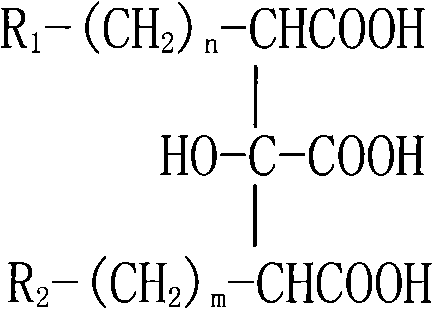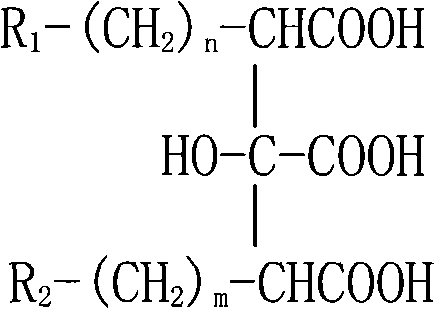Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
71 results about "Medicinal chemistry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medicinal chemistry and pharmaceutical chemistry are disciplines at the intersection of chemistry, especially synthetic organic chemistry, and pharmacology and various other biological specialties, where they are involved with design, chemical synthesis and development for market of pharmaceutical agents, or bio-active molecules (drugs).
Combination therapy
InactiveUS20150165021A1Enhance immune responseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsPharmaceutical drugINKT Cells
Owner:NKT THERAPEUTICS
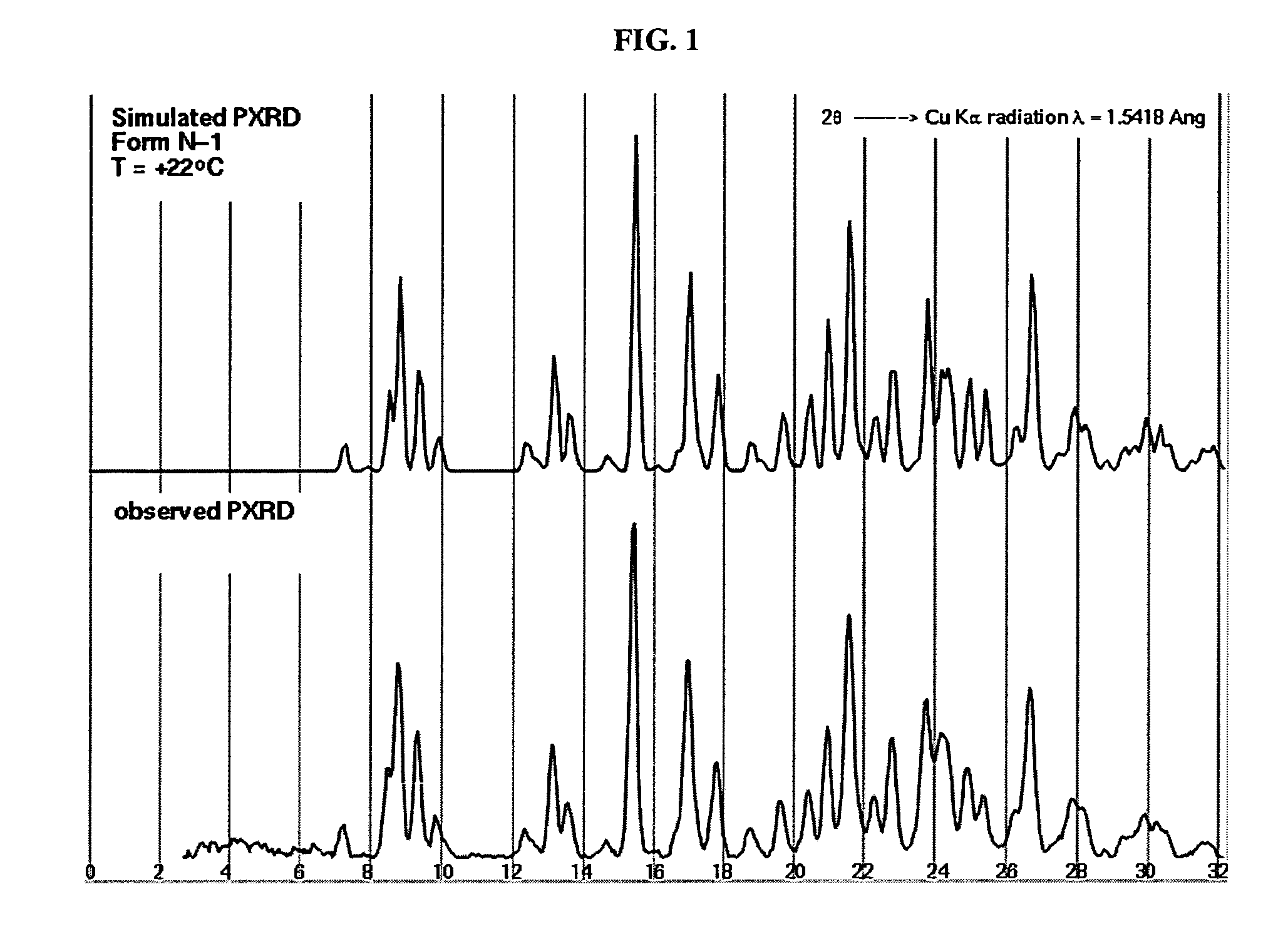
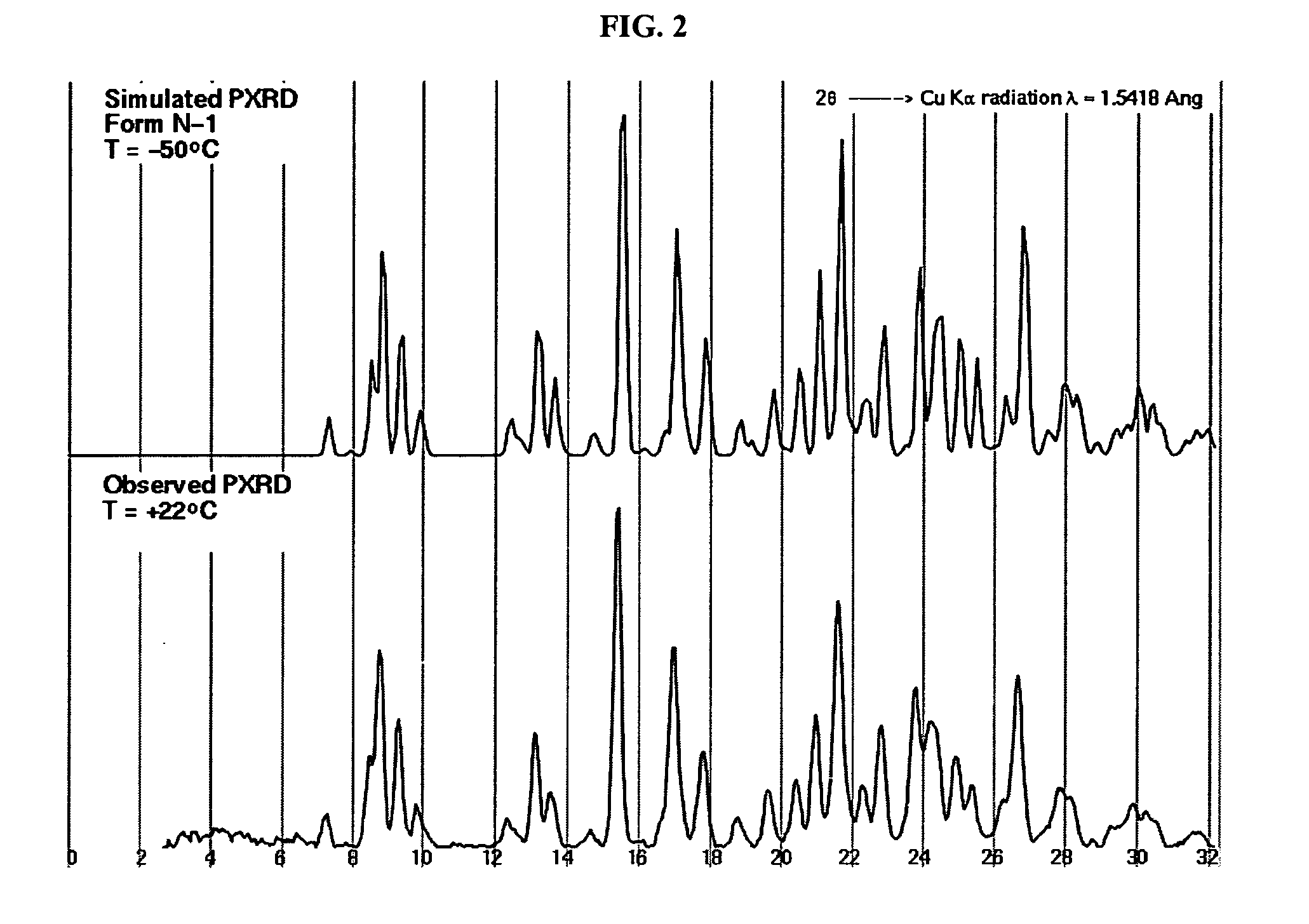
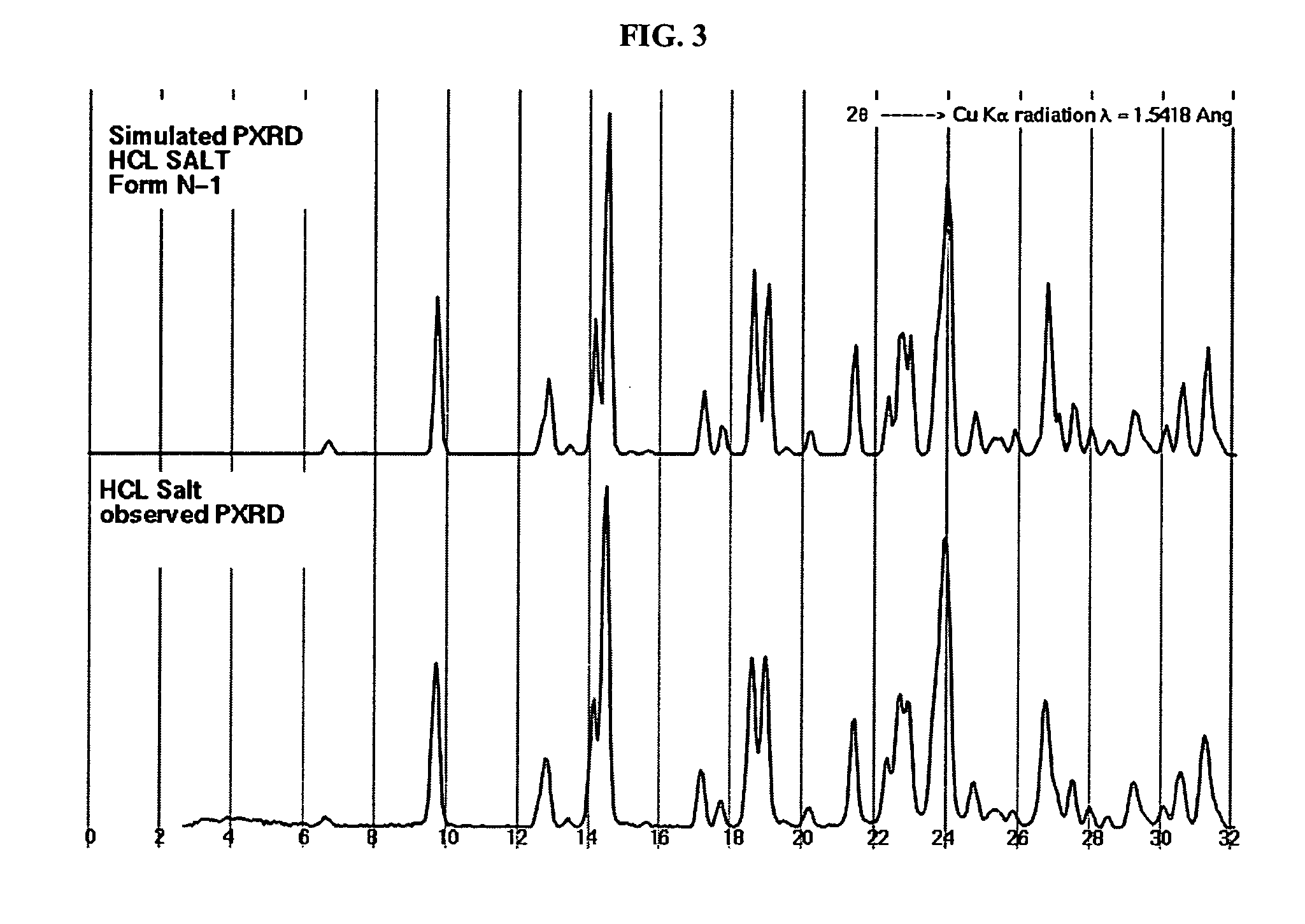
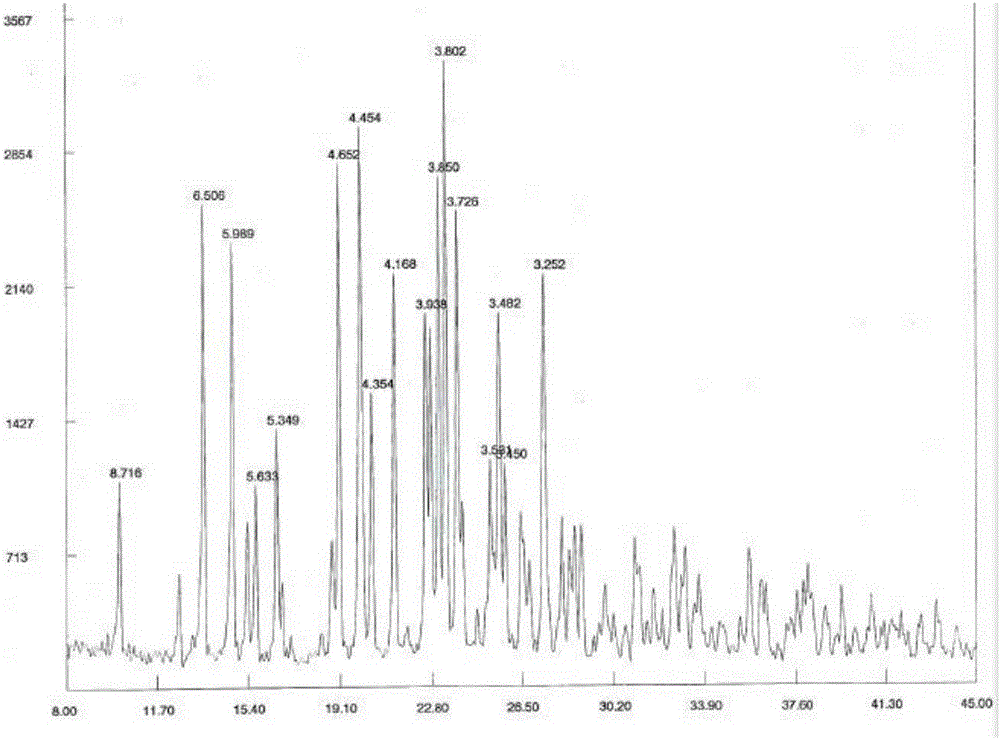
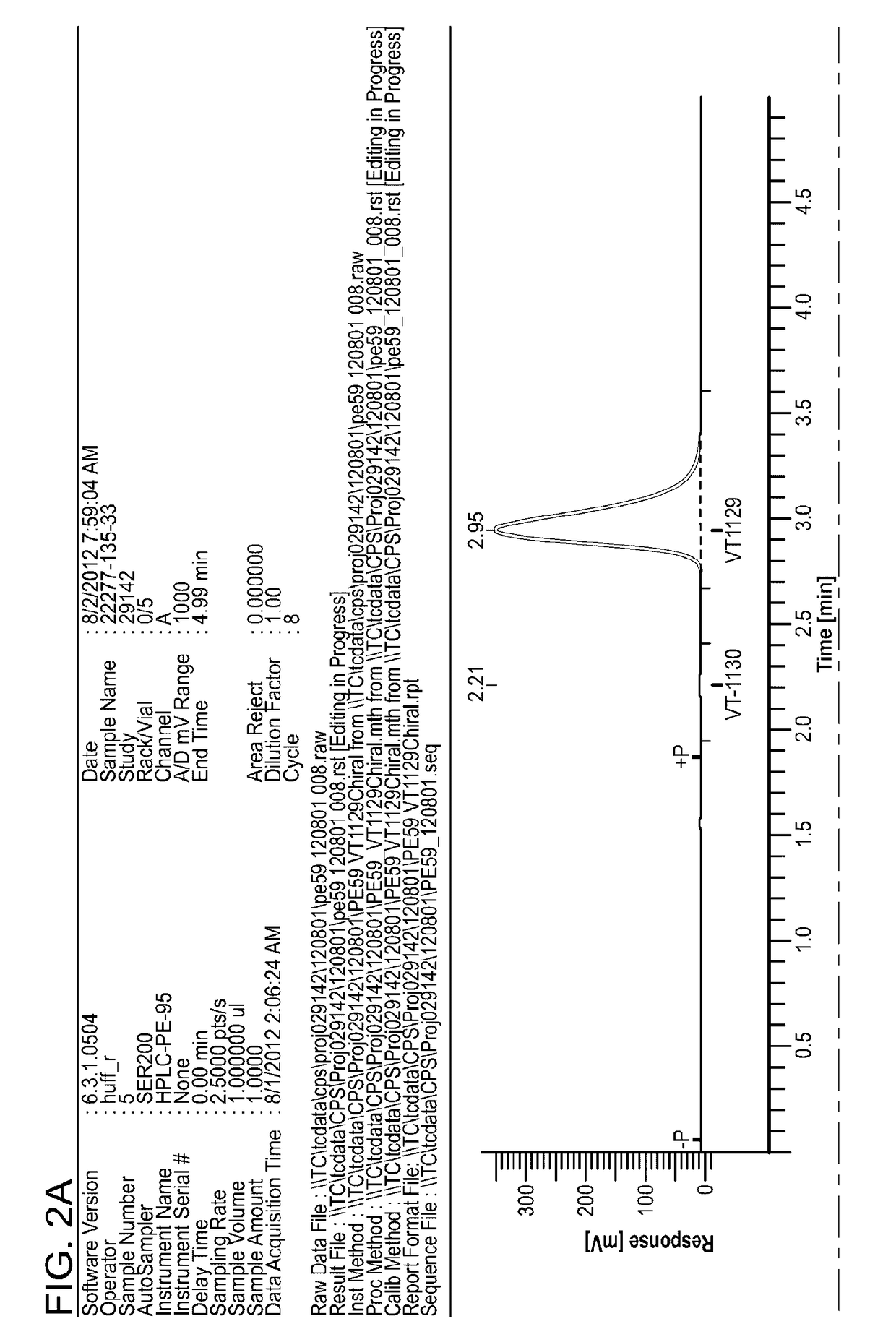
Crystalline forms and process for preparing spiro-hydantoin compounds
Owner:BRISTOL MYERS SQUIBB CO
Cariprazine tartrate, preparation method therefor and medical use thereof
ActiveCN105218484ANot easy to absorb moistureImprove solubilityNervous disorderOrganic chemistry methodsCariprazinePharmaceutical drug
Owner:ANHUI HEALSTAR PHARM CO LTD
Antifungal compound process
Owner:MYCOVIA PHARMA INC +1
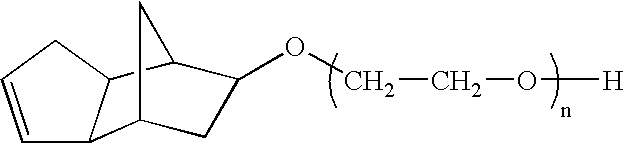
Dispersions containing alkoxylates of alicyclic polycyclic compounds
Owner:ETHOX CHEM LLC
Pentadienone compound containing quinazolinone aryloxy as well as preparation method and application thereof
The invention discloses a compound of resisting plant viruses: a pentadienone compound containing quinazolinone aryloxy and a preparation method and biological activity. The invention introduces a series of novel pentadienone derivatives containing quinazolinone aryloxy which are synthesized by six steps by taking substituted o-aminobenzoic acid, formamide, 35% formalin, 1, 4-dioxane, thionyl chloride, hydroxyl benzaldehyde, acetone, sodium hydroxide, hydrochloric acid, potassium carbonate, potassium iodide, substituted aromatic aldehyde, substituted heterocyclic aldehyde and the like as raw materials. The compound disclosed by the invention further has higher treating, protecting and passivating and inhibiting effects to cucumber mosaic virus (CMV), tobacco mosaic virus (TMV), southern rice black streaked dwarf virus (SRBSDV) and rice stripe virus (RSV), shows higher anti-plant virus activity, and can be used for preparing anti-plant virus pesticides.
Owner:GUIZHOU UNIV
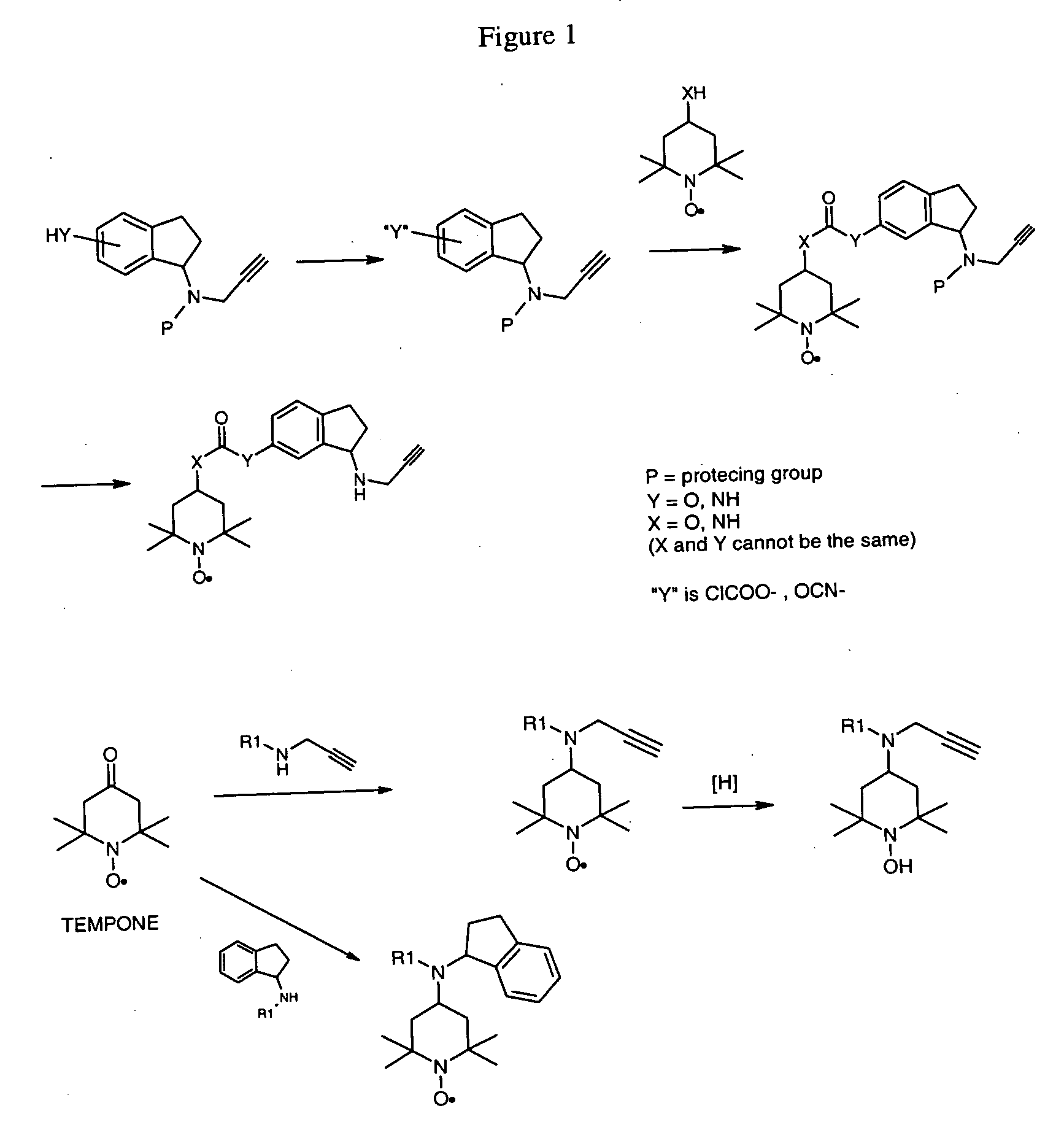
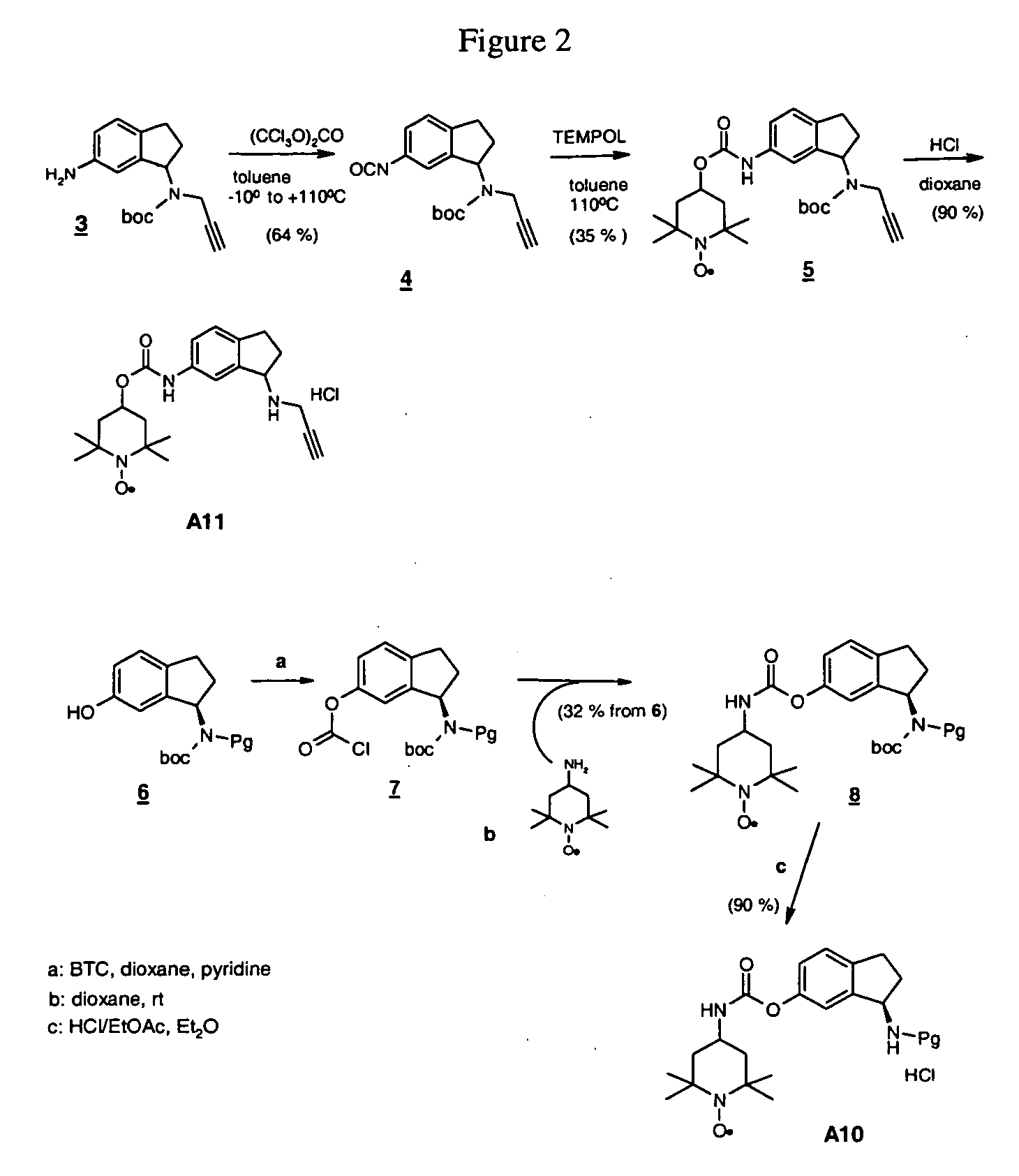
Propargyl nitroxides and indanyl nitroxides and their use for the treatment of neurologic diseases and disorders
Owner:TEVA PHARMA IND LTD
Propofol injection and its preparing method
InactiveCN101045042ANo side effectsLess irritatingHydroxy compound active ingredientsPharmaceutical delivery mechanismDisodium EdetateHigh pressure
Owner:SHANGHAI INST OF PHARMA IND
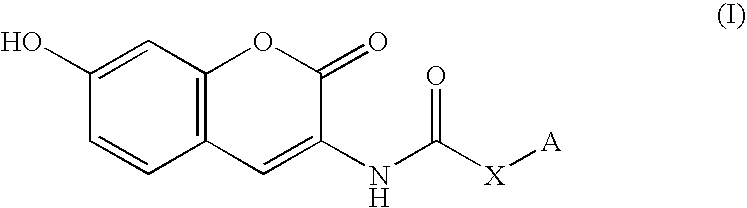
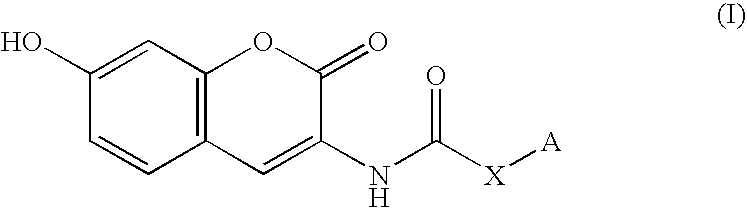
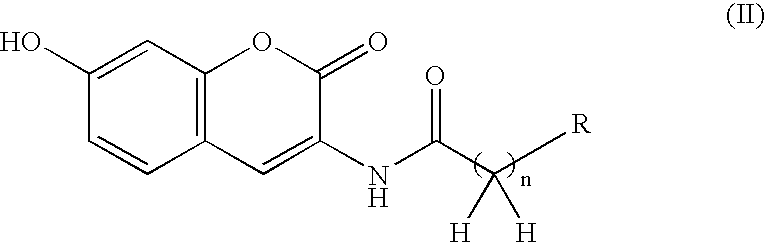
Coumarin derivative
InactiveUS20050054717A1Good effectEliminate side effectsBiocideHeavy metal active ingredientsArylSulfur
Owner:INST OF MEDICINAL MOLECULAR DESIGN
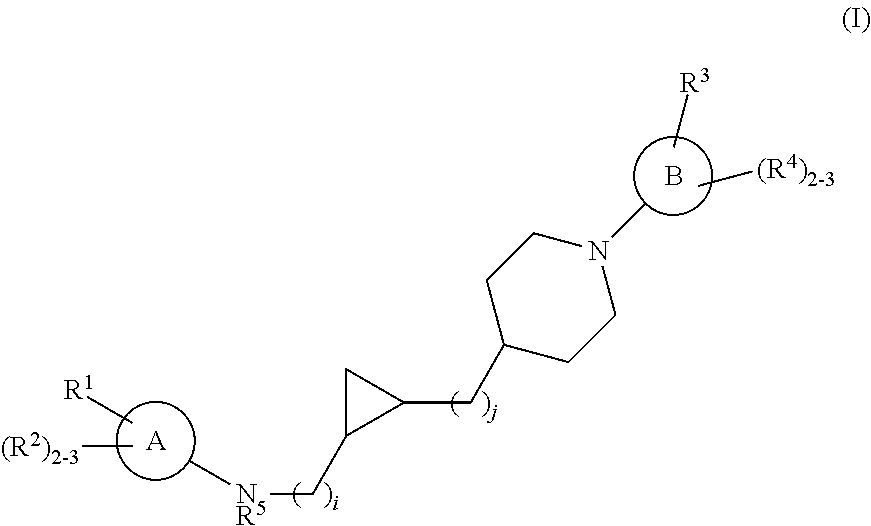
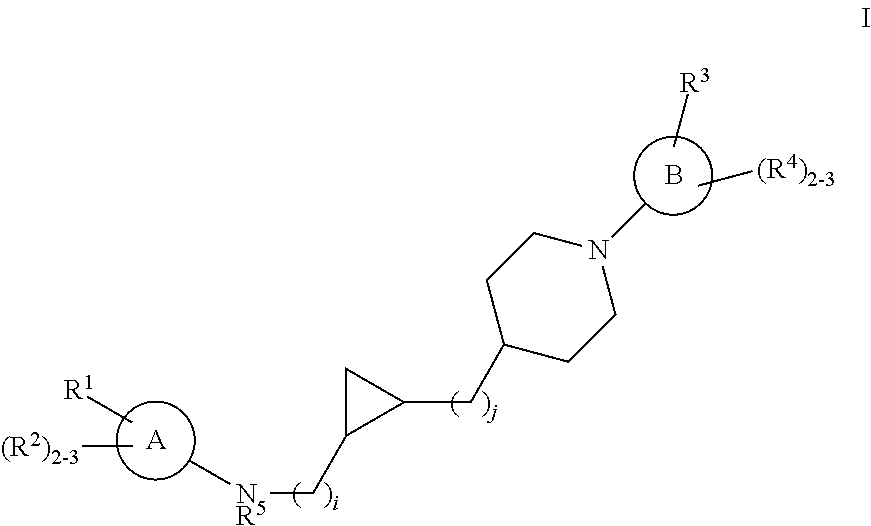
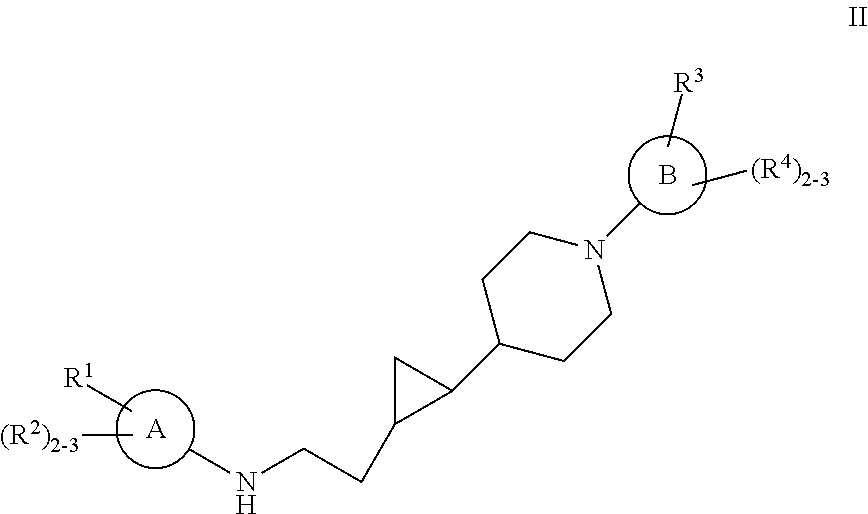
Substituted cyclopropyl compounds, compositions containing such compounds and methods of treatment
Owner:MERCK SHARP & DOHME LLC
Controlled release formulation for treating sleep disorders
The invention relates to a controlled-release formulation for preventing and / or treating sleep disorders comprising Zaleplon or a pharmaceutically acceptable salt thereof in immediate release form and Zolpidem or a pharmaceutically acceptable salt thereof in sustained release form, wherein Zaleplon or a pharmaceutically acceptable salt thereof and Zolpidem or a pharmaceutically acceptable salt thereof are released in two phases where the first phase is a immediate release phase of Zaleplon or a pharmaceutically acceptable salt thereof and the second phase is a sustained release phase of Zolpidem or a pharmaceutically acceptable salt thereof.
Owner:TAIWAN BIOTECH
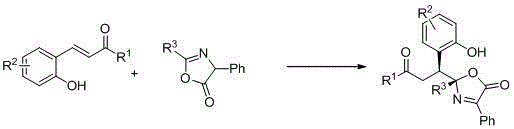
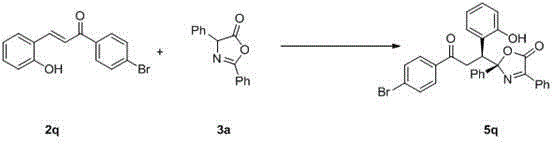
Synthetic method for chiral quaternary carbon oxazolidinone compound
InactiveCN104447604ASimple and fast operationHigh yieldOrganic chemistryThioureaCombinatorial chemistry
Owner:SUZHOU UNIV
Di-substituted dinaphtho-[2,1-b:1',2'-d] furan derivative as well as preparation method and application thereof
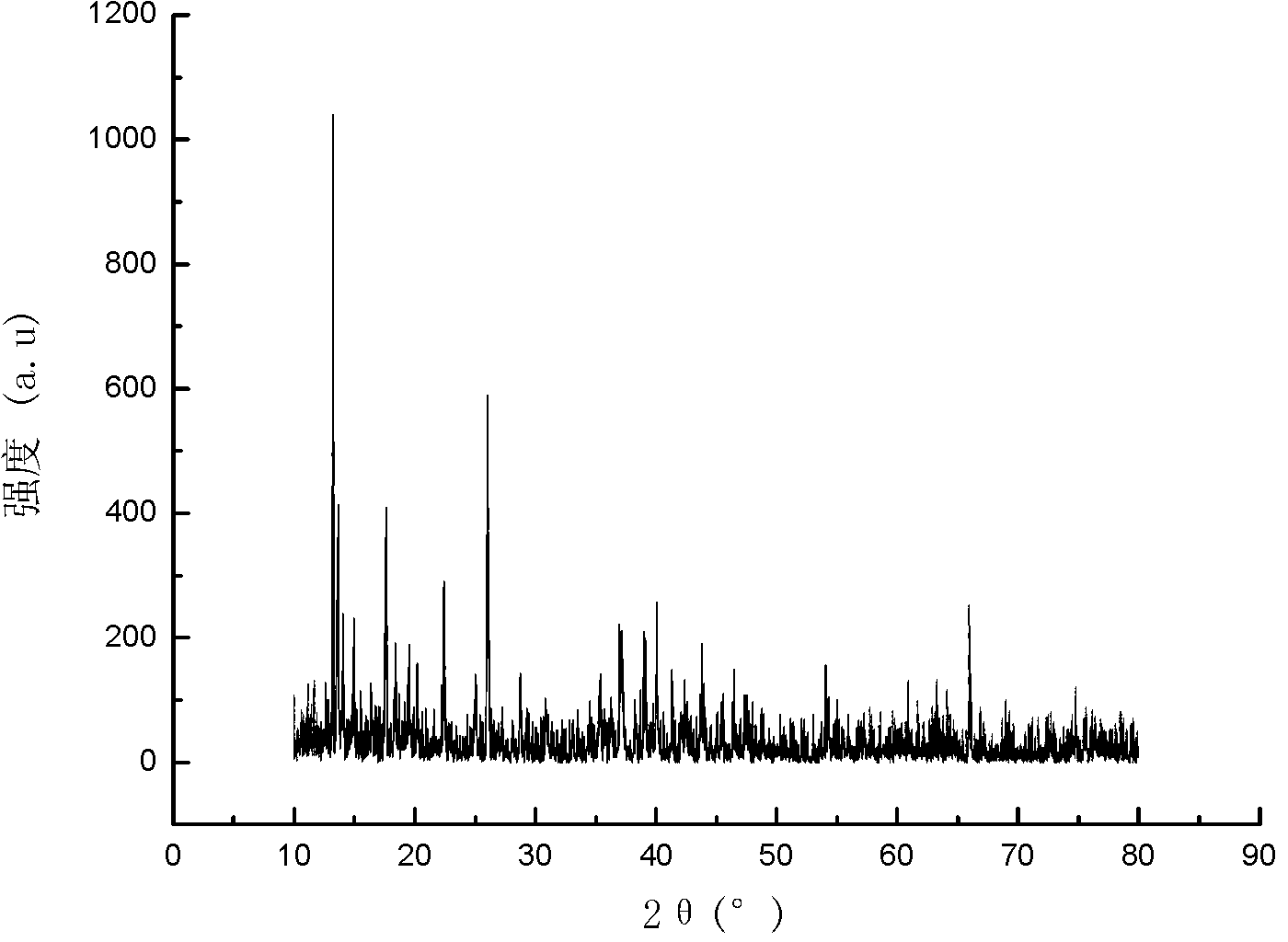
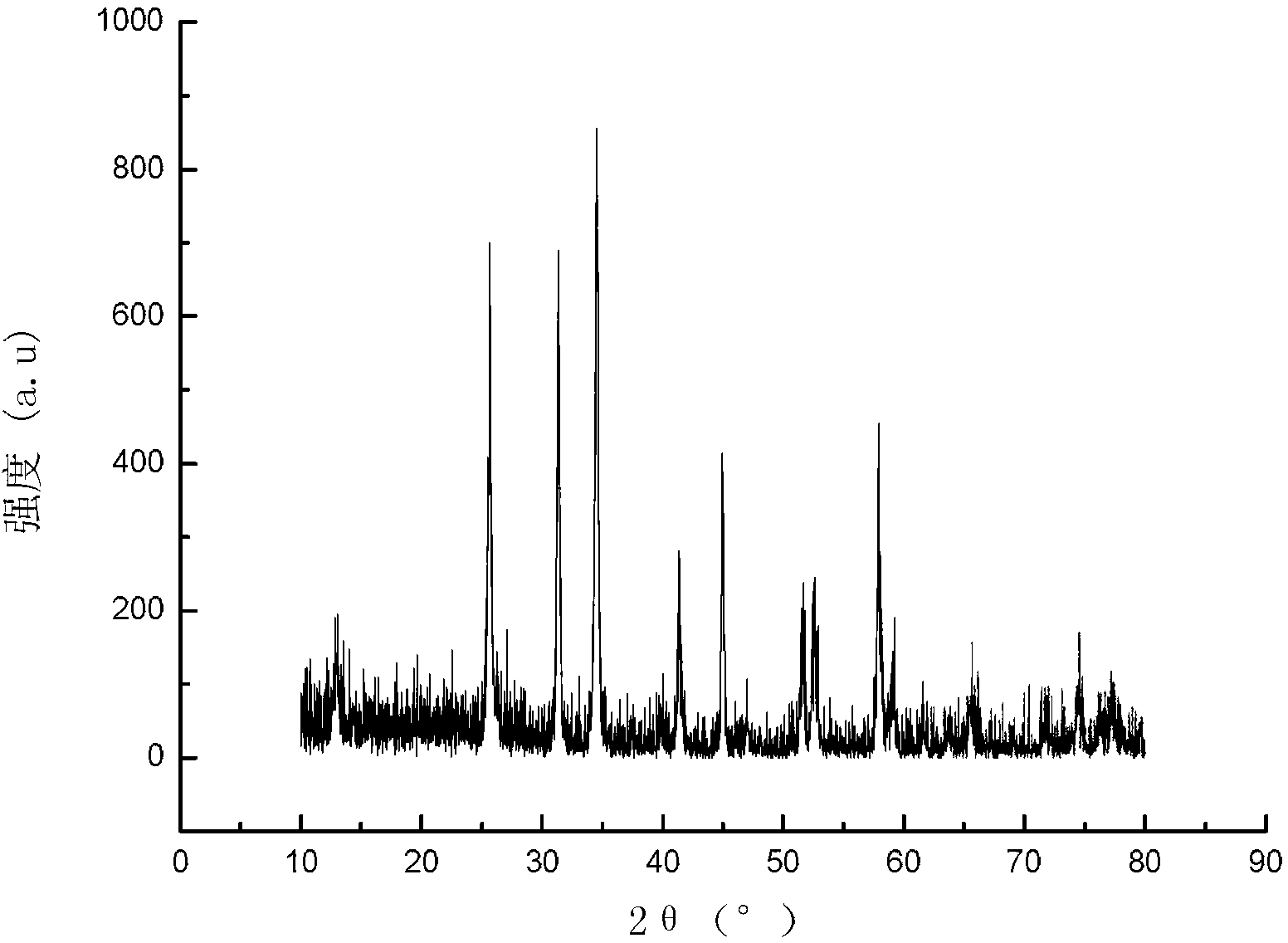
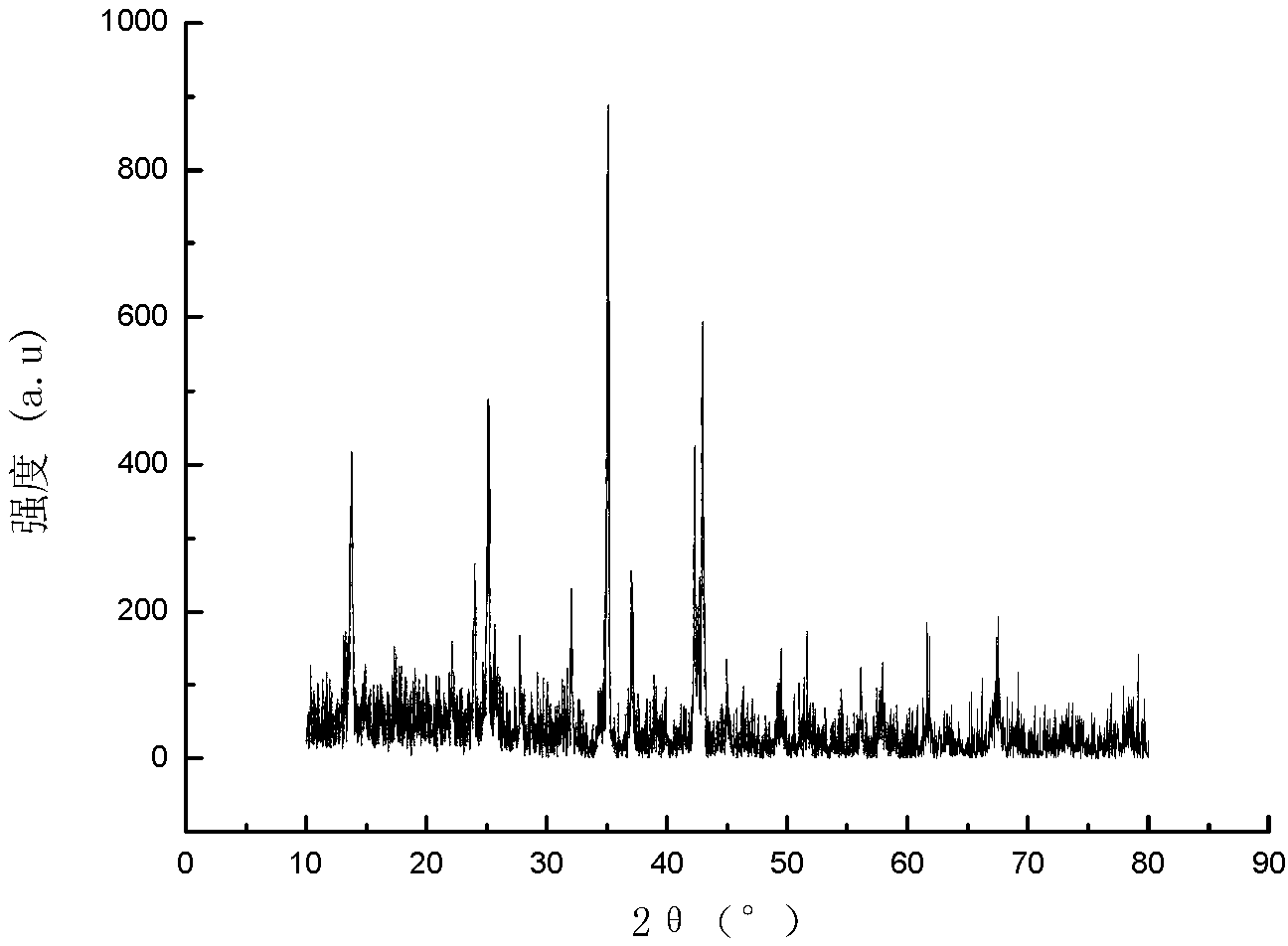
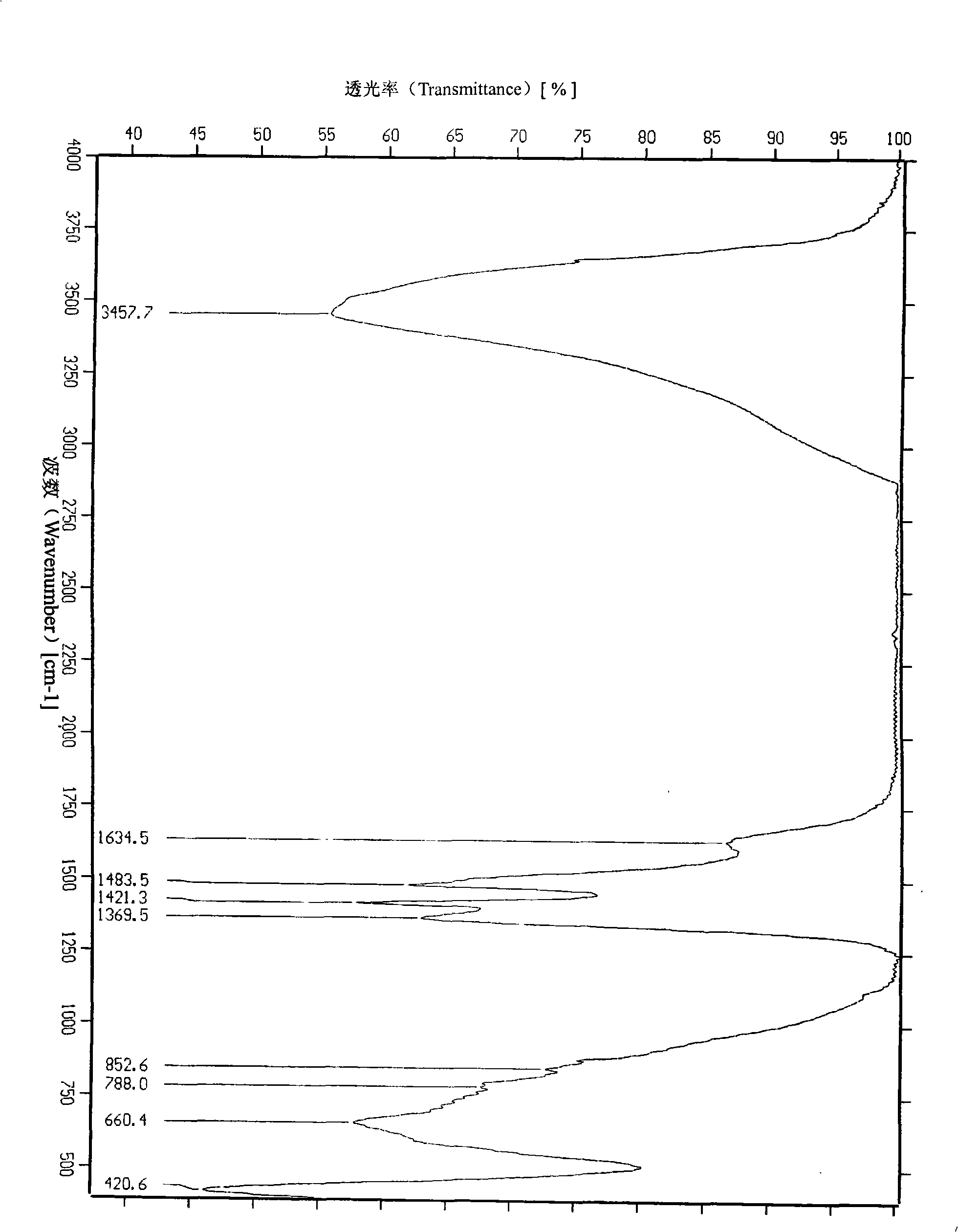
ActiveCN103396386APrevent proliferationReasonable designOrganic active ingredientsOrganic chemistryFuranAnti-Tumor Drugs
The invention relates to a di-substituted dinaphtho-[2,1-b:1',2'-d] furan derivative as well as a preparation method and an application thereof. The preparation method comprises the following steps of: synthesizing a series of di-substituted dinaphtho-[2,1-b:1',2'-d] furan derivatives through a series of reactions by using dibromo-dinaphtho-[2,1-b:1',2'-d] furan as the material. The invention further provides an application of the di-substituted dinaphtho-[2,1-b:1',2'-d] furan derivative in preparing an anti-tumor drug. By virtue of in-vitro pharmacological activity screening, the compound which is synthesized by the preparation method disclosed by the invention is shown to have an effect for inhibiting proliferation of tumor cells, wherein the compound 16 has an obvious inhibiting effect for the liver cancer cells SMMC-7721; and the IC50 value reaches 0.57mu M. Besides, the di-substituted dinaphtho-[2,1-b:1',2'-d] furan derivative is reasonable in design, simple and convenient in preparation method and strong in practicability.
Owner:HARBIN MEDICAL UNIVERSITY
Method for preparing anhydrous neodymium chloride under open system
InactiveCN103193259AAvoid hydrolysisSimplify the conditions of the vacuum environmentRare earth metal compoundsNeodymium chlorideMedicinal chemistry
Owner:HEILONGJIANG UNIV
Heparin-modified adriamycin liposome preparation and preparation method thereof
InactiveCN103720658AEasy to prepareHeparin has good hydrophilicityPowder deliveryOrganic active ingredientsSide effectCholesterol
The invention relates to the field of medicinal preparations, and in particular relates to a heparin-modified adriamycin liposome (Hep-DOX-Lip) preparation. The preparation is characterized by consisting of 1 part of adriamycin, 1-4 parts of heparin, 5-30 parts of soybean lecithin, 0.5-4 parts of cholesterol and 0.5 part of a cationic material. The invention further discloses a preparation method of the heparin-modified adriamycin liposome preparation. The heparin-modified adriamycin liposome preparation has an effect similar to pegylation, and can remarkably enhance the stability of an adriamycin liposome, prolong the in-vivo half-life period of a medicament, and enhance the bioavailability of the medicament. Meanwhile, the heparin-modified adriamycin liposome preparation can remarkably lower the toxic and side effects of chemotherapeutic drugs and enhance the compliance of patients.
Owner:CHINA PHARM UNIV
Supercritical extraction method of conic gymnadenia tuber element
The invention relates to a supercritical extraction method of a conic gymnadenia tuber element, and in particular relates to a method for extracting a natural active ingredient with an anti-aging effect, i.e. the conic gymnadenia tuber element, from a Tibetan medicine conic gymnadenia tuber. According to the invention, through creating a series of new extraction process conditions of pressure, temperature, entrainer and the like and the method, the traditional extraction contradiction of active ingredient purity and extraction rate are solved. Anti-aging medicines, health care products and human hand and face skin care products, which are manufactured through taking the conic gymnadenia tuber element as a basic formula, have the characteristics of nature, safety, transdermal absorption, no side effect and the like.
Owner:SHENZHEN RAPOS LLC
Method of preparing aluminum magnesium carbonate
InactiveCN101343078AHigh purityHigh yieldAluminium compoundsMagnesium carbonatesMedicinal chemistryCarbonate
Owner:IL YANG PHARMA CO LTD
Preparation method of sodium ibandronate
InactiveCN102898466AEasy to operateMeet the quality requirements of raw materialsGroup 5/15 element organic compoundsSkeletal disorderPhosphorous acidChlorobenzene
The invention relates to the technical field of pharmaceutical chemistry, particularly relates to a method of pharmaceutical synthesis, and specifically relates to a preparation method of sodium ibandronate. To overcome the disadvantages of high content of chlorides and phosphites in sodium ibandronate prepared by a conventional preparation method of sodium ibandronate, the preparation method of sodium ibandronate with extremely low content of chlorides and phosphites is provided. In the preparation method, 3-(N-methylpentylamino) propionic acid hydrochloride, phosphorus trichloride and phosphorous acid are employed as raw materials and reacted in a chlorobenzene solvent, so as to obtain sodium ibandronate with extremely low content of the chlorides and the phosphites. The obtained sodium ibandronate can not only meet impurity control standards of the chlorides and the phosphites in a sodium ibandronate crude drug, but also prevent low yield, long period and huge harm to human body and environment which are brought by a lot of refining steps.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
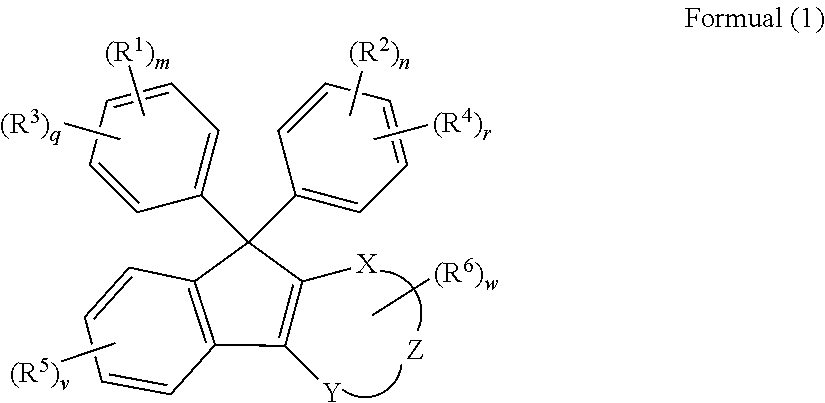
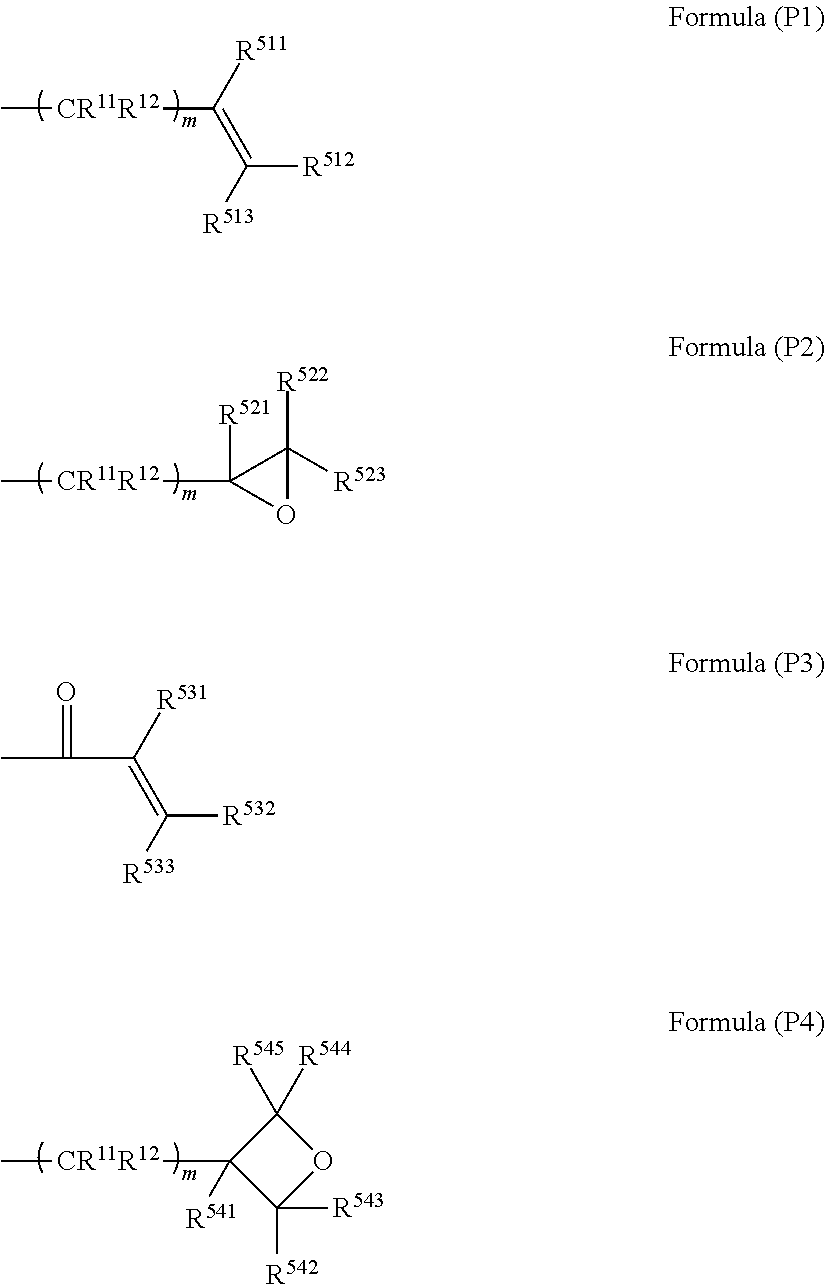
Compound, curable composition, cured product, optical member, and lens
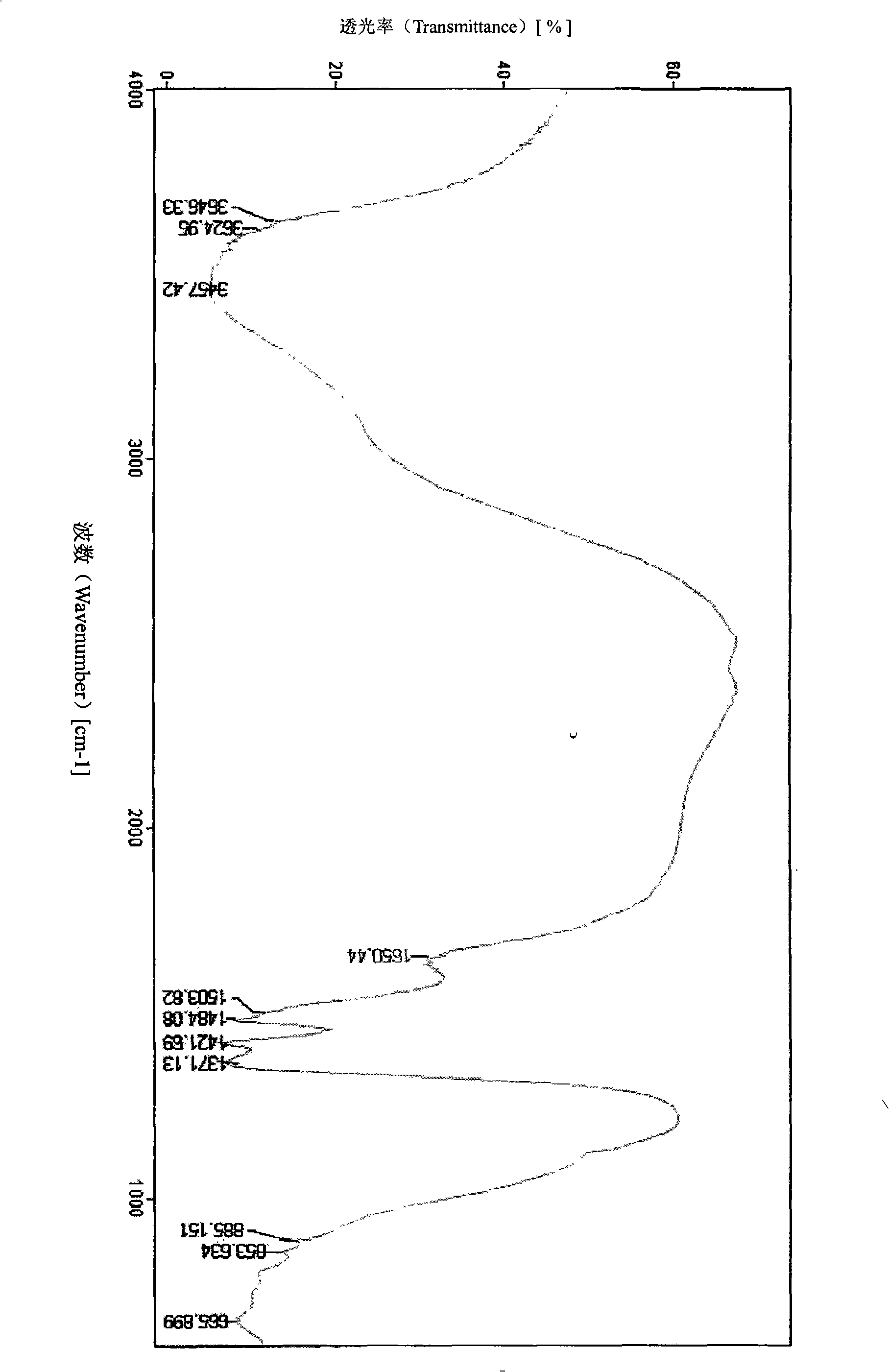
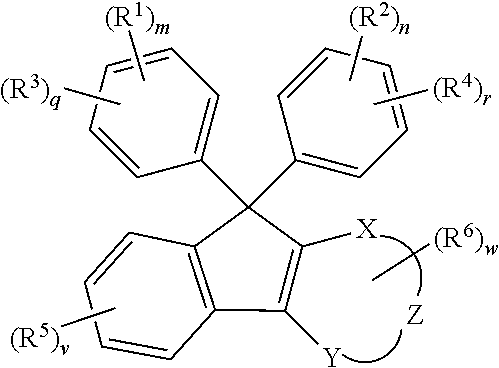
ActiveUS20180305486A1Low Abbe numberHigh abnormal dispersibilityOrganic chemistryOptical elementsMedicinal chemistryAbbe number
Owner:FUJIFILM CORP
Milnacipran hydrochloride intermediate as well as preparation method and application thereof
ActiveCN103613513AEconomical method of preparationEfficient preparation methodOrganic compound preparationCarboxylic acid esters preparationMedicinal chemistryMilnacipran hydrochloride
Owner:SHANGHAI SHYNDEC PHARMA CO LTD
Florfenicol/colistin sulfate soluble powder and preparation method thereof
ActiveCN103550755AImprove synergistic antibacterial effectEasy to usePowder deliveryOrganic active ingredientsPyrrolidinonesPolyvinylpyrrolidone
The invention discloses a florfenicol / colistin sulfate soluble powder which is composed of the following components in percentage by weight: 5-20% of florfenicol, 2-20% of colistin sulfate, 0.5-2% of borax, 5-30% of citric acid, 0.1-2% of potassium bicarbonate, 7.5-30% of polyvinylpyrrolidone, 1-5% of poloxamer 188 and the balance of anhydrous glucose. The invention also discloses a preparation method of the soluble powder, which comprises the following steps: dissolving the polyvinylpyrrolidone in anhydrous ethanol, adding the florfenicol, citric acid, borax, potassium bicarbonate and poloxamer 188, evenly mixing, recovering the ethanol, drying, pulverizing, adding the colistin sulfate and anhydrous glucose, and evenly mixing. The soluble powder ensures that the two reagents simultaneously enter the organism and better display the synergic antibacterial action; the method is simple and convenient to operate, and enhances the production efficiency; and the anhydrous ethanol is recovered, so the method protects the environment and is suitable for industrial production.
Owner:HENAN SOAR VETERINARY PHARMA
Process for preparing Pd/carbon catalyst
ActiveCN101347727AGood dispersionHigh microcrystalline contentMetal/metal-oxides/metal-hydroxide catalystsCarboxylic compound separation/purificationActivated carbonBenzaldehyde
Owner:CHINA PETROLEUM & CHEM CORP +1
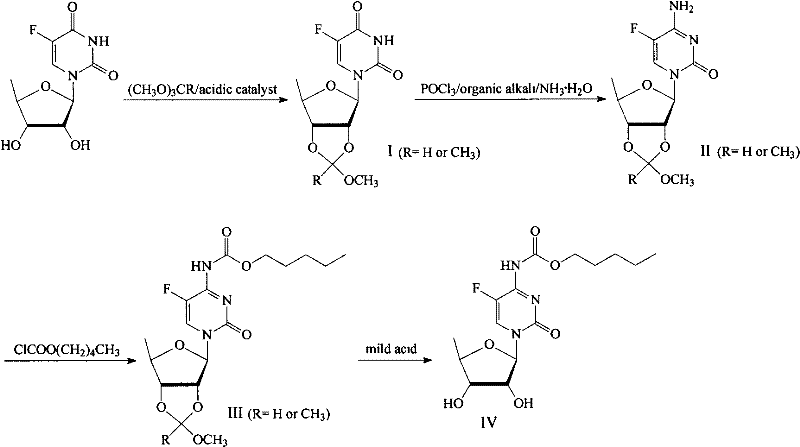
A new process for preparing capecitabine from fluoride
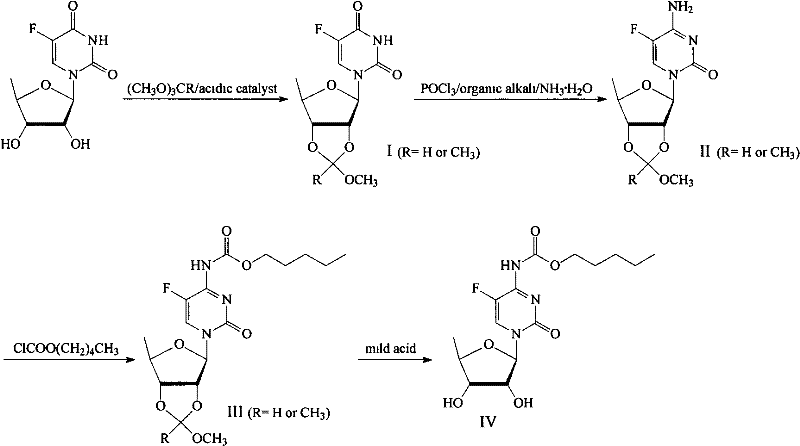
InactiveCN102260310AMild reaction conditionsHigh yieldSugar derivativesSugar derivatives preparation5-fluorocytidineChemical reaction
Owner:JIANGNAN UNIV
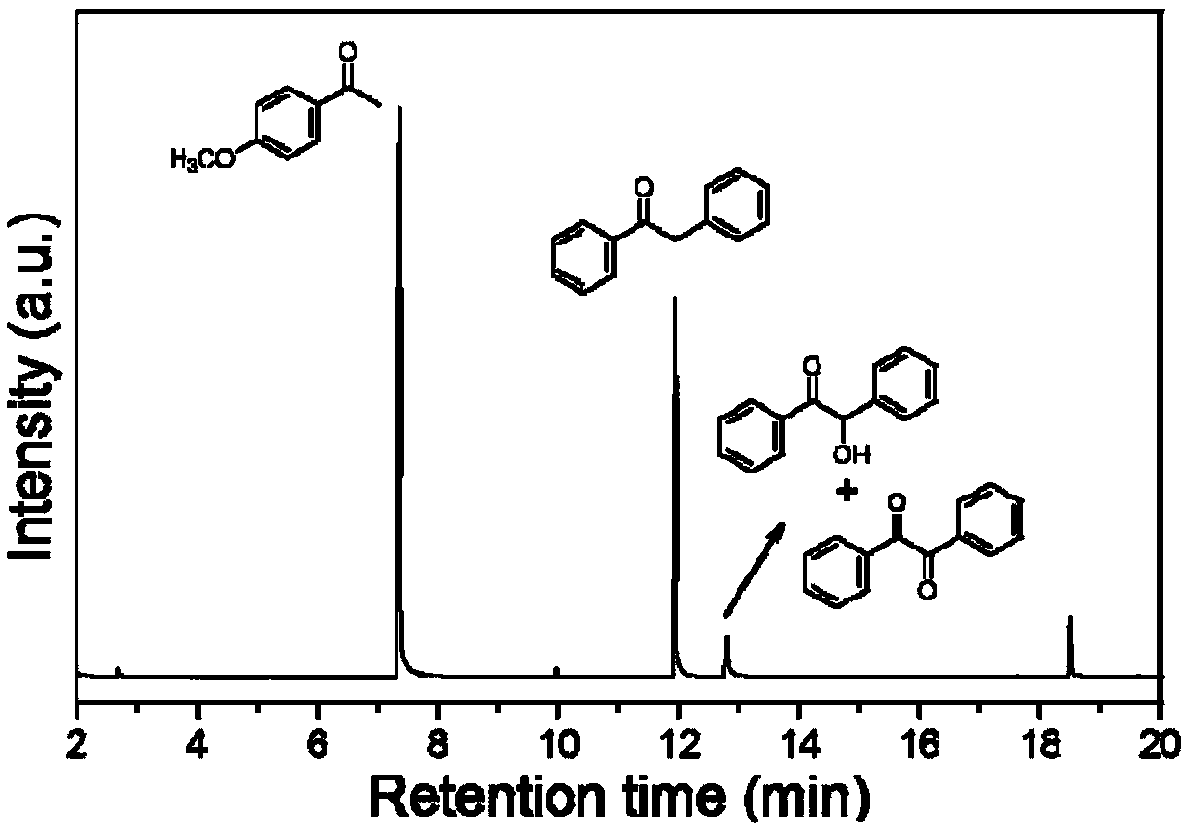
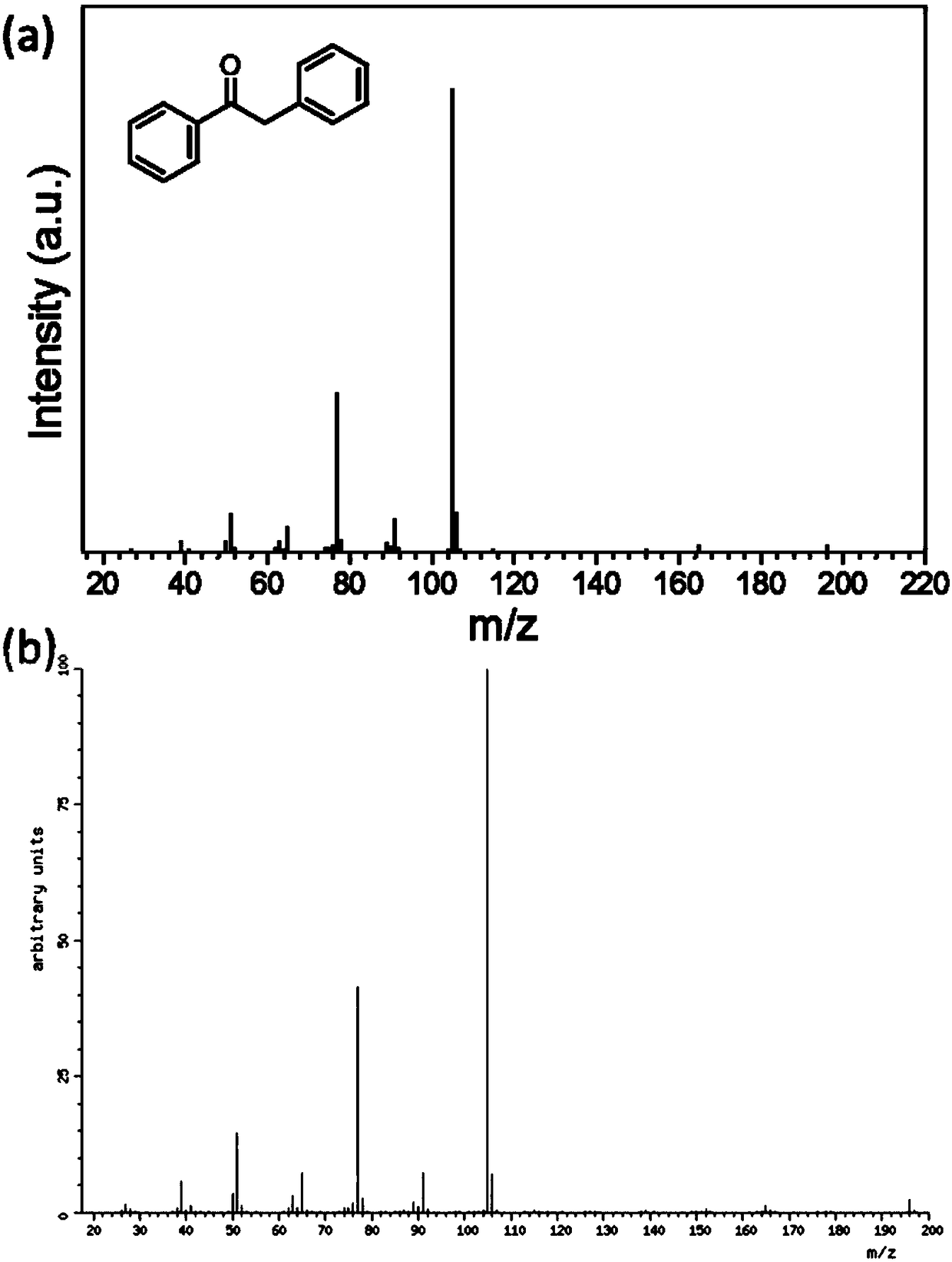
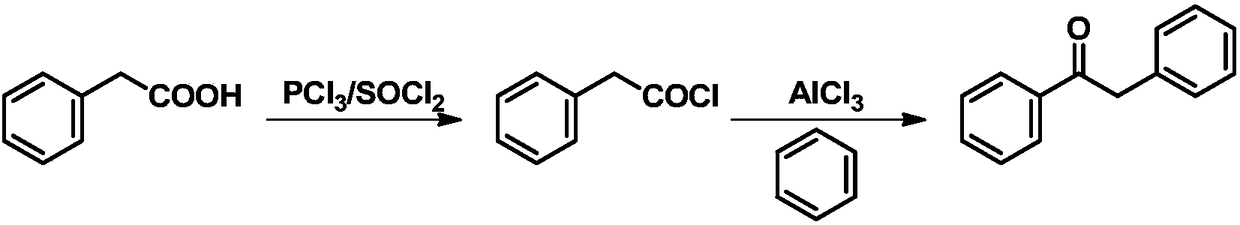
Method for preparing diphenylethanone from benzyl alcohol through photocatalytic one-step method
InactiveCN108440259ALow costAvoid dangerOrganic compound preparationHydroxy compound preparationRoom temperatureAcetonitrile
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Alcohol-soluble nitrocotton optically variable ink and preparation process thereof
The invention discloses an alcohol-soluble nitrocotton optically variable ink. The alcohol-soluble nitrocotton optically variable ink is characterized in that: ethanol is adopted as a main solvent, and other components include: 16.0-20.0% of alcohol-soluble nitrocotton, 4.0-8.0% of alcohol-soluble rosin, 0.0-5.0% of acetyl tributyl citrate, 8.5-9.5% of an optically variable pigment, 45.0-62.0% ofethanol, 8.0-12.0% of ethyl acetate, 2.0-5.0% of dipropylene glycol methyl ether, and 0-0.5% of alcohol-soluble color ink. Resin dissolution, optically variable pigment dispersion, color matching andinspection process methods are adopted to prepare the alcohol-soluble optically variable ink, the invention aims to prepare the optically variable ink that can save energy, reduce emission, lower thecost, meets the requirements of the existing market, and at the same time meets the requirements of environmental protection condition for VOC emission indexes, and the alcohol-soluble nitrocotton optically variable ink has higher economic benefits and social benefits.
Owner:JIANGSU TIGER INK CO LTD
Method of Treating Cancer With Atpenin A5 Derviatives
The present invention includes molecules, composition, and methods for making and using a molecule having the formula (I), wherein R′ is selected from H, methoxy, or methoxymethyl; X is selected from H, OH, methoxy, or methoxymethyl or O—O methoxymethyl; Y is O; and R″ is selected from H, OH, 2-furan, ethyl, propyl, pentyl, hexyl, heptyl, octyl, nonly, decyl, or dodecyl, that are saturated or unsaturated.
Owner:UNIV OF ROCHESTER MEDICAL CENTER +1
Saponins antineoplastic compound in Bohadschia argus Jaeger
The invention relates to medical technique, which in detail relates to a saponin antineoplastic compound Arguside B or Arguside C. The R1 in structure general formula represents OH or H, R2 represents CH3 or CH2OH, when R1 is OH and R2 is CH3, the compound is Arguside B; when R1 is H and R2 is CH2OH, the compound is Arguside C. The chemical structure and spatial configuration of said two compounds are determined through various modern spectral analysises, especially through analysis with multiple advanced nuclear magnetic resonance spectroscopy. It is tested that said two compounds are apparently inhibitive to seven cancers such as A- 549 lung cancer and MCF- 7 breast cancer. It can be used to prepare antineoplastic medicine. The invention provides leading compound for new antineoplastic medicine development, and is valuable for ocean biological resource utilization in China.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Triarylmethane compound and its preparation method and use
InactiveCN103275060ALow costLow photoelectric conversion performanceLight-sensitive devicesOrganic chemistryCyanoacetic acidEthylic acid
The invention discloses a triarylmethane compound and its preparation method and use. The preparation method comprises the following steps that 1, R2-CHO and R1-H undergo a reaction to produce a compound I; and 2, the compound I and a Vilsmeier reagent undergo a reaction to produce a compound II; and the compound II and cyanoacetic acid undergo a reflux reaction in an acetic acid solution to produce the triarylmethane compound; or 3, the compound I and N-bromosuccinimide undergo a bromination reaction to produce a compound III; the compound III and (OH)2B-R3-CHO undergo a reflux reaction in methylbenzene or chloroform to produce a compound IV; and the compound IV and cyanoacetic acid undergo a reflux reaction in an acetic acid solution to produce the triarylmethane compound. The triarylmethane compound is used as a photosensitive dye and is used for preparation of a dye-sensitized solar cell material. The triarylmethane compound has wide and cheap raw material sources. The preparation method has simple processes and a low cost, can be industrialized and can be used as a novel dye-sensitized solar cell material.
Owner:CENT SOUTH UNIV
Synthesis of Azo Bonded Immunoregulatory Compounds
Methods are disclosed for preparing compounds of Formula I: where R1, R3, and R4are independently hydrogen or C1 to C4 alkyl, and R2 is: where R5 is selected from the group consisting of hydrogen and C1 to C4 alkyl, or where R6, R7 and R8 are independently hydrogen or C1 to C4 alkyl; or the esters or pharmacologically acceptable salts thereof. The methods can involve converting a suitably functionalized aniline compound to a diazonium salt (which aniline compound can be first formed by reduction of a nitrobenzene) and coupling the diazonium salt with a suitably functionalized benzene compound. The suitably functionalized aniline compound either includes a primary alcohol or aldehyde group, which is then oxidized to a carboxylic acid group, or includes a nitrile or amide group, which is hydrolyzed to a carboxylic acid group. The methods can also involve the direct coupling (via reduction of nitro groups to form an azo linkage) of suitably functionalized nitrobenzenes. The compounds and or their metabolites can be used to treat or prevent various diseases, particularly inflammatory conditions of the GI tract.
Owner:BIOCON LTD
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap
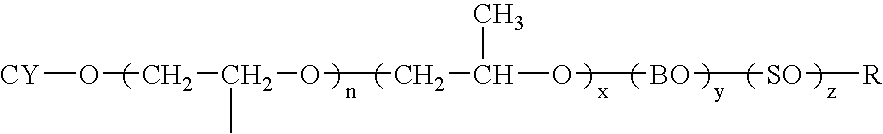
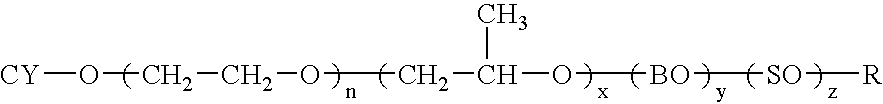

![Di-substituted dinaphtho-[2,1-b:1',2'-d] furan derivative as well as preparation method and application thereof Di-substituted dinaphtho-[2,1-b:1',2'-d] furan derivative as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img_release/76474e7d-018b-4771-88ed-408de08d9a74/FDA00003464943900011.png)
![Di-substituted dinaphtho-[2,1-b:1',2'-d] furan derivative as well as preparation method and application thereof Di-substituted dinaphtho-[2,1-b:1',2'-d] furan derivative as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img_release/76474e7d-018b-4771-88ed-408de08d9a74/FDA00003464943900021.png)
![Di-substituted dinaphtho-[2,1-b:1',2'-d] furan derivative as well as preparation method and application thereof Di-substituted dinaphtho-[2,1-b:1',2'-d] furan derivative as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img_release/76474e7d-018b-4771-88ed-408de08d9a74/BDA00003464944000021.png)