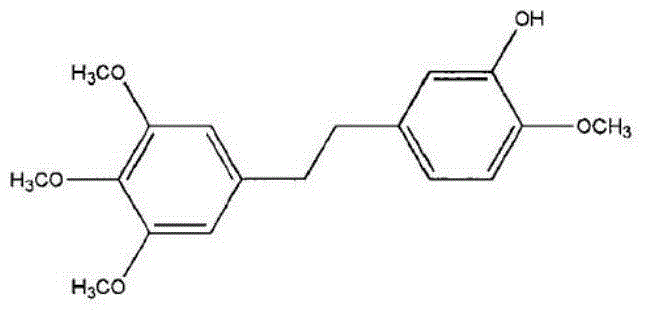
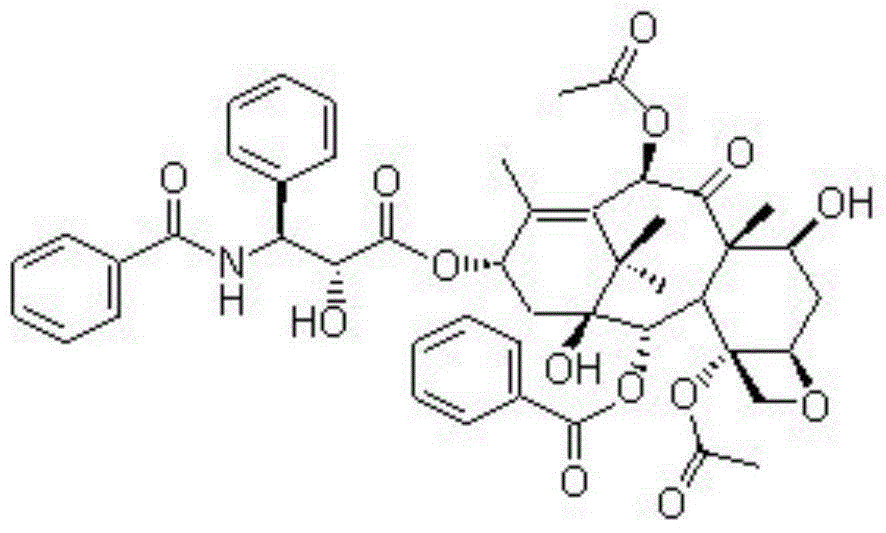
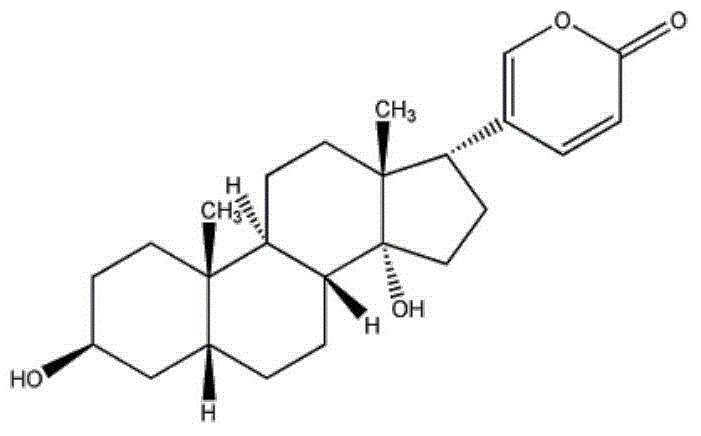
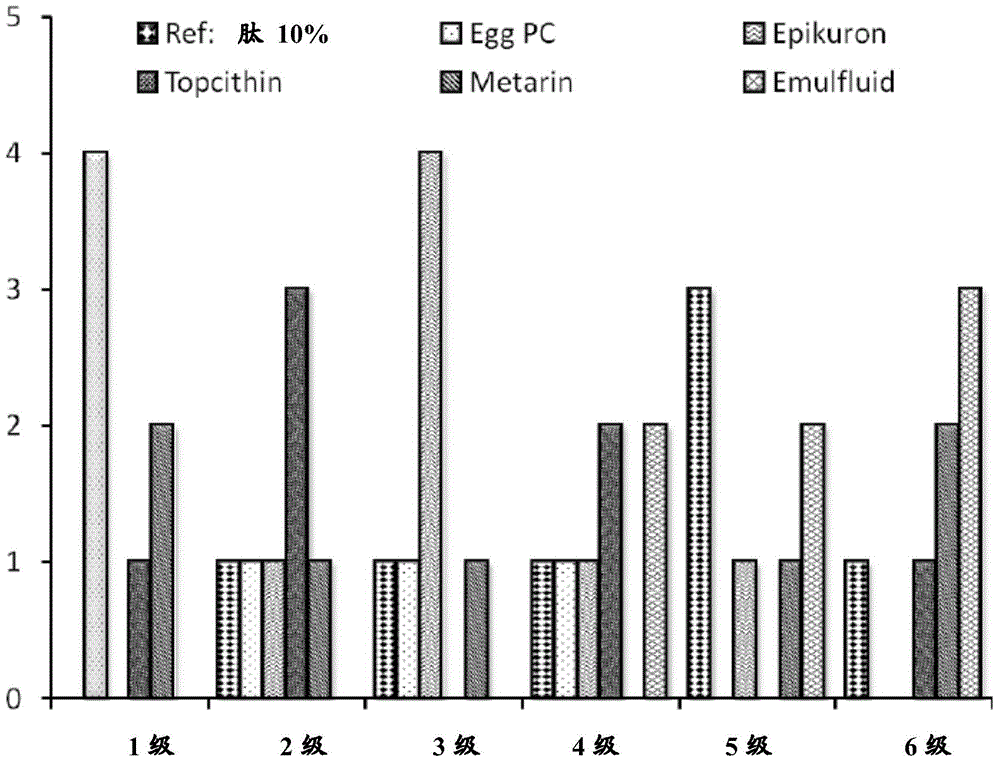
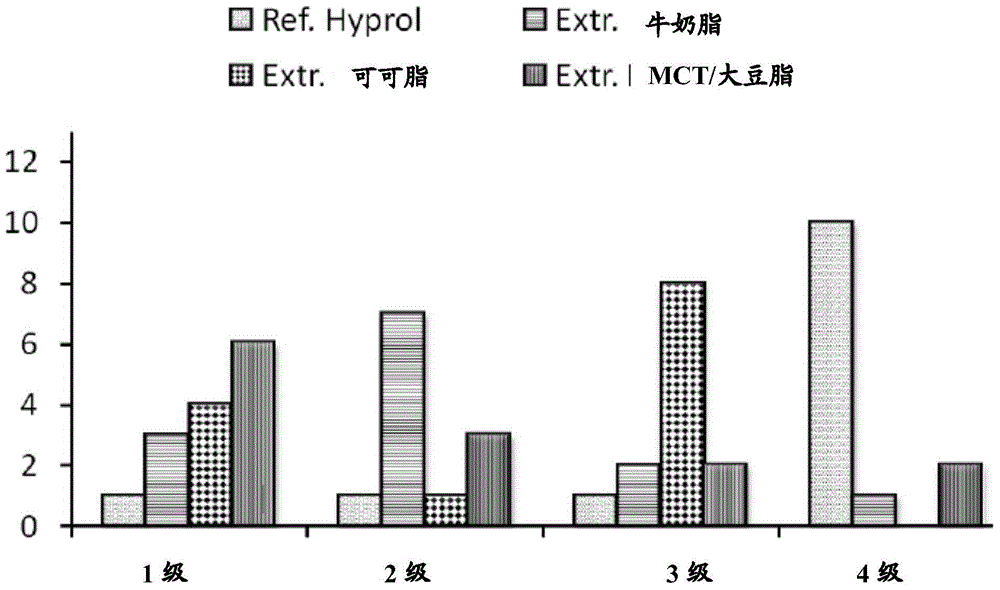
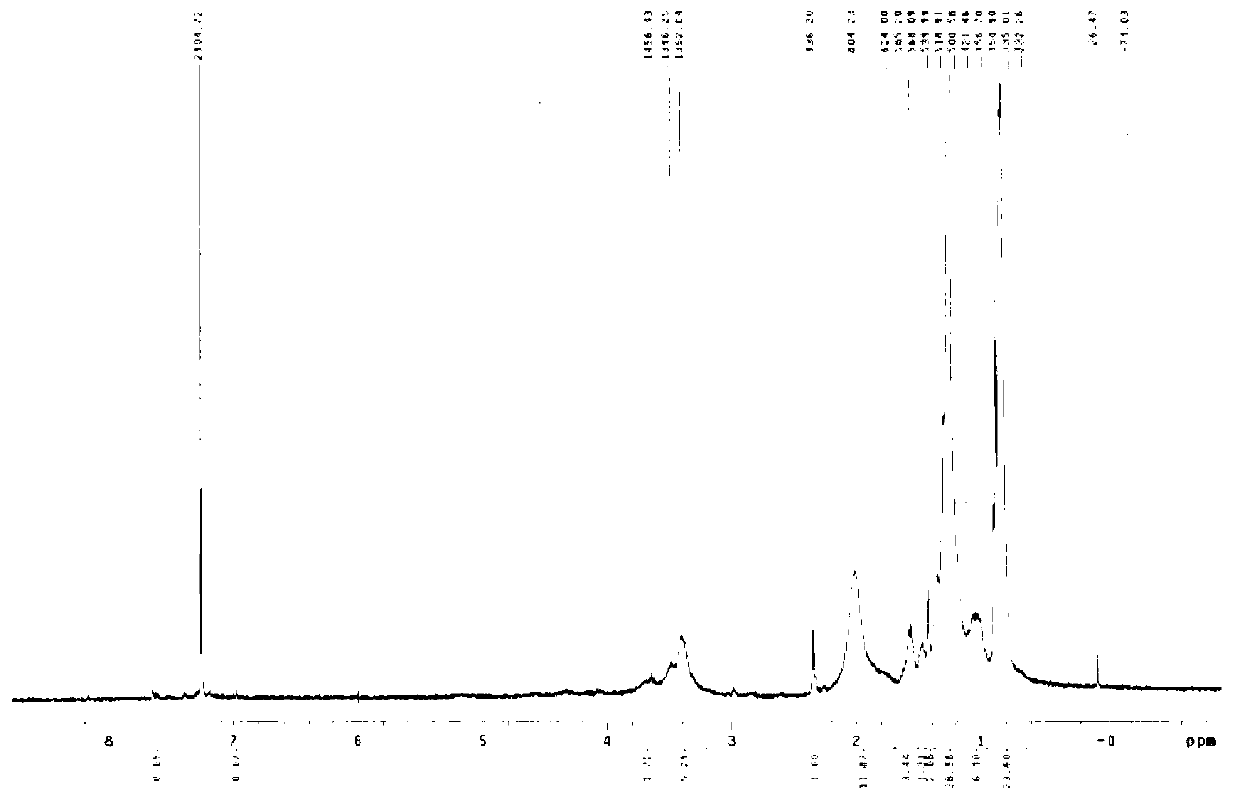
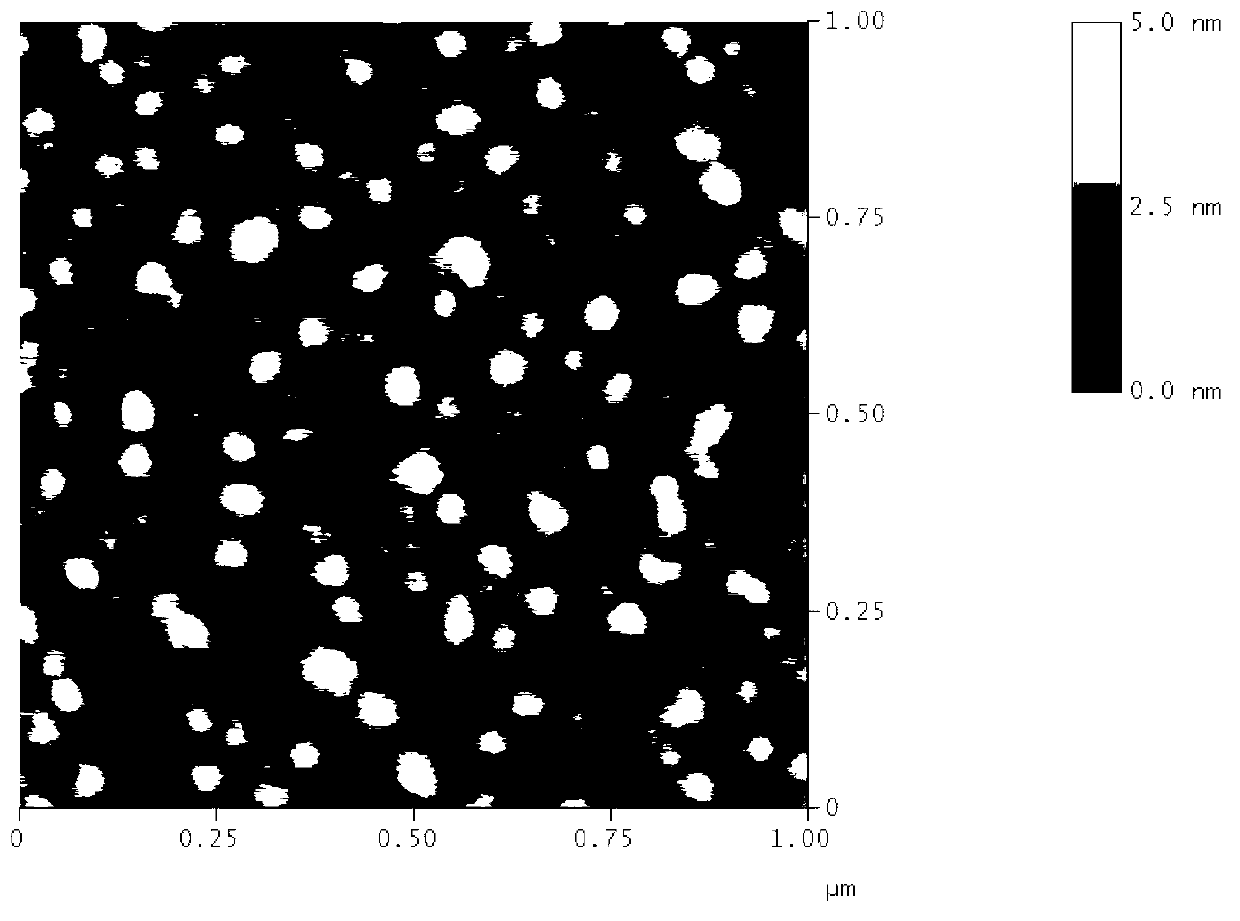
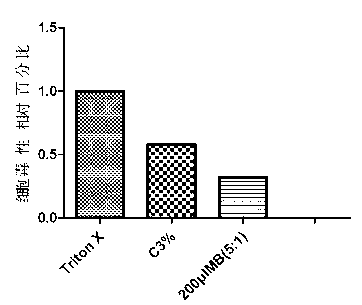Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
11 results about "Liposome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A liposome is a spherical vesicle having at least one lipid bilayer. The liposome can be used as a vehicle for administration of nutrients and pharmaceutical drugs. Liposomes can be prepared by disrupting biological membranes (such as by sonication).
Novel reagents for intracellular delivery of macromolecules
InactiveUS20050260757A1Improve delivery efficiencyIncrease rangeSugar derivativesMicroencapsulation basedLiposomeCompound (substance)
Owner:LIFE TECH CORP
Oxygen-fluorine liposome microbubble and preparation method thereof
InactiveCN103212094AImprove performanceUniform particle sizeNervous disorderEnergy modified materialsPolyethylene glycolPhospholipid
Owner:CHILDRENS HOSPITAL OF CHONGQING MEDICAL UNIV
Cefmetazole sodium proliposome preparation
ActiveCN101623264AThe encapsulation efficiency has not decreasedImprove stabilityOrganic active ingredientsAntibacterial agentsCholesterolPhospholipid
The invention provides a cefmetazole sodium proliposome preparation which comprises the following components by weight part: 1 part of cefmetazole sodium, 3-15 parts of liposome carrier and 2-10 parts of proppant, wherein the liposome carrier comprises polyene phosphatidyl choline, cholesterol and oleinic acid according to a weight ratio of (4-20):(1-5):1. The cefmetazole sodium proliposome preparation has good preparation stability and cannot crack because of dewatering, fusion, ice crystal generation, and the like in a freeze-drying process; and after hydrated re-dissolution, the cefmetazole sodium proliposome preparation still can maintain good entrapment rate.
Owner:HAINAN LINGKANG PHARMA CO LTD
Heparin-modified adriamycin liposome preparation and preparation method thereof
InactiveCN103720658AEasy to prepareHeparin has good hydrophilicityPowder deliveryOrganic active ingredientsSide effectCholesterol
The invention relates to the field of medicinal preparations, and in particular relates to a heparin-modified adriamycin liposome (Hep-DOX-Lip) preparation. The preparation is characterized by consisting of 1 part of adriamycin, 1-4 parts of heparin, 5-30 parts of soybean lecithin, 0.5-4 parts of cholesterol and 0.5 part of a cationic material. The invention further discloses a preparation method of the heparin-modified adriamycin liposome preparation. The heparin-modified adriamycin liposome preparation has an effect similar to pegylation, and can remarkably enhance the stability of an adriamycin liposome, prolong the in-vivo half-life period of a medicament, and enhance the bioavailability of the medicament. Meanwhile, the heparin-modified adriamycin liposome preparation can remarkably lower the toxic and side effects of chemotherapeutic drugs and enhance the compliance of patients.
Owner:CHINA PHARM UNIV
Lung cancer and breast cancer treatment compound preparation containing active ingredients of traditional Chinese medicine and preparation method of lung cancer and breast cancer compound preparation
InactiveCN105079005AGood inhibitory effectHigh anticancer activityEther/acetal active ingredientsRespiratory disorderPolyethylene glycolIn vivo
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Kit for detecting hepatitis B virus and detection method of hepatitis B virus
Owner:NANJING AGRICULTURAL UNIVERSITY +1
Encapsulated bitter peptides, methods of encapsulating bitter peptides, and nutritional compositions including encapsulated bitter peptides
ActiveCN104703487AHave sensory propertiesCover up the sensesPeptide/protein ingredientsOintment deliveryCrystallographyEmulsion
Owner:SOC DES PROD NESTLE SA
Gene recombination human interferon alpha 2b liposome and injection, suppository, eye drop, sparying preparation and ointment thereof
Owner:TIANJIN CITY HENGTIAN BIOLOGICAL TECH
Cationic macromolecular proteolipid gene medicine carrier, preparation method and application
InactiveCN102716500ARich varietyHigh transfection efficiencyGenetic material ingredientsMacromolecular non-active ingredientsLipid formationPositive control
Owner:SHANGHAI INST OF ONCOLOGY
Spla2 hydrolysable liposomes with improved storage stability
Owner:BIO BEDST
Liposomal formulation for use in the treatment of cancer
ActiveUS20200054557A1Good physical stabilityComplicates and prevents manipulationOrganic active ingredientsInorganic non-active ingredientsPyrimidineLiposome
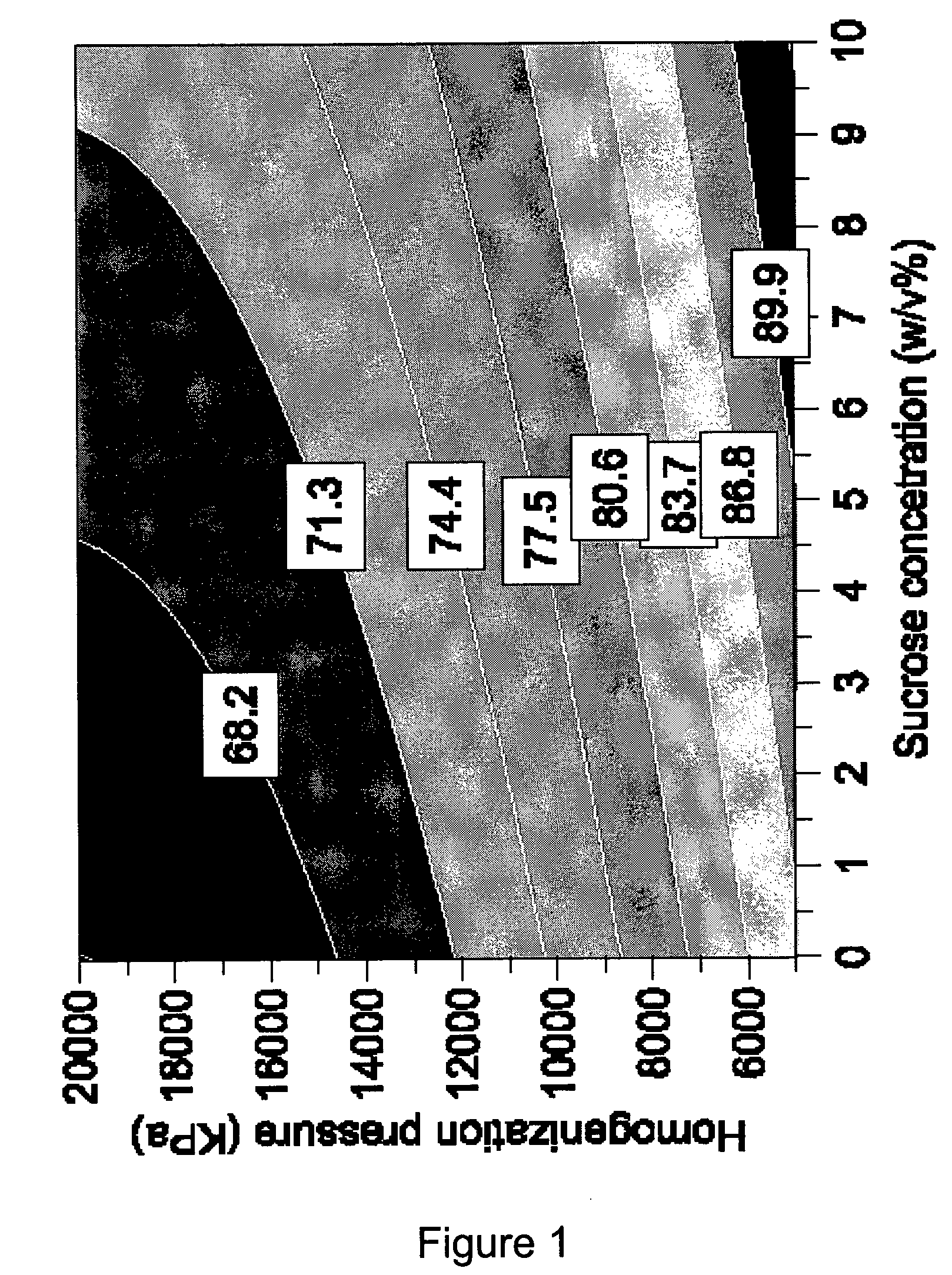
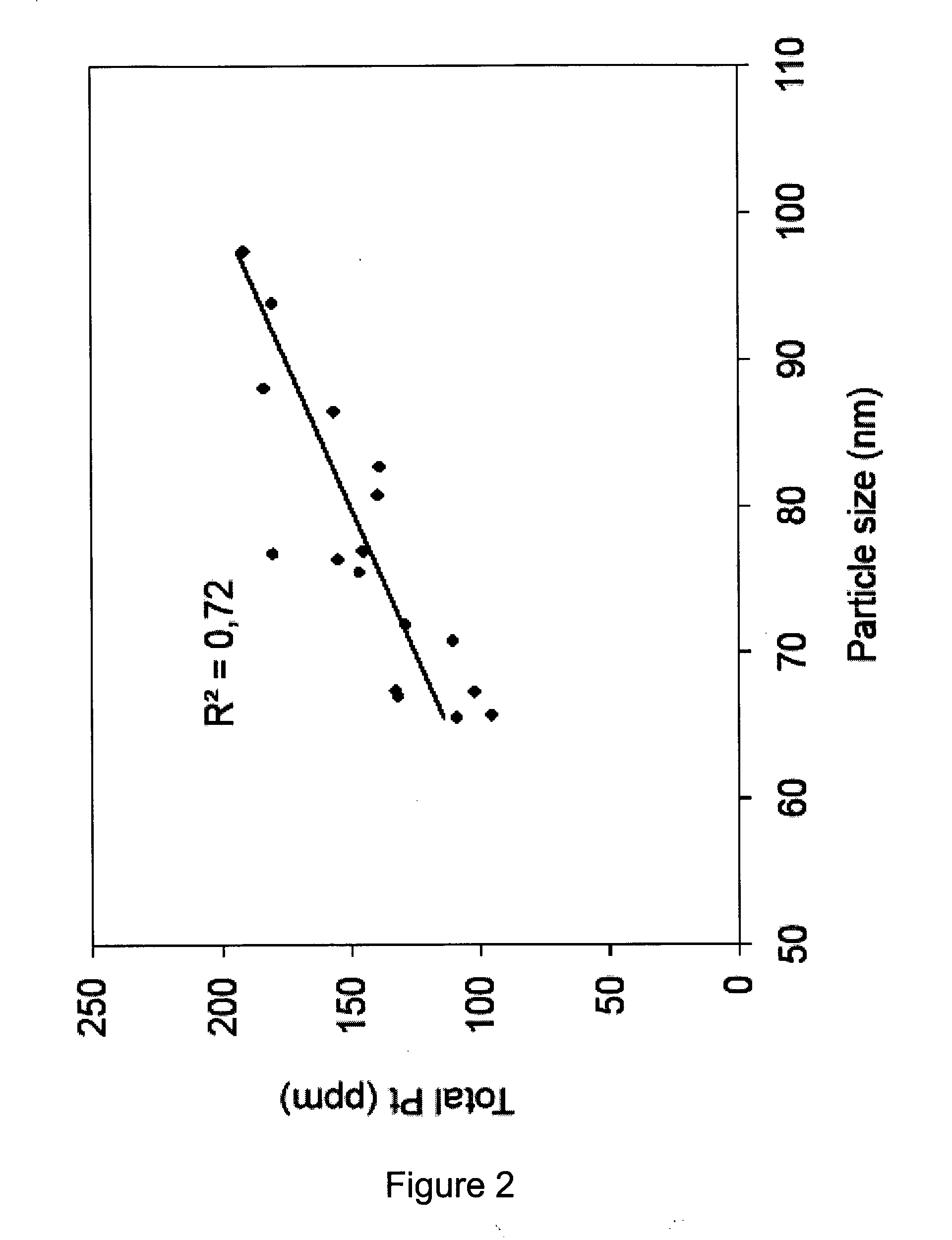
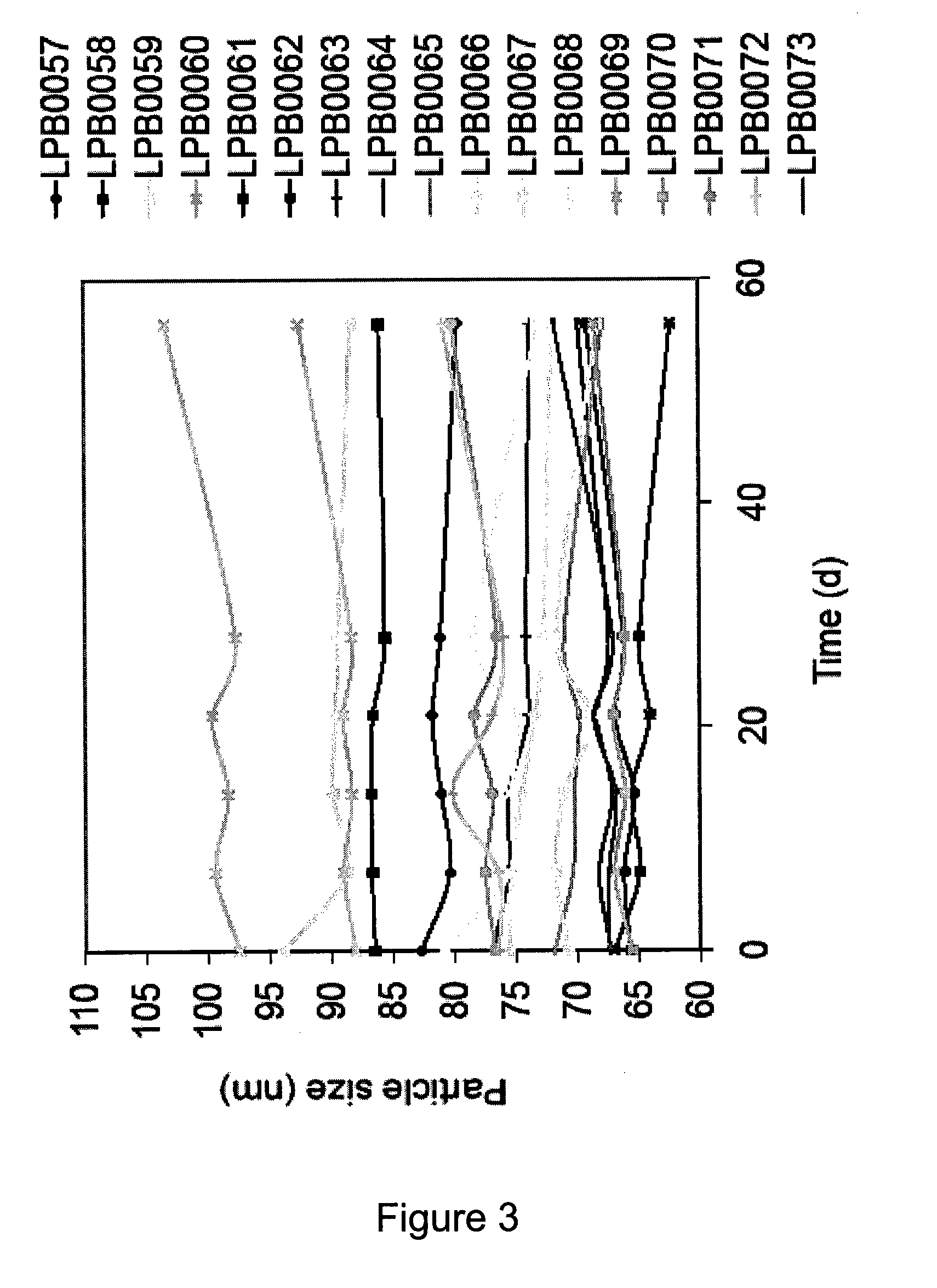
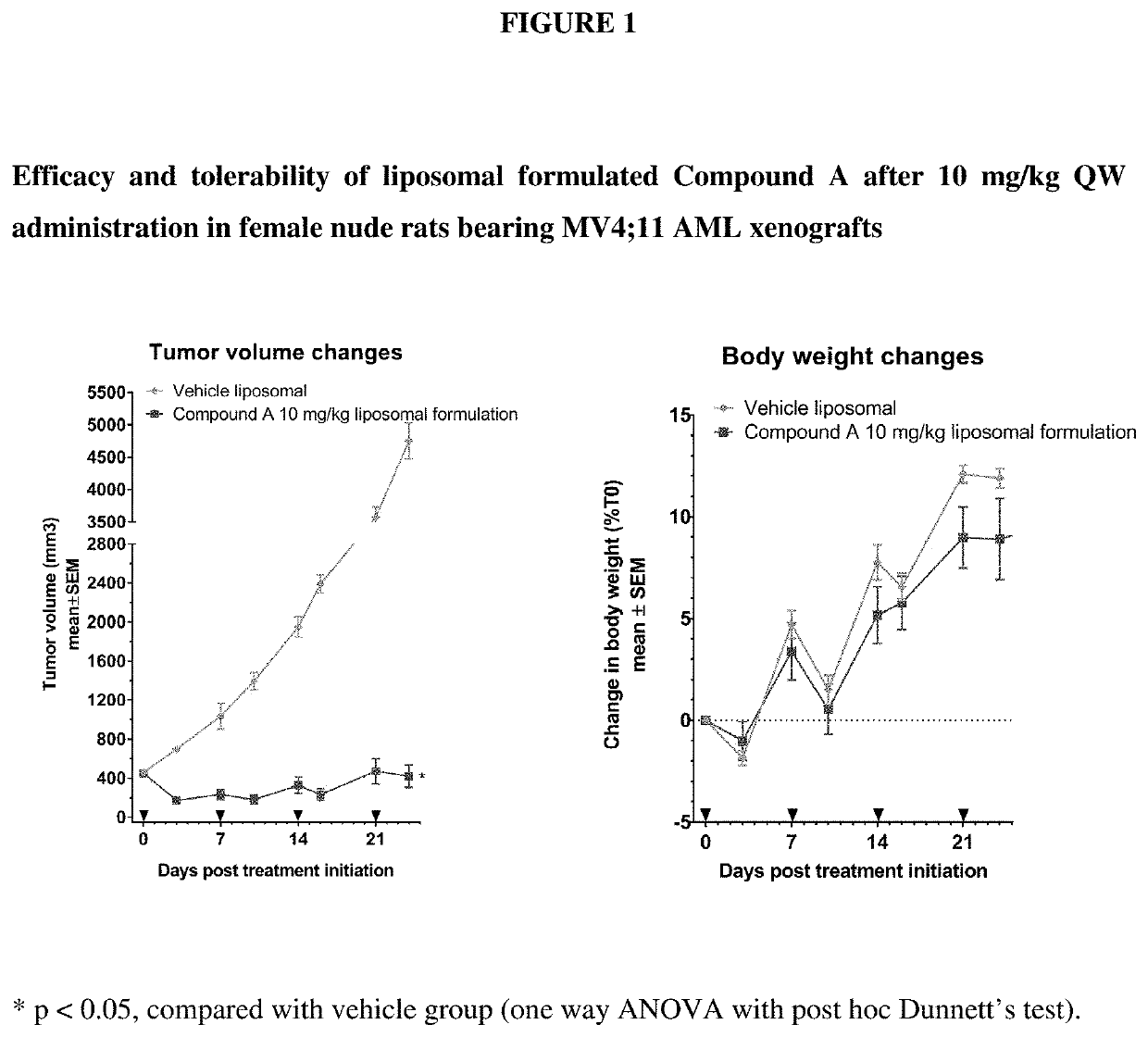
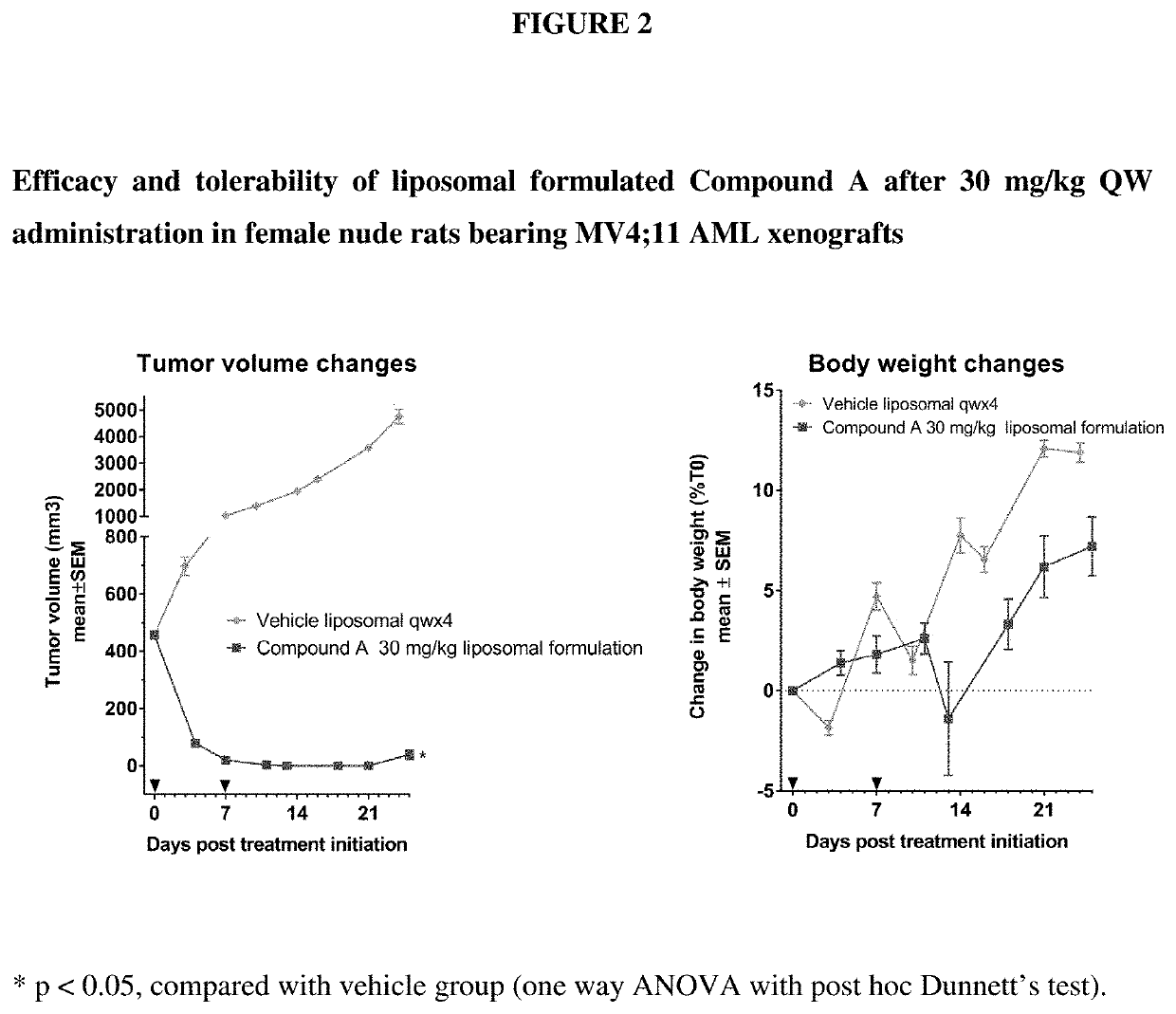
The invention relates to a pharmaceutical liposomal composition comprising 2-{[5-{3-chloro-2-methyl-4-[2-(4-methylpiperazin-1-yl)ethoxy]phenyl}-6-(4-fluorophenyl)thieno [2,3-d]pyrimidin-4-yl]oxy}-3-(2-{[2-(2-methoxyphenyl)pyrimidin-4-yl]methoxy}phenyl) propanoic acid, referred to herein as ‘Compound A’, or a pharmaceutically acceptable salt thereof. More specifically the invention relates to a liposomal vehicle, an organic concentrate composition comprising Compound A, and a pharmaceutical composition for parenteral administration comprising liposomes and Compound A. Furthermore, the invention relates to the use of such compositions for the treatment of cancer. ‘Compound A’ as used herein includes all enantiomers, diastereoisomers, and atropisomers thereof, or mixtures thereof, and also optionally includes the pharmaceutically acceptable salts thereof.
Owner:LES LAB SERVIER +1
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap