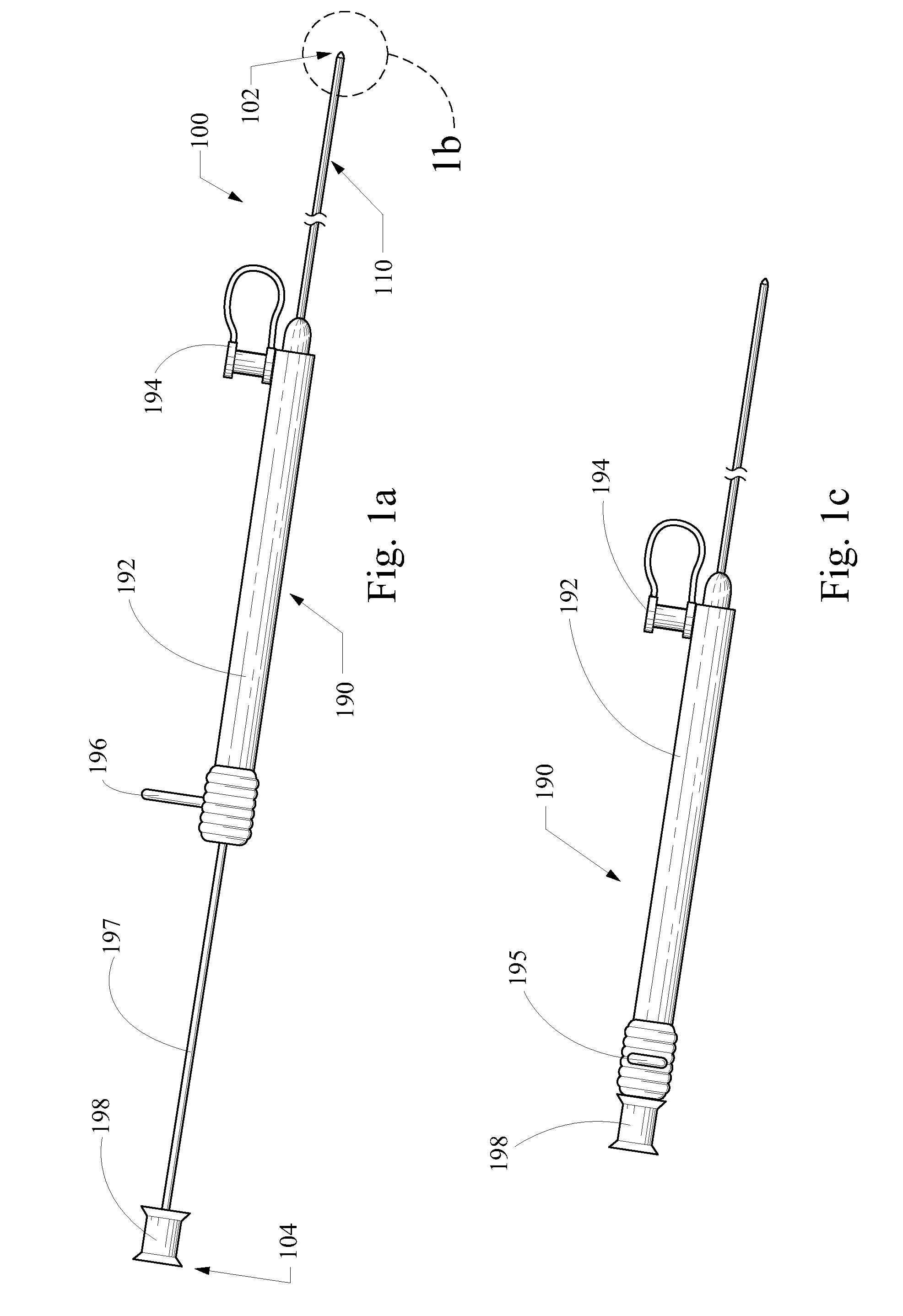
Syringe assembly for a catheter
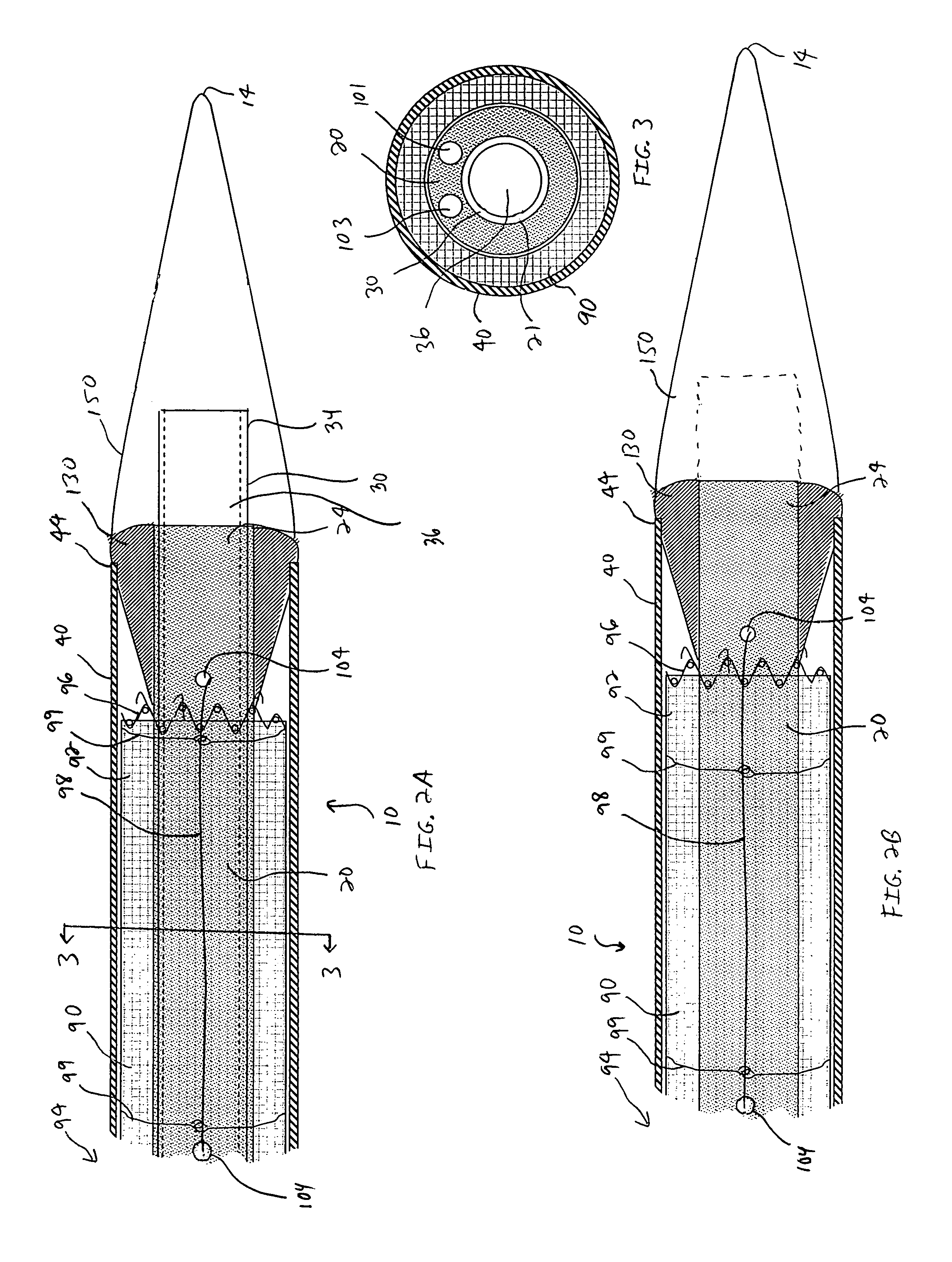
A substance delivery apparatus for a catheter assembly is provided. The delivery apparatus includes a base and a needle connected to the base. The needle is capable of pivotally moving with respect to the base for penetrating into tissues of a passageway for administering a therapeutic or bioactive substance to the subject. The needle can pivot in response to inflation of a balloon incorporated with the catheter assembly.
Owner:ABBOTT CARDIOVASCULAR
Devices and methods for treating aortic valve stenosis
Fluid delivery devices having a porous applicator, as well as methods for using the same in the treatment of aortic valve stenosis, are provided. The subject devices further include a ventricular occlusion balloon and a compliant element that is configured to envelope the occlusion balloon. Also provided are systems and kits that include the subject fluid delivery devices.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Apparatus & method for determining physiologic characteristics of body lumens
Disclosed are a system, apparatus, and method for determining a physiologic characteristic of a body lumen that include determining, at one or more selected pressures, the volume of incompressible medium infused into a balloon 54 of catheter 24 while the balloon is placed in each of (a) a lumen having a predetermined, fixed diameter and (b) a desired location of a body lumen having an unknown diameter. In certain variations, the physiologic characteristic (e.g., diameter, cross-sectional area) is determined by calculating the difference in the volume of infused medium between (a) and (b) at at least one static pressure. Other physiologic characteristics (e.g., compliance) are determined by calculating the difference in infused medium for (b) at at least two static pressures.
Owner:ANGIOMETRX
Vascular prothesis having flexible configuration
InactiveUS20050033410A1Increase flexibilityReduced delivery profileStentsBlood vesselsBiomedical engineeringHinge point
Owner:NOVOSTENT CORP
Stent Delivery System for Detecting Wall Apposition of the Stent During Deployment
Owner:MEDTRONIC VASCULAR INC
Steerable endovascular graft delivery system
Owner:LIFESHIELD SCI
Mechanical propulsion catheter apparatus and methods
Owner:MICHIGAN SKUNK WORKS LLC +1
Heart valve prosthesis delivery system
PendingCN110013358AFor proper placementFacilitated releaseStentsBalloon catheterRat heartDelivery system
Owner:SUZHOU JIECHENG MEDICAL INC
Patient-specific intraluminal implants
ActiveUS20140088698A1Increase opportunitiesPrecise positioningStentsAdditive manufacturing apparatusProsthesisPatient specific
Owner:MATERIALISE NV
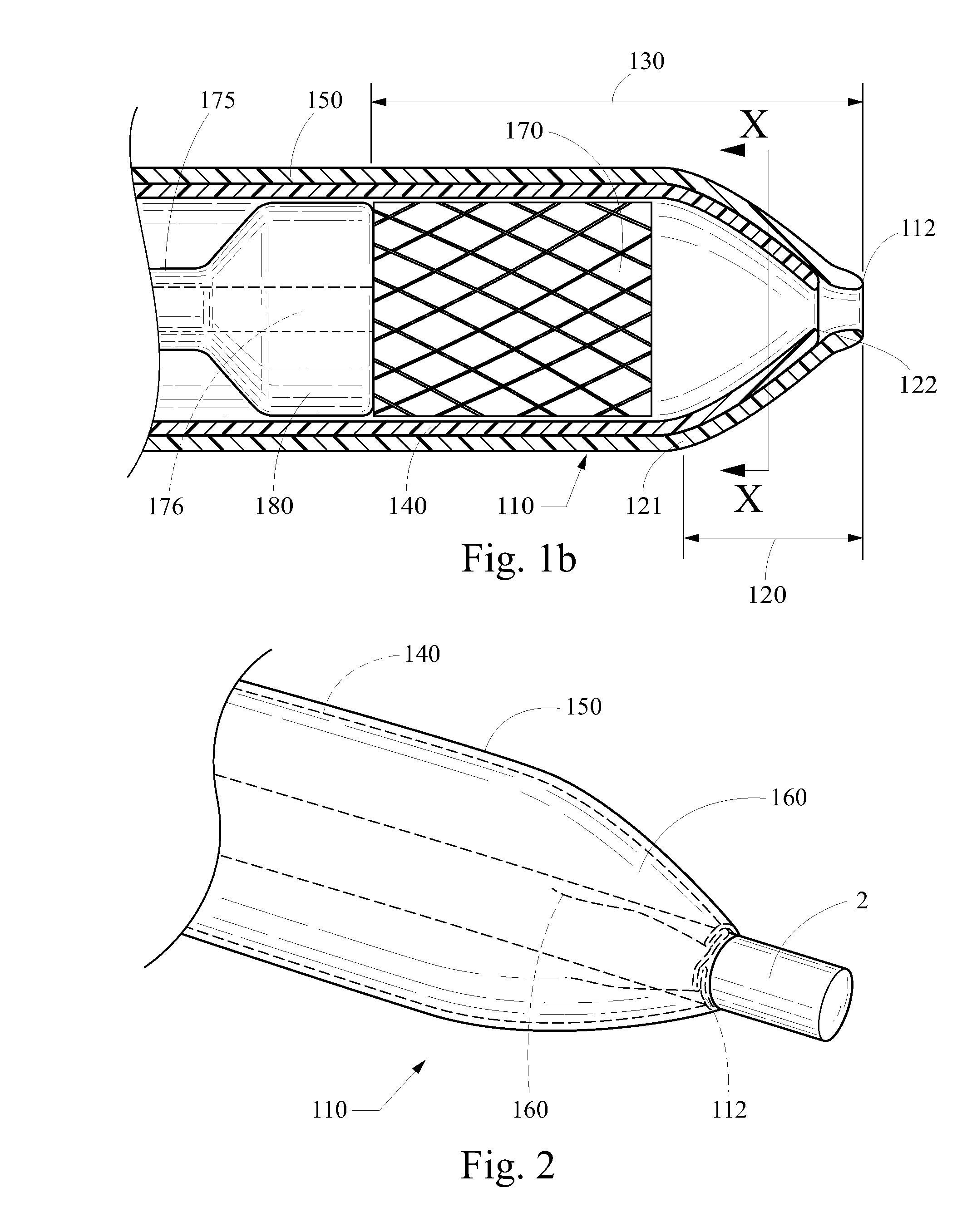
Stent delivery and retention apparatus
A stent delivery system comprises an inner member and an expandable balloon mounted in a collapsed state on the inner member, the expandable balloon having a first and a second end. A compressible stent having a first diameter is mounted in a compressed state around the expandable balloon between the first and second ends of the balloon. At least a first retainer pillow is formed in the expandable balloon at its first end and has an outer diameter which is at least substantially equal to the diameter of the compressed stent. A first pillow support member is mounted on the inner member and supports the first retainer pillow to maintain the pillow's outer diameter.
Owner:MEDTRONIC VASCULAR INC
Stent graft
Owner:COOK MEDICAL TECH LLC
Dilation stent system
Owner:青岛智辰生物科技有限公司
Branched frozen elephant trunk device and method
Owner:COOK MEDICAL TECH LLC
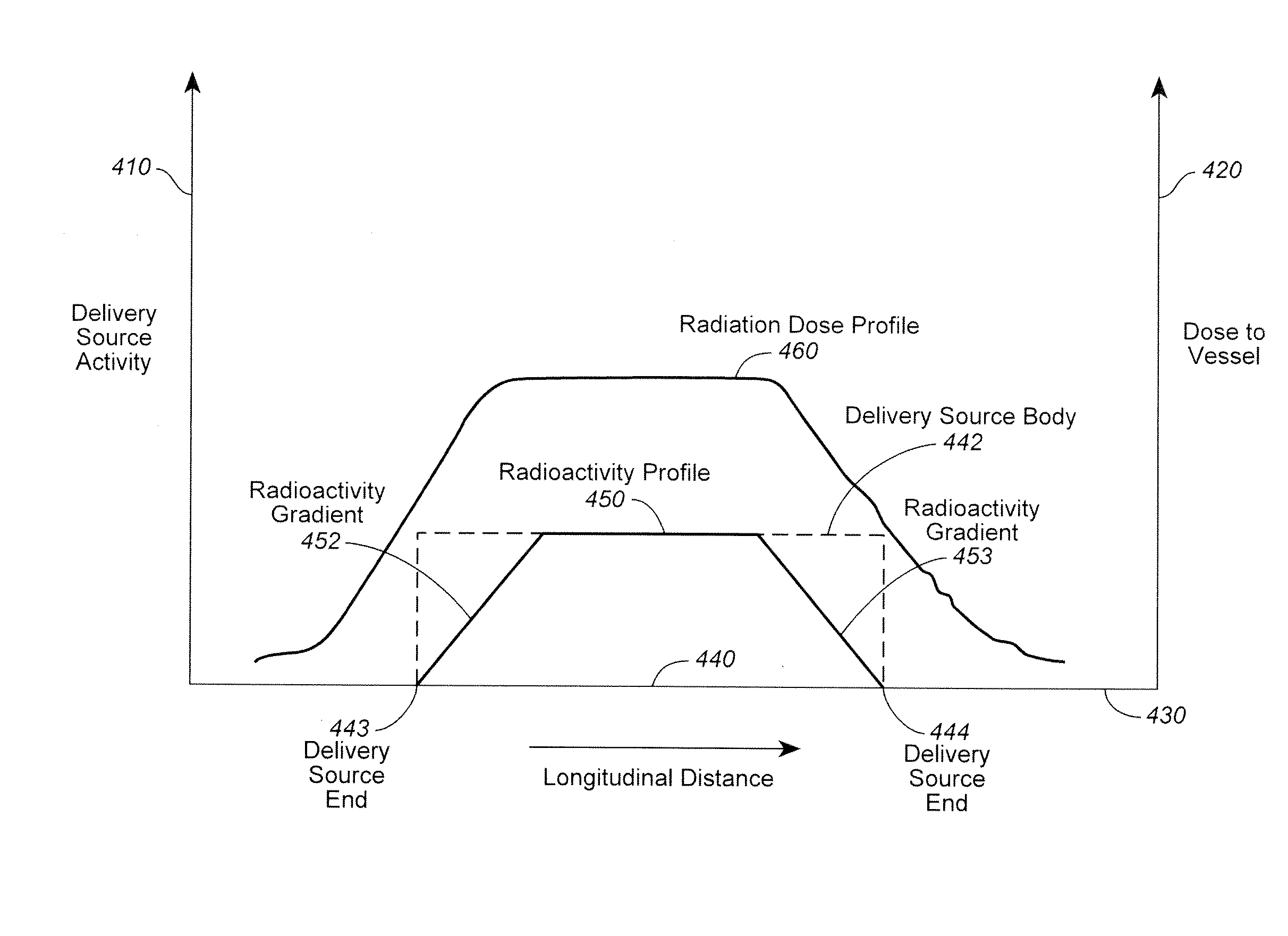
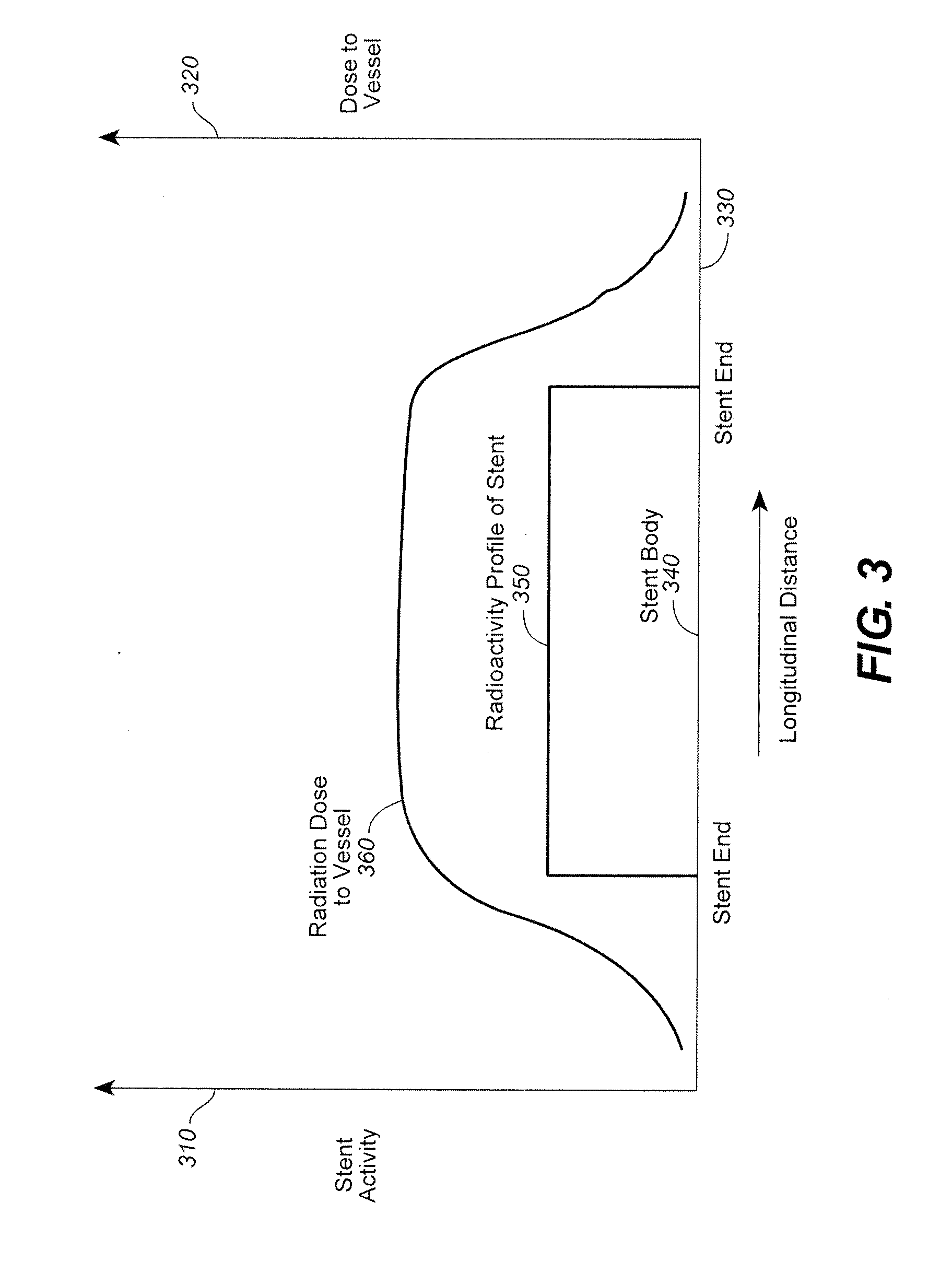
Radiation or drug delivery source with activity gradient to minimize edge effects
Owner:ABBOTT CARDIOVASCULAR
Stent assembly
A stent assembly including an upstream portion adapted to modify a flow characteristic of embolic material disposed in a blood stream flowing through the upstream portion, and a downstream portion in fluid communication with the upstream portion and adapted for the blood stream to flow therethrough, the downstream portion including a trapping region for trapping therein the embolic material. The downstream portion may extend from the upstream portion, or alternatively, may be distanced from the upstream portion.
Owner:NESSTENT
Telescopic stent for esophagus
Owner:HENAN UNIV OF SCI & TECH
Stent for Protecting Bifurcated Blood Vessels in Bifurcation Lesion
InactiveUS20120283821A1Precise positioningEasy and accurate mannerStentsDilatorsInsertion stentGuide wires
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
Stent having expandable elements
Owner:MOB TECH GMBH
Degradable magnesium alloy stent
Owner:SHANGHAI KINDLY MEDICAL INSTR CO LTD +1
Device and method for safely positioning a coronary stent in the coronary arteries
The invention is intended for use in medicine and enables maximally precise, quick and safe positioning of a coronary stent in the case of uncomplicated and complicated anatomical injuries of the coronary vascular bed, and also when stenting renal and visceral arteries. The claimed device consists of a body with a truncated anterior portion and a cylindrical posterior portion, which are interconnected such that the cylindrical posterior portion of the body is rotatable about its own axis. The inside surface of the cylindrical posterior portion of the body is provided with a thread that is capable of engaging a lip of a slider disposed on guides inside the posterior portion of the body. The slider is connected to a barrel of a gripper having vanes with elastic elements at a delivery systemgripping point such that the gripper is rotatable about its own axis, wherein the vanes of the gripper have protuberances disposed in a clamping cylinder which is arranged inside the body of the device and squeezes and releases the vanes of the gripper under the action of a release member consisting of a pressure surface on the outer truncated portion of the body and vanes arranged inside the bodyat an angle to one another and to the clamping cylinder, respectively releasing or locking the delivery system in the device. When preparing the device for use, the coronary stent is first inserted on a delivery system into the device; the coronary stent is then manually manoeuvred on the delivery system to the injured site of the coronary artery, the delivery system is locked by the vanes of thegripper, and the stent is moved in a longitudinal direction on the delivery system and positioned in the coronary artery by circumferential rotation of the cylindrical posterior portion of the device.
Owner:SEVEN SONS LTD R N 515985570 THECO
Carotid artery in-situ fenestration balloon sheath
InactiveCN114367000ANot easy to oppressPrecise positioningStentsBalloon catheterBiomedical engineeringGeneral surgery
The invention provides a carotid artery in-situ fenestration balloon sheath. The carotid artery in-situ fenestration balloon sheath comprises a balloon sheath body, a sheath core and an injection device. The balloon sheath body is of a hollow structure with a through head and tail and comprises a first balloon, a sheath tube front section, a second balloon, a sheath tube rear section and a sheath tail, and the first balloon is an annular balloon and can expand outwards after being filled; the second balloon is of a hollow flexible bendable structure; the sheath tube front section, the sheath tube rear section and the sheath tail are of hollow tubular structures; the first balloon, the sheath tube front section, the second balloon, the sheath tube rear section and the sheath tail are fixedly connected and communicated in sequence; a third balloon is fixed in the sheath tail; the sheath core is of a hollow structure with holes in the head and the tail, and the head of the sheath core is conical. The sheath core is inserted into the balloon sheath body through the third balloon, and the head of the sheath core penetrates out of the first balloon; the injection device is communicated with the first balloon, the second balloon and the third balloon and is responsible for injecting liquid into the first balloon, the second balloon and the third balloon; after the third balloon is filled, the inner wall of the balloon is tightly attached to the sheath core.
Owner:NANJING DRUM TOWER HOSPITAL
Double stent
Owner:BENTLEY INNOMED
Single use devices with integrated vision capabilities
PendingUS20220000341A1Improve reliabilityMinimally invasiveStentsSurgical needlesVisual functionSingle-Use Device
An integrated single use device with vision capabilities is provided. The device may comprise: an endoscope comprising: i) a disposable elongate member comprising a proximal end and a distal end and ii) a camera module located at the distal end, and the proximal end is removably attached to a supporting member; and one or more disposable instruments integrated to the endoscope, and the device is configured to perform functions of both the endoscope and the one or more disposable instruments.
Owner:NOAH MEDICAL CORP
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap