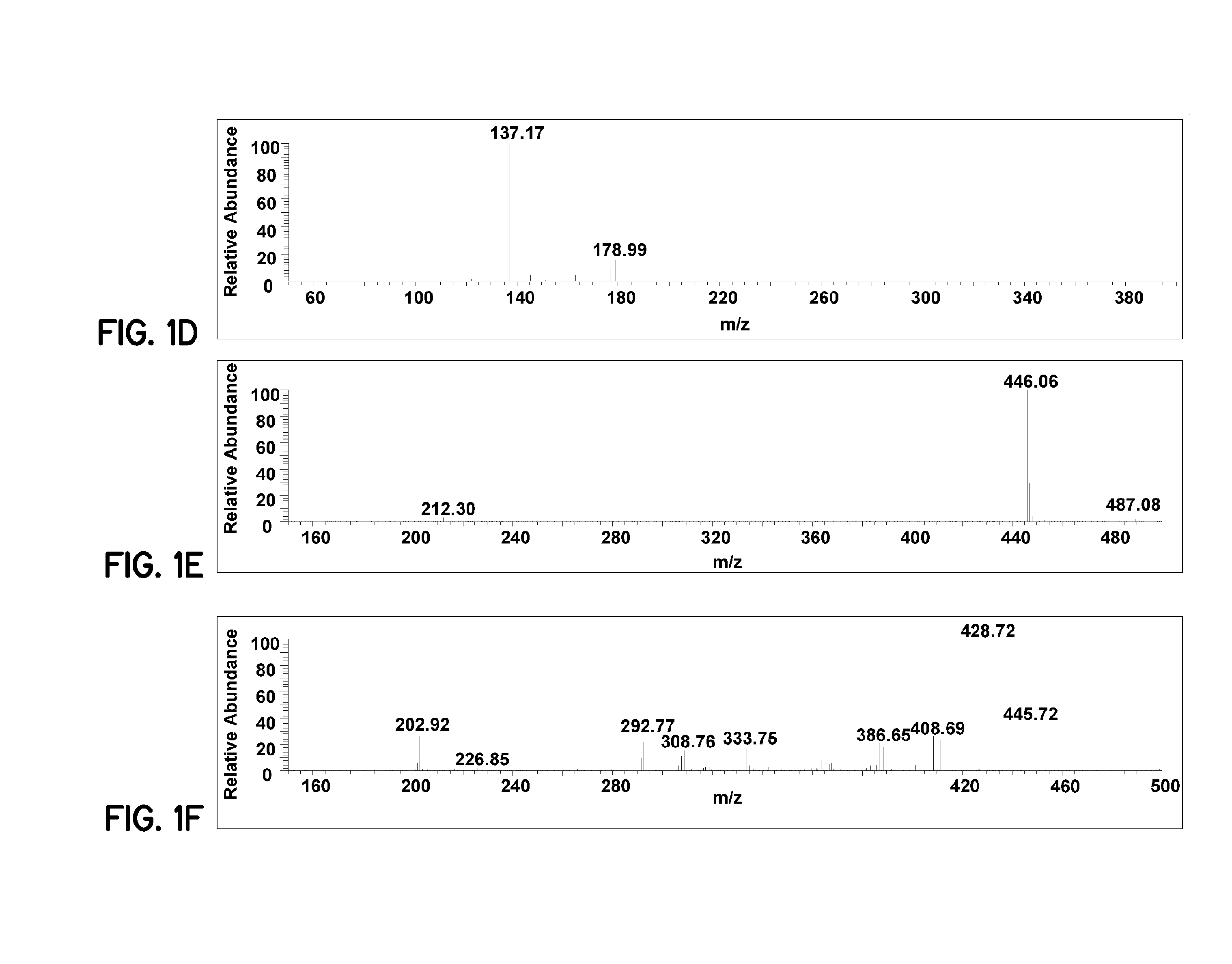
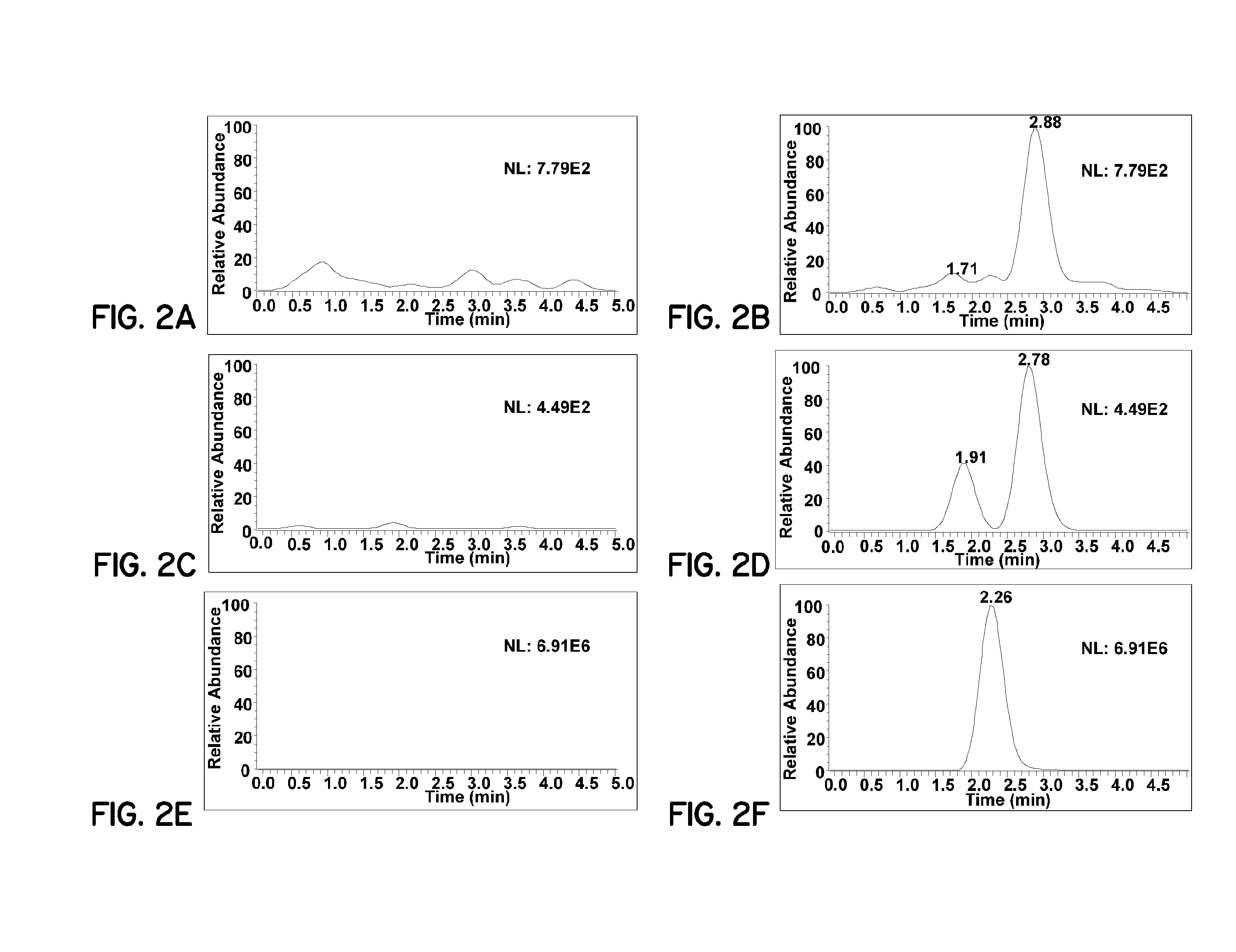
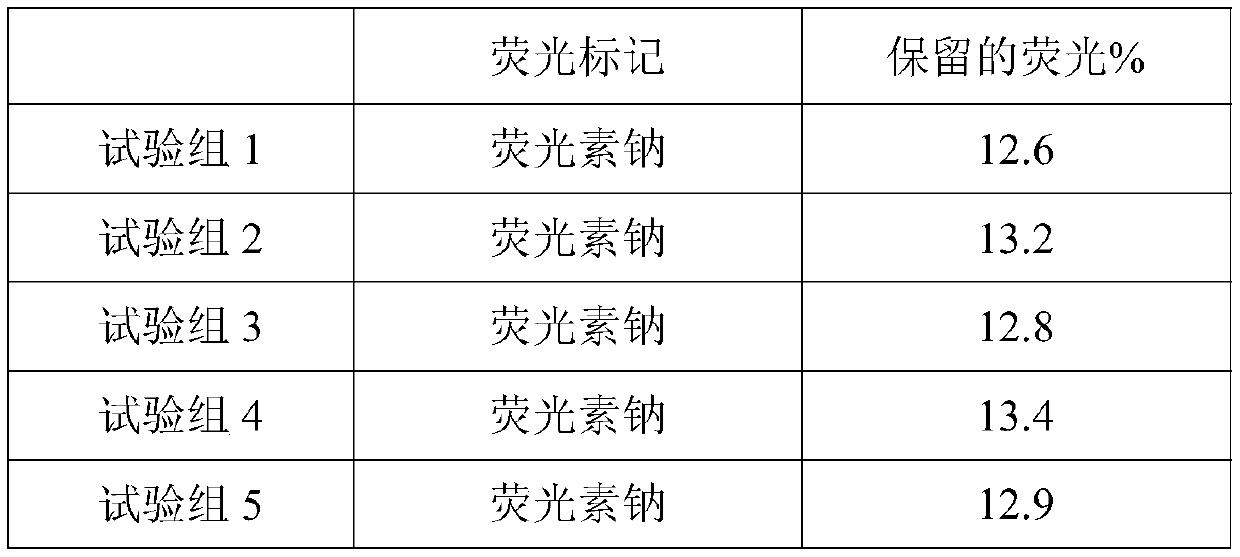
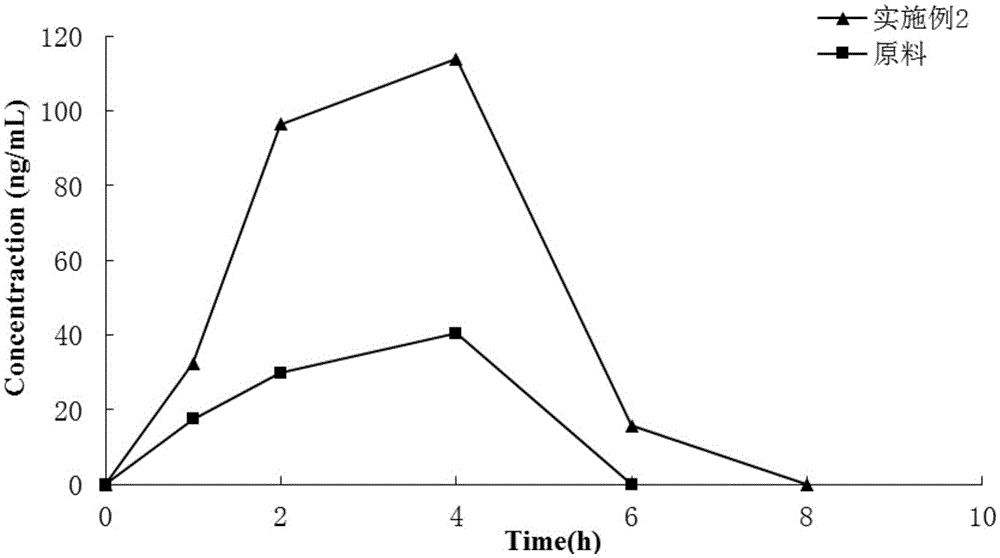
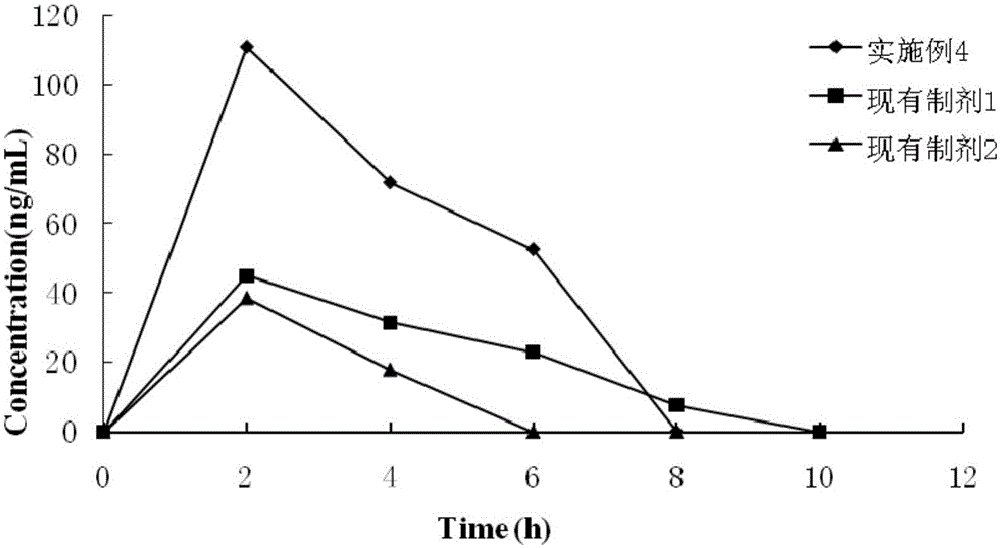
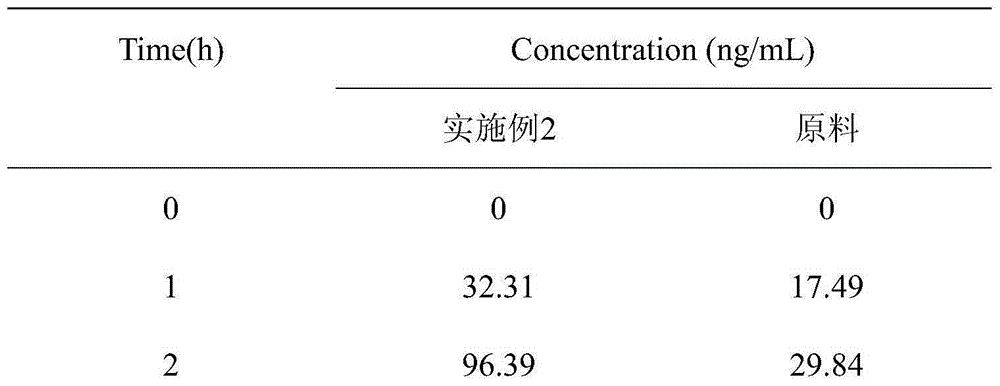
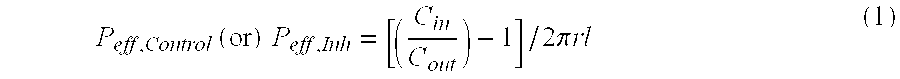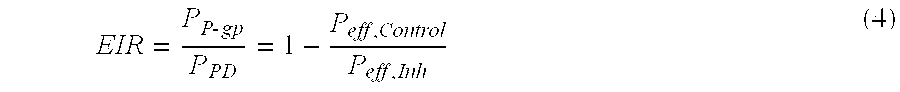Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
17 results about "Bioavailability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In pharmacology, bioavailability (BA or F) is a subcategory of absorption and is the fraction of an administered dose of unchanged drug that reaches the systemic circulation, one of the principal pharmacokinetic properties of drugs. By definition, when a medication is administered intravenously, its bioavailability is 100%. However, when a medication is administered via other routes (such as orally), its bioavailability generally decreases (due to incomplete absorption and first-pass metabolism) or may vary from patient to patient. Bioavailability is one of the essential tools in pharmacokinetics, as bioavailability must be considered when calculating dosages for non-intravenous routes of administration.
Crystal form G of ibrutinib and preparation method
InactiveCN105646499AImprove stabilityHigh purityOrganic active ingredientsOrganic chemistry methodsSolubilitySolvent
The invention discloses a crystal form G of ibrutinib. The crystal form G is characterized in that X-ray powder diffraction (X-RPD) which adopts Cu-Kalpha radiation and is represented with a 2theta angle has diffraction peaks in positions at angles of 5.0 degrees plus or minus 0.2 degrees, 7.3 degrees plus or minus 0.2 degrees, 10.1 degrees plus or minus 0.2 degrees, 12.0 degrees plus or minus 0.2 degrees, 13.2 degrees plus or minus 0.2 degrees, 17.1 degrees plus or minus 0.2 degrees, 19.5 degrees plus or minus 0.2 degrees, 20.8 degrees plus or minus 0.2 degrees, 22.3 degrees plus or minus 0.2 degrees, 24.3 degrees plus or minus 0.2 degrees, 27.4 degrees plus or minus 0.2 degrees and 31.2 degrees plus or minus 0.2 degrees. Related solvents in a preparation process of the crystal form G are cheap, the conditions are mild, the operation is simple, good controllability and reproducibility are realized, further, the prepared crystal form has great stability, the HPLC (high performance liquid chromatography) purity is higher than 99%, and the phenomenon of crystal transformation can be avoided; besides, the solubility is high, the dissolubility is good, and the bioavailability is high.
Owner:孙霖
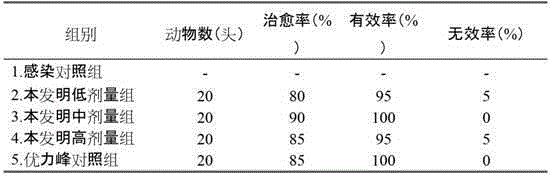
Medicine for treating cold grasserie and method of preparing the medicine
ActiveCN101584809ADrug effectEffectiveComponent separationSkeletal disorderMirabilis jalapaPharmaceutical drug
The invention discloses a medicine for treating cold grasserie, comprising the raw medicine by weight parts: 50 to 150 parts of Tibet pleurospermum, 50 to 150 parts of Himalayan four-o'clock root, 50 to 150 parts of caltrop, 50 to 150 parts of Polygonati Rhizoma and 50 to 150 parts of asparagus; grain diameter of the medicine is 0.1 to 100 microns. The medicine of the invention has higher effective dissolution of medicine, medicine penetration power and bioavailability, and better medicine effect. The invention further discloses a method of preparing the medicine for treating cold grasserie and special quality examination method thereof such that the quality examination method effectively controls the stability of the medicine, and also effectively identifies the specificity of pharmaceutical ingredients in medicine.
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
Heparin-modified adriamycin liposome preparation and preparation method thereof
InactiveCN103720658AEasy to prepareHeparin has good hydrophilicityPowder deliveryOrganic active ingredientsSide effectCholesterol
The invention relates to the field of medicinal preparations, and in particular relates to a heparin-modified adriamycin liposome (Hep-DOX-Lip) preparation. The preparation is characterized by consisting of 1 part of adriamycin, 1-4 parts of heparin, 5-30 parts of soybean lecithin, 0.5-4 parts of cholesterol and 0.5 part of a cationic material. The invention further discloses a preparation method of the heparin-modified adriamycin liposome preparation. The heparin-modified adriamycin liposome preparation has an effect similar to pegylation, and can remarkably enhance the stability of an adriamycin liposome, prolong the in-vivo half-life period of a medicament, and enhance the bioavailability of the medicament. Meanwhile, the heparin-modified adriamycin liposome preparation can remarkably lower the toxic and side effects of chemotherapeutic drugs and enhance the compliance of patients.
Owner:CHINA PHARM UNIV
Preparation method of traditional Chinese medicinal submicron powder for animals
ActiveCN103463347AAvoid destructionNo overheatingPowder deliveryUnknown materialsBiotechnologyMedicinal herbs
Owner:SICHUAN KANGSIHAI ANIMAL MEDICINE
Zinc oxide-based nano-drug composition, and preparation method and application thereof
ActiveCN105853373AIncrease the difficultyIncrease costOrganic active ingredientsZinc oxides/hydroxidesSynthesis methodsPotassium hydroxide
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Qingkailing dispersing tablet
ActiveCN1682836AHas the function of clearing heat, detoxifying, calming and calming the nervesAntipyreticAnalgesicsDiseaseAcute Pharyngitis
Owner:哈药集团三精千鹤制药有限公司
Curcumin compositions and uses thereof
Owner:THE OHIO STATES UNIV
Veterinary compound tilmicosin injection and preparation method thereof
InactiveCN104161768AImprove performanceSimple processOrganic active ingredientsAntibacterial agentsDiseaseBULK ACTIVE INGREDIENT
The invention discloses a veterinary compound tilmicosin injection and a preparation method thereof. 100ml of the veterinary compound tilmicosin injection comprises 15-30g of tilmicosin, 4-10g of florfenicol, 0.5-1.0g of an anti-oxidant, 3.3-8.0g of a cosolvent and the balance injection water. The veterinary compound tilmicosin injection having stable performances is prepared from tilmicosin and florfenicol as main raw materials and the anti-oxidant and the cosolvent as auxiliary materials. After intramuscular injection, the veterinary compound tilmicosin injection can be easily absorbed by animals and even completely absorbed, improves bioavailability, has long drug active ingredient action time, can be used for clinical treatment on livestock and poultry respiratory tract system disease caused by a plurality of pathogenic bacteria, has good curative effects and is convenient for use. The preparation method of the veterinary compound tilmicosin injection has simple processes, is convenient for operation and is suitable for large-scale industrial production.
Owner:ZHENGZHOU HOUYI PHARMA
Dihydroergotoxine methanesulfonate sustained release tablet and preparation method thereof
Owner:珠海天翼医药技术开发有限公司
Composition for eyes and having improved drying protection and retention
InactiveCN110090294AGood for healthImprove complianceSenses disorderAntipyreticSide effectPatient compliance
Owner:嘉兴市爵拓科技有限公司
Multi-element lycopene phosphatide compound and preparation method thereof
ActiveCN106551920AImprove bioavailabilitySignificant absorption advantageCosmetic preparationsHydrocarbon active ingredientsLycopenePhospholipid
Owner:NANJING HEALSOUL LIFE SCI & TECH CO LTD
Clopidol controlled-release microparticle preparation and preparation method thereof
ActiveCN105168179AAvoid sudden releaseLess irritatingInorganic non-active ingredientsAntiparasitic agentsMicroparticleClopidol
Owner:ZHEJIANG ESIGMA BIOTECH CO LTD
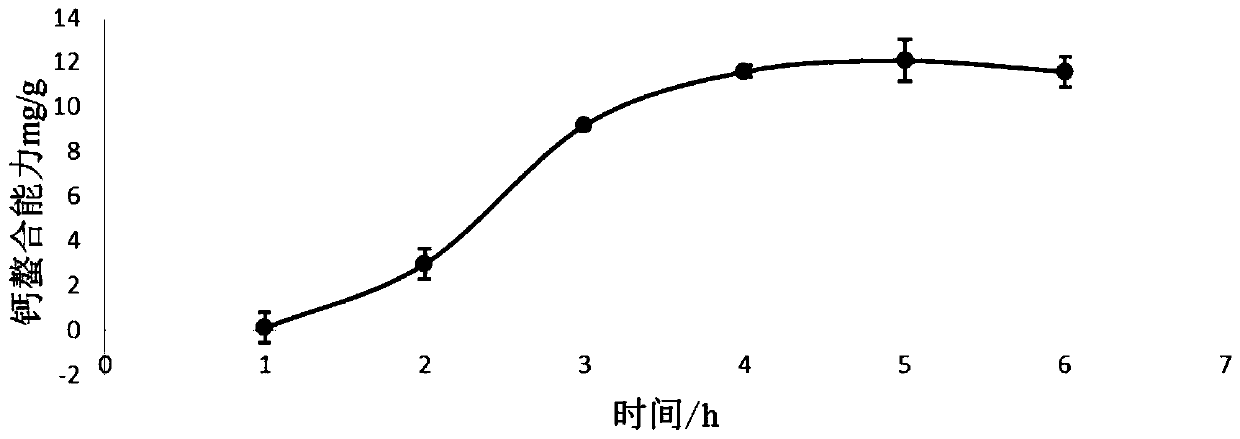
Novel calcium chelating peptide as well as preparation method and application thereof
ActiveCN110669127AEasy to transportImprove absorption and utilizationConnective tissue peptidesPeptide preparation methodsPhysiochemical ActivityPharmaceutical Substances
Owner:SOUTH CHINA AGRI UNIV
Effervescent traditional Chinese medicine composition having intense heat-purging and detoxicating function and preparation method thereof
InactiveCN101816726ADissolve evenlyPromote absorptionAntipyreticAnalgesicsSide effectAdditive ingredient
Owner:TIANJIN REBATE SCI & TECH DEV
Pharmaceutical composition containing glucosamine and having weight reducing function
ActiveCN105520947AImprove solubilityImprove bioavailabilityOrganic active ingredientsMetabolism disorderOrlistatSide effect
The invention belongs to the field of medicine, and in particular, relates to a pharmaceutical composition containing glucosamine and having a weight reducing function. According to the pharmaceutical composition containing glucosamine and having the weight reducing function, tests find out that glucosamine can significantly increase the orlistat dissolution degree, and greatly improves the orlistat bioavailability. At the same time, the pharmaceutical composition containing glucosamine and having the weight reducing function has a significant weight reducing effect on obesity sensitive type rats; after drug discontinuance, a weight rebound phenomenon does not appear, moreover, diarrhea or loose stools or other side effects do not appear during drug taking, and the pharmaceutical composition is a healthy weight reducing drug having a significant weight reducing effect.
Owner:GUANGDONG PHARMA UNIV
Traditional Chinese medicine composition for treating porcine encephalitis and preparation method of traditional Chinese medicine composition
InactiveCN106692869AGood control effectAntibacterial effect is obviousAntibacterial agentsNervous disorderSide effectAdditive ingredient
Owner:DINGZHENG ANIMAL PHARMA TIANJIN
Methods and compositions for treating gastrointestinal conditions
InactiveUS20090062242A1Minimizing systemic effect of drugBiocideDigestive systemPhysiologyBioavailability
Owner:AGI THERAPEUTICS RES
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap