Curcumin compositions and uses thereof
a technology of compositions and curcumin, which is applied in the direction of drug compositions, biocide, animal husbandry, etc., can solve the problems of affecting the degree of chemical degradation of drugs by intestinal microflora, the inability to predict the bioavailability of orally administered chemicals, and the inability to reduce the availability of drugs. , to achieve the effect of increasing the bioavailability of curcumin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
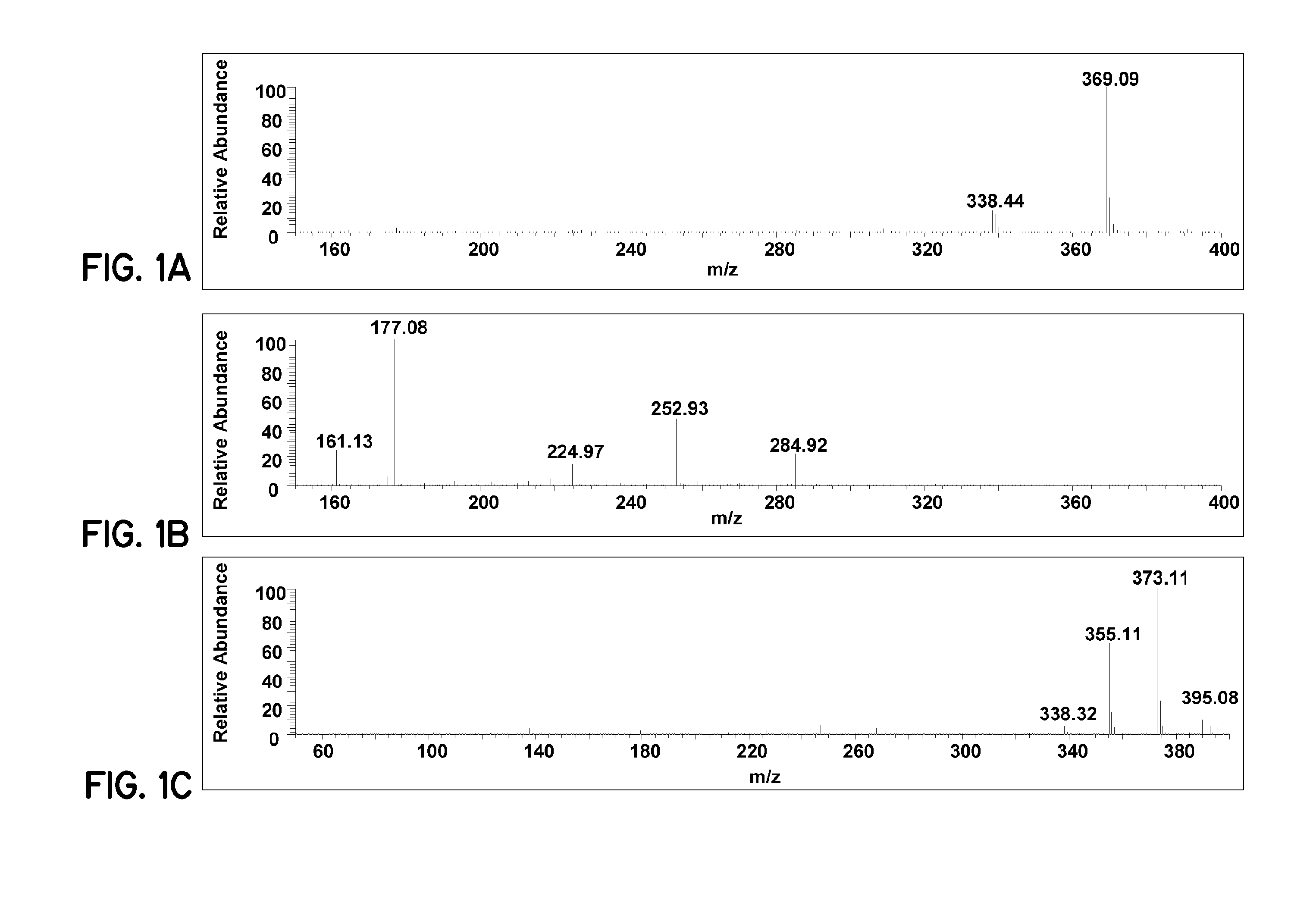
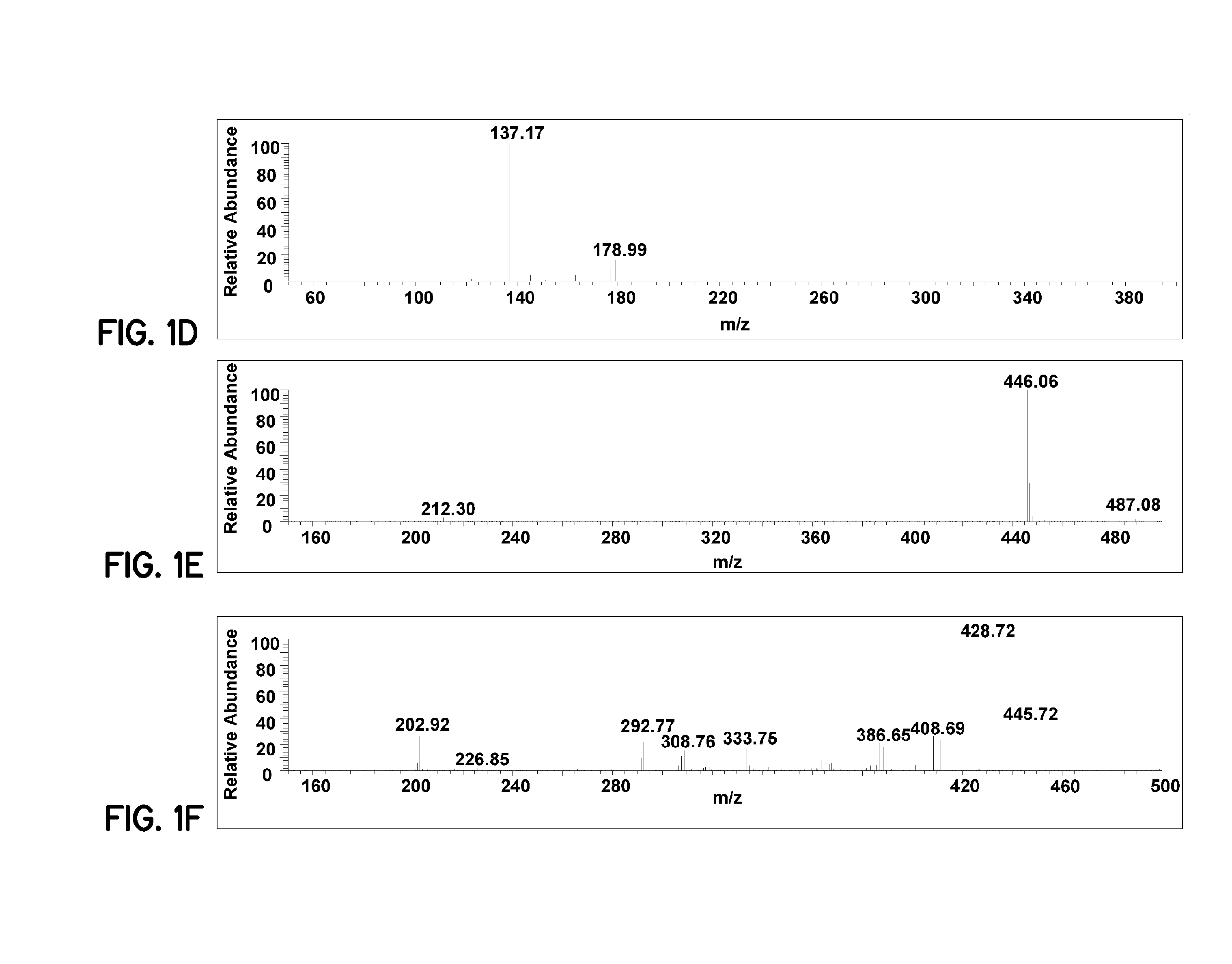
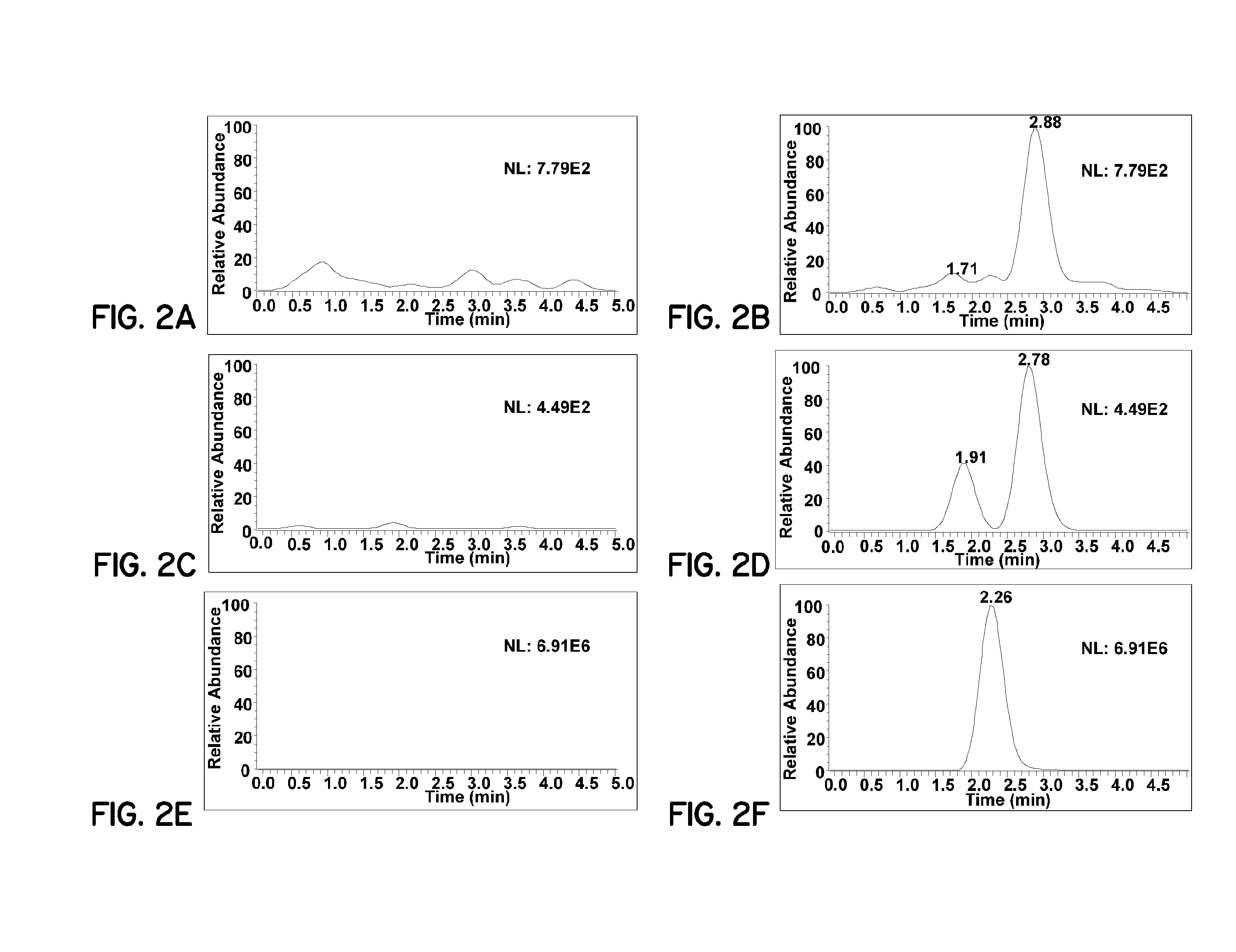
[0078]Pharmaceutical formulations prepared according to embodiments of the invention improve bioavailability of curcumin. For these examples, curcumin (95%) was purchased from Acros Organics. Tetrahydrocurcumin (THC) was prepared according to a published procedure (Ohtsu, et al., J. Med. Chem., 2002, 45(23), 5037-5042). The internal standard OSU-Arg was obtained from National Cancer Institute (Bethesda, Md., USA). Analytical HPLC grade methanol, acetonitrile, ethyl acetate and formic acid were purchased from Fisher Scientific (Pittsburgh, Pa., USA). Heparin-treated and EDTA-treated mouse plasma were purchased from Harlan Bioproducts (Indianapolis, Ind., USA). The phosphate buffer saline (PBS, pH 7.4) was purchased from Sigma-Aldrich (St. Louis, Mo., USA). All chemicals and reagents were used as received. An E-pure water purification system (Barnstead, Dubuque, Iowa) was used to obtain HPLC grade water (>18 mΩ).
[0079]The LC-MS system used consisted of a Finnigan TSQ Quantum EMR Triple Q
example 2
[0113]Curcumin formulations according to embodiments of the invention has anti-tumor activity. For these studies, the curcumin gel formulation was tested in a breast cancer model using MDA-MB-231 breast cancer cell engrafted nude mice. Female athymic nu / nu mice (4-6 weeks old, 18-22 g) were obtained from Charles River Laboratory (Wilmington, Mass.) and acclimated for 1 week in a pathogen-free enclosure before start of study. Animals were given sterile rodent chow and water ad libitum and were housed in sterile filter-top cages with 12 hour light / dark cycles. All experiments were conducted in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). MDA-MB-231 cells (5×106 cells per mouse) were suspended with cell culture medium, and subcutaneously implanted into the right flank of the athymic nu / nu mice. When tumors were grown between 100 to 200 mm3, treatments were initiated. Mice were randomly assigned i
example 3
[0116]Abnormal DNA methylation has been observed in some types of cancers. As such, we evaluated the effect of the curcumin formulations according to embodiments of the invention on DNA methylation.
[0117]Materials: Decitabine (DAC) was obtained from the National Cancer Institute and used without further purification. Curcumin, methanol, acetonitrile (HPLC grade), ammonium formate, ammonium acetate, ammonium bicarbonate, 5-methyl-2-deoxycytidine (5mdC), 2-deoxycytidine (2dC), 2-deoxyguanosine (2dG), nucleophosphatase (NP1), snake venom phosphatase (SVP), and alkaline phosphatase (AP), deoxynucleotide triphosphate (2.5 mM), AmpliTaqGold polymerase and 10×PCR buffer were purchased from Sigma-Aldrich (St. Louis, Mo.). The primers for amplification of p15INK4B and its bisulfite-converted promoter region, p21, DNMT1, DNMT3a and DNMT3b, and Sp1 binding promoter region in DNMT1 were purchased from either Sigma-Aldrich (St. Louis, Mo.) or Integrated DNA Technology (IDT, Coralville, Iowa). M. Ss
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap