Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Pharmaceutical Substances" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gastric retention controlled drug delivery system
ActiveUS20040180088A1Maintain physical integrityFast swellingOrganic active ingredientsNervous disorderControlled drugsControl release
The present invention provides a gastric retention controlled drug delivery system comprising: (a) a controlled release core comprising a drug, a highly swellable polymer and a gas generating agent, said core being capable of swelling and achieving floatation rapidly while maintaining its physical integrity in gastrointestinal fluids for prolonged periods, and (b) a rapidly releasing coat composition comprising the same drug as in the core and pharmaceutically acceptable excipients, wherein the coating composition surrounds the core such that the system provides a biphasic release of the drug in gastrointestinal fluids.
Owner:SUN PHARMA INDS
Nucleoside prodrug and application thereof
ActiveCN113999237AImprove oral bioavailabilityImprove performanceOrganic chemistryAntiviralsAnimal virusOral treatment
The invention relates to a nucleoside prodrug capable of being orally taken for treating mammalian virus infection, and especially relates to a compound shown as a formula (I) or pharmaceutically acceptable salt or stereoisomer thereof, or a pharmaceutical composition thereof, and application of the compound or the composition in preparation of drugs for treating, inhibiting or preventing diseases caused by virus infection.
Owner:RISEN SUZHOU PHARMA TECH CO LTD
Comb amphiphilic polymer using pulullan as main chain, synthetic process and application thereof
InactiveCN104231246AShort synthesis timePharmaceutical non-active ingredientsPolyesterPolymer science
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of mesoporous silica in situ doped acrylic resin bone cement composite for enhancing durable release capacity of medicines
Owner:NANJING DRUM TOWER HOSPITAL
Preparation method of sodium ibandronate
InactiveCN102898466AEasy to operateMeet the quality requirements of raw materialsGroup 5/15 element organic compoundsSkeletal disorderPhosphorous acidChlorobenzene
The invention relates to the technical field of pharmaceutical chemistry, particularly relates to a method of pharmaceutical synthesis, and specifically relates to a preparation method of sodium ibandronate. To overcome the disadvantages of high content of chlorides and phosphites in sodium ibandronate prepared by a conventional preparation method of sodium ibandronate, the preparation method of sodium ibandronate with extremely low content of chlorides and phosphites is provided. In the preparation method, 3-(N-methylpentylamino) propionic acid hydrochloride, phosphorus trichloride and phosphorous acid are employed as raw materials and reacted in a chlorobenzene solvent, so as to obtain sodium ibandronate with extremely low content of the chlorides and the phosphites. The obtained sodium ibandronate can not only meet impurity control standards of the chlorides and the phosphites in a sodium ibandronate crude drug, but also prevent low yield, long period and huge harm to human body and environment which are brought by a lot of refining steps.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Chiral spiro indole-pyran pyridine alkaline compound and preparation and application thereof
The invention discloses a chiral spiro indole-pyran pyridine alkaline compound and a chiral synthesis method thereof, belonging to the field of biochemical technology. In the invention, a novel spiro indole-pyran pyridine alkaline compound is synthesized three-dimensionally and selectively by taking a 4-hydroxy coumarin compound or a 1-naphthol compound and a dicyan oxoindole olefin compound as reaction substrates and taking rosin-derived chiral thiourea as a catalyst. As proved by a biological activity experiment, the chiral spiro indole-pyran pyridine alkaline compound can selectively kill tumor cells, has strong actions on tumors of different types and has high selective broad spectrum anti-tumor activity. The chiral spiro indole-pyran pyridine alkaline compound can be taken as an anticancer active substance, and has a good application prospect in preparation of anticancer medicaments.
Owner:LANZHOU UNIV
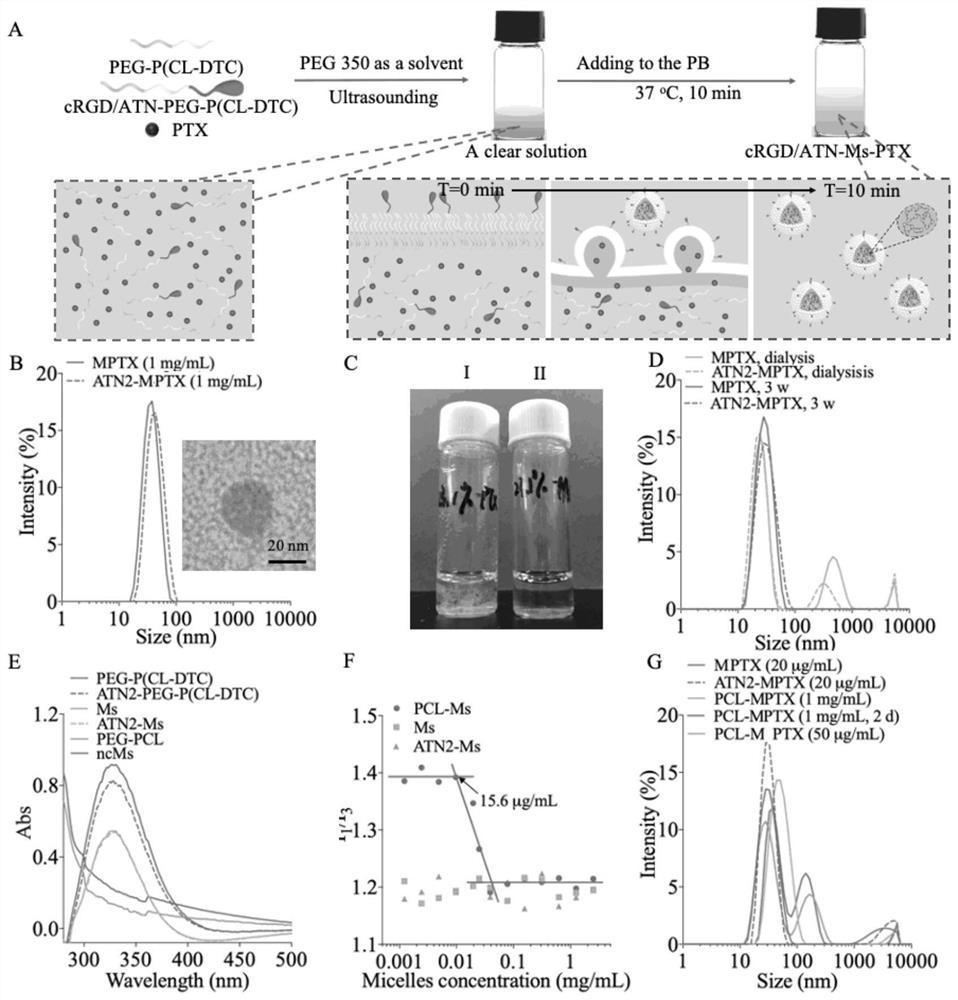
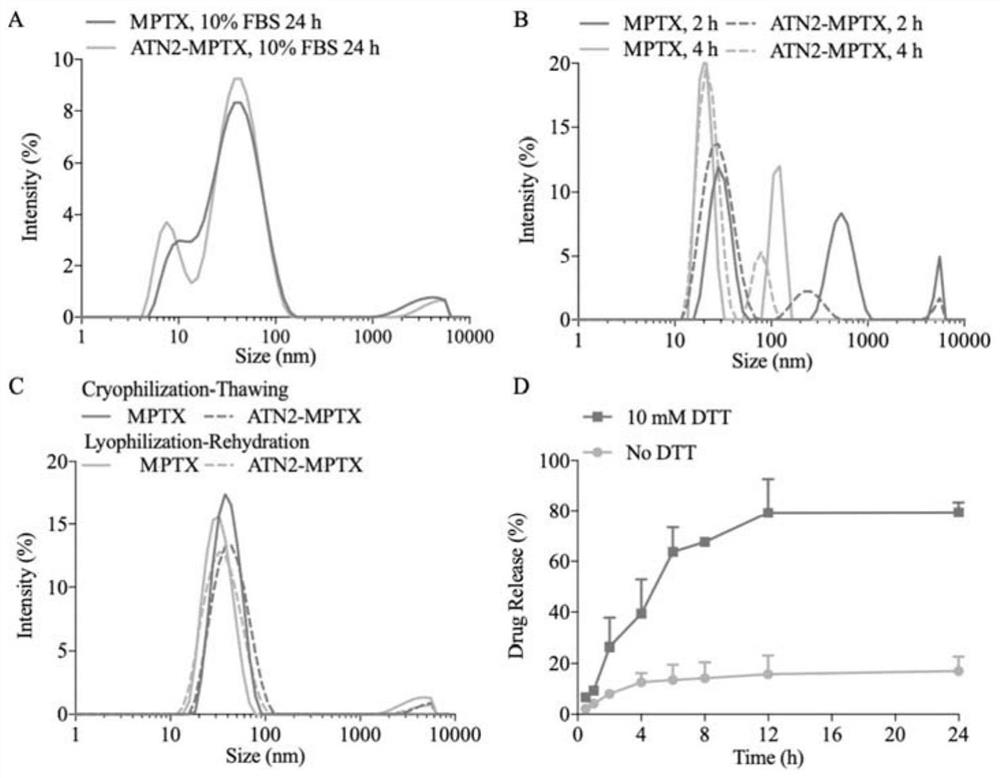
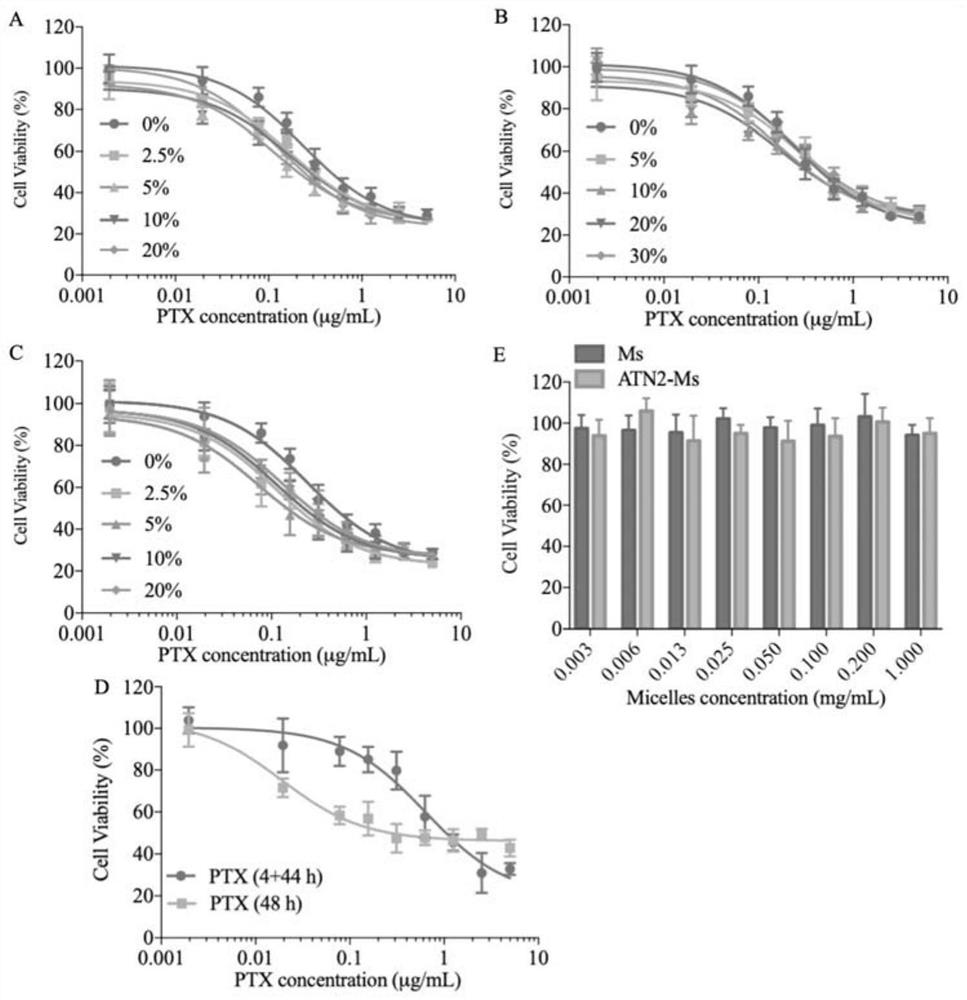
Small micelle nano-drug as well as preparation method and application thereof
PendingCN113332241AHigh biodistributionPromote value-addedOrganic active ingredientsPharmaceutical non-active ingredientsPolythylene glycolCombinatorial chemistry
Owner:SUZHOU UNIV
Compound clobetasol propionate nano-medicament and preparation method thereof
InactiveCN102327273ASystem stabilityOrganic active ingredientsAntimycoticsPropanoic acidEczematous rash
Owner:NORTHWEST A & F UNIV
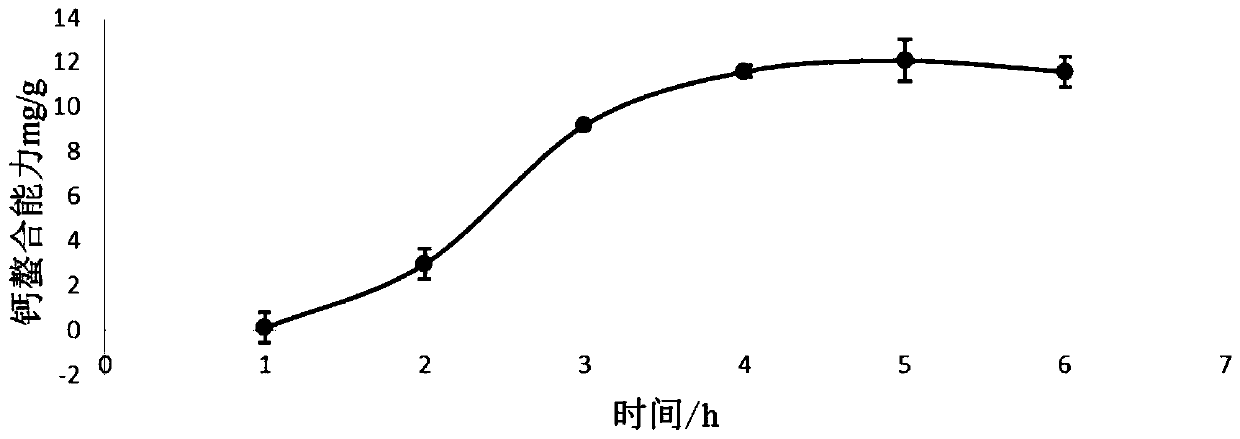
Novel calcium chelating peptide as well as preparation method and application thereof
ActiveCN110669127AEasy to transportImprove absorption and utilizationConnective tissue peptidesPeptide preparation methodsPhysiochemical ActivityPharmaceutical Substances
Owner:SOUTH CHINA AGRI UNIV
Albumin oil-in-water emulsion capable of generating flexible deformation as well as preparation method and application of albumin oil-in-water emulsion
ActiveCN112999154AExtended half-lifeSmall particle sizePeptide/protein ingredientsHydroxy compound active ingredientsOil emulsionOil phase
The invention provides an albumin oil-in-water emulsion capable of generating flexible deformation as well as a preparation method and application of the albumin oil-in-water emulsion. The albumin oil-in-water emulsion comprises an oil phase, a water phase and albumin, the albumin is dispersed in the water phase and / or adsorbed on an oil-water interface. The emulsion provided by the invention is an oil-in-water emulsion which is stable in albumin and can generate flexible deformation, has bionic flexibility, and can penetrate through tissue gaps through deformation, so that a stronger tissue permeation effect is realized. The albumin and the oil-in-water emulsion are combined to prepare the oil-in-water emulsion which has deformability and can enhance tissue permeability, the oil-in-water emulsion is applied to the fields of drug adjuvants or carriers and the like, the lymph node delivery and tissue permeation efficiency can be improved, and the effectiveness of vaccines or drugs is improved.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
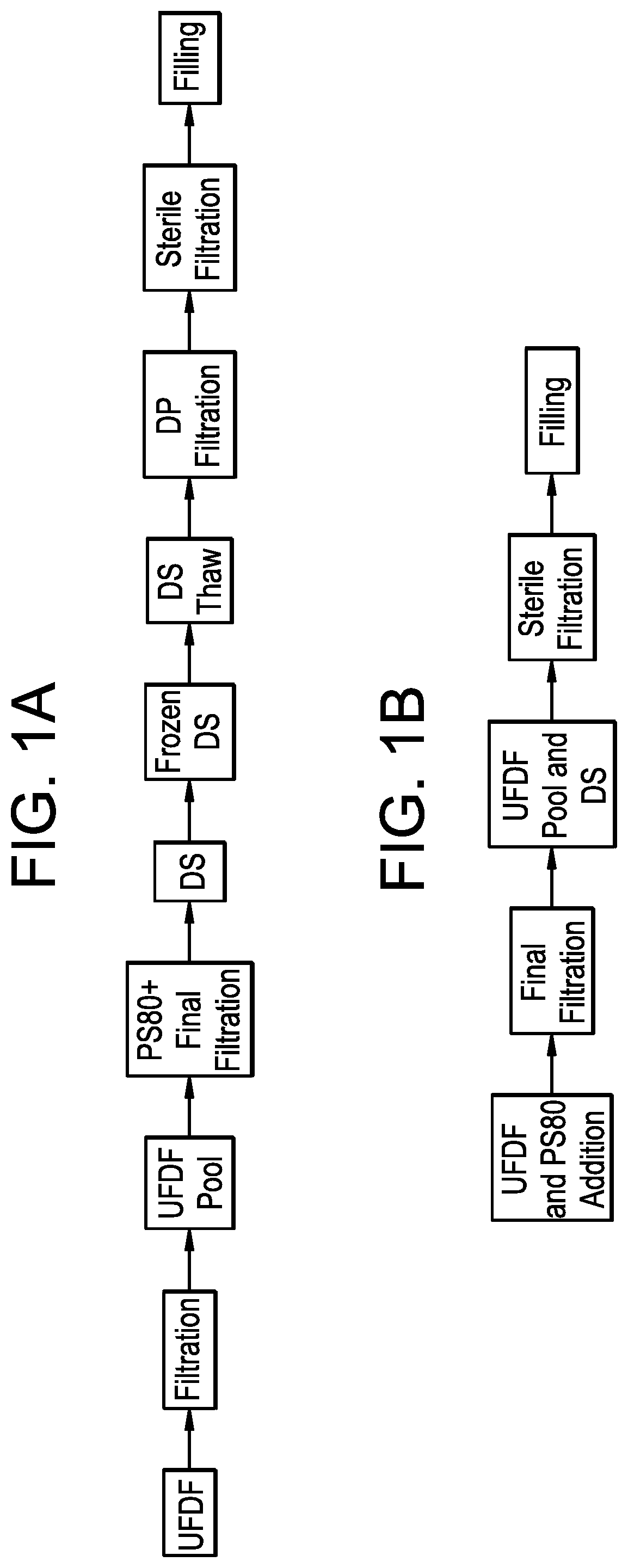
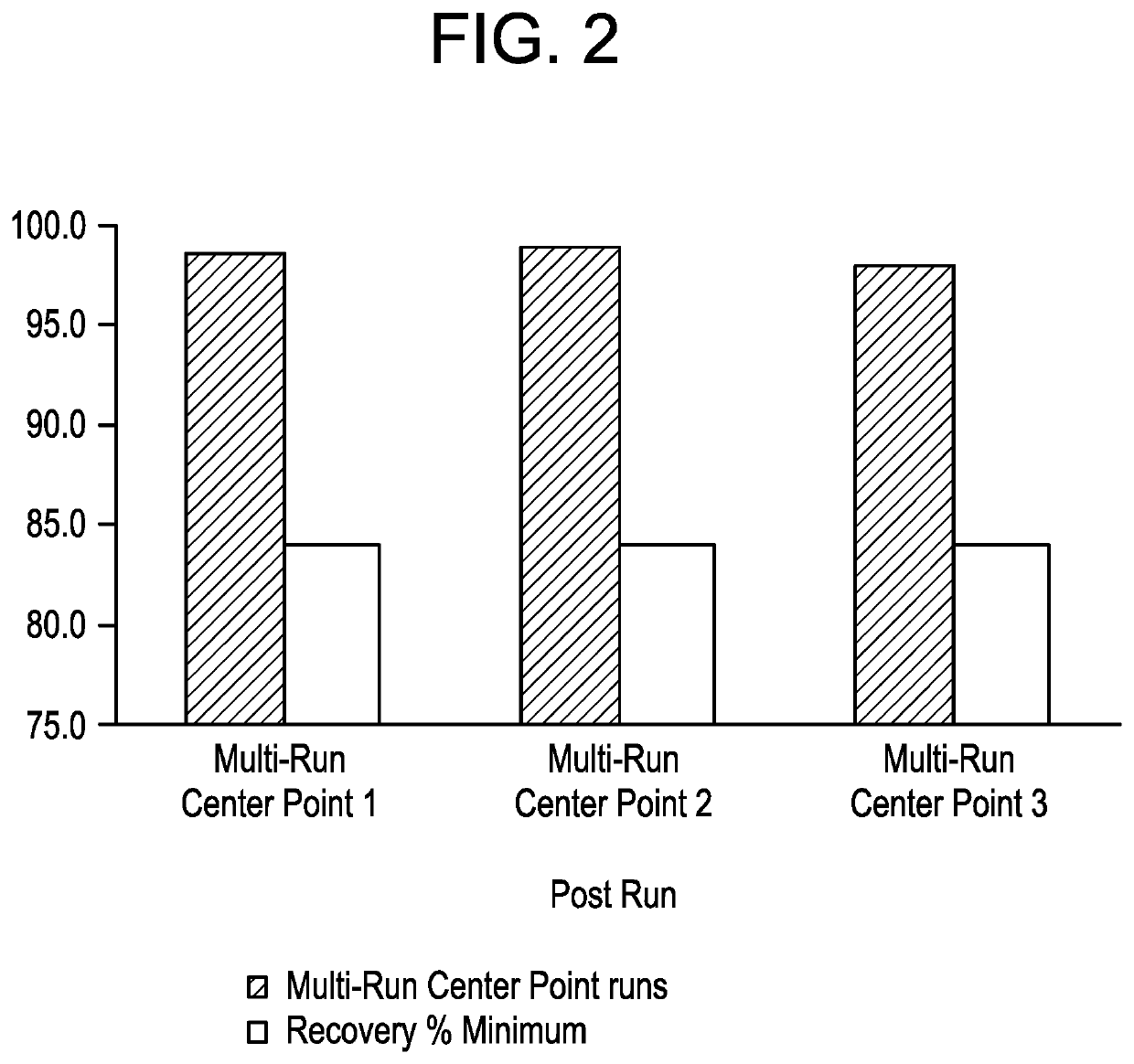
A continuous manufacturing process for biologics manufacturing by integration of drug substance and drug product processes
PendingUS20220119526A1Reduced footprintReducing drug substance lossSemi-permeable membranesMembranesProcess engineeringDrug product
Owner:AMGEN INC
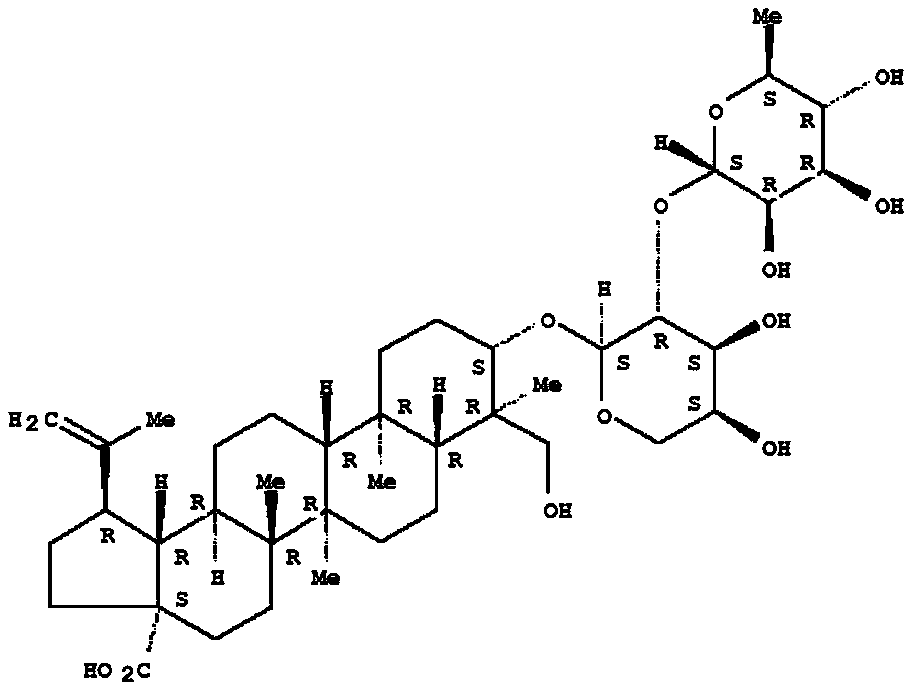
Application of pulsatilla saponin A3 in inhibiting growth of multi-drug-resistant Providencia rettgeri
Owner:SHAANXI UNIV OF SCI & TECH
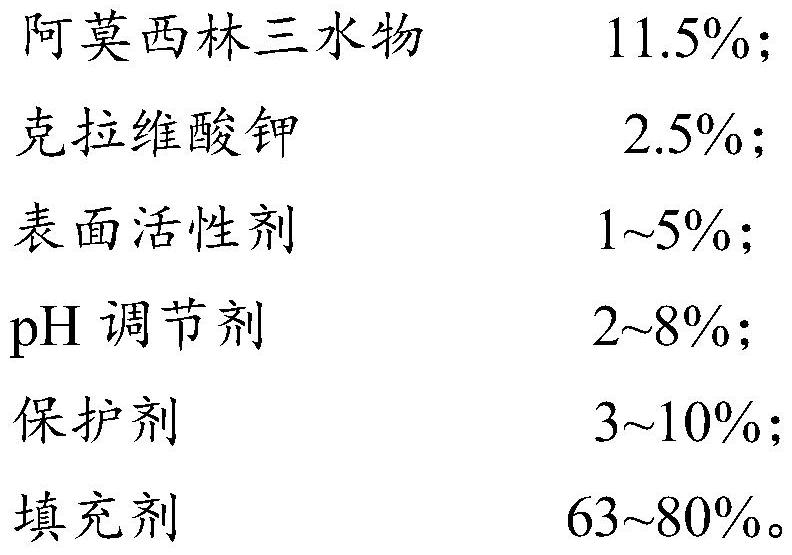
Compound amoxicillin soluble powder and preparation method thereof
InactiveCN113712923ASolubility Promotion and StabilizationImprove solubilityPowder deliveryAntibacterial agentsActive agentPharmaceutical Substances
Owner:济南鑫宝星动物药业有限公司
Method of synthesizing antimicrobial silver nanoparticles using pigeon dung
InactiveUS20200268807A1Readily apparentInorganic active ingredientsBird material medical ingredientsPharmaceutical SubstancesAnti bacterial
Owner:KING SAUD UNIVERSITY
Ferulic acid derivative as well as preparation method and application thereof
ActiveCN114181193AGood nephritis curative effectHigh activityOrganic active ingredientsOrganic chemistryEfficacyPharmaceutical Substances
Owner:成都亨达药业有限公司
Application of KMT2D in preparation of antitumor drugs
ActiveCN112057608ARegulation of proliferationRegulation of apoptosisPeptide/protein ingredientsTransferasesProtein structureProtein level
The invention discloses an application of KMT2D in the preparation of antitumor drugs, and belongs to the technical field of biological medicines. A protein structure of KMT2D is analyzed through a bioinformatics means, two essentially disordered structures LCD1 and LCD2 of the KMT2D protein are finally obtained through screening, and the influence of the two structures on the KMT2D function is researched by combining the gene editing technology, the change (including mRNA and protein levels) of a KMT2D enzymatic complex is further researched, and thereby a foundation is laid for the deep understanding of a mechanism of KMT2D promoting tumor.
Owner:NANTONG UNIVERSITY
7-(3-aminomethyl-4-substituted-benzyloxyimino-1-pyrrolidinyl)naphthyridinone carboxylic acid compounds
InactiveCN104892600AHigh activityExcellent in vitro activityOrganic active ingredientsAntibacterial agentsNaphthyridinoneAntituberculosis drug
The invention relates to 7-(3-aminomethyl-4-substituted-benzyloxyimino-1-pyrrolidinyl)naphthyridinone carboxylic acid compounds disclosed as Formula (I), a preparation method and medical application thereof, and an antituberculosis drug composition using the compounds as effective components, particularly, 6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridinyl-3-carboxylic acid compounds, wherein the 1- substituent group is (1R,2S)-2-fluorocyclopropyl group, the 7- substituent group is 3-aminomethyl-4-substituted-benzyloxyimino-1-pyrrolidinyl group, and R represents fluorine, chlorine, bromine, methyl, methoxy, dimethoxy or methynedioxy group.
Owner:ZHEJIANG STARRY PHARMA +1
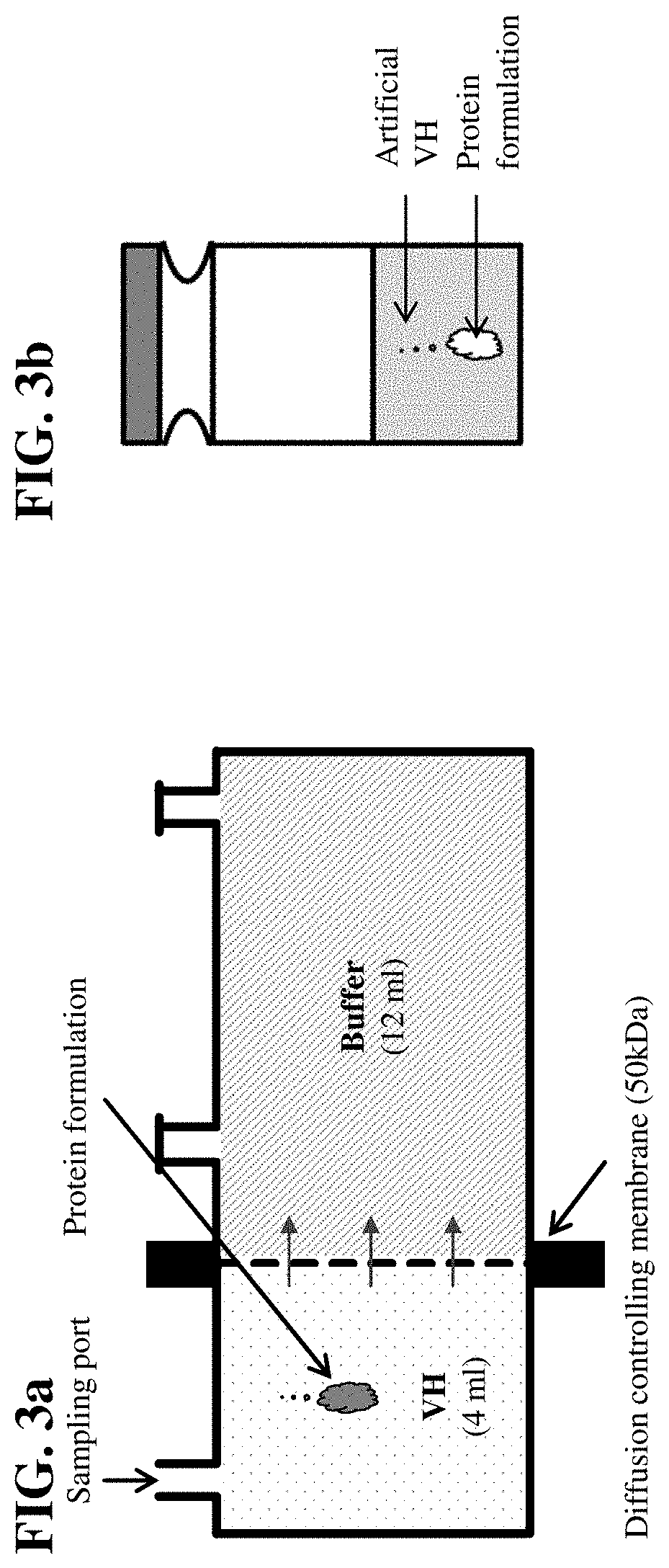
Artificial Vitreous Humor for the Investigation of Drugs and Drug Formulations
InactiveUS20200345636A1Inorganic non-active ingredientsPharmaceutical delivery mechanismVitreous HumorsP phosphate
Owner:F HOFFMANN LA ROCHE INC
Application of stilbene compounds in treating and preventing AIDS
The invention provides an application of stilbene compounds in treating and preventing AIDS. The stilbene compounds have a structural formula represented by the general formula (I), wherein R1, R2, R3, R4, R5 or R6 independently represents hydrogen, hydroxyl, C1-C4 alkyl, C1-C4 alkoxy, C1-C4 acyloxy, halogen, nitro, trifluoromethyl, or cyano, provided that at least one of R1, R2 and R3 is hydroxyl and at least one of R4, R5 and R6 is hydroxyl. In the compounds represented by the general formula (I), the inhibition activity IC50 of a stilbene compound piceatannol against HIV-1 protease reaches 59mum.
Owner:TIANJIN INT JOINT ACADEMY OF BIOTECH & MEDICINE
Solid disperse granules for treating peptic ulcers
InactiveCN106421147AGood pain reliefLittle side effectsPowder deliveryDigestive systemDrugUlcer care
The invention relates to solid disperse granules for treating peptic ulcers. The solid disperse granules are prepared from 1 part by weight of solid dispersoids and 1-3 parts by weight of filler, wherein the solid dispersoids are prepared from raw material medicine powder of polyethylene glycol-6000 equal to (1-2):(3-5) w / w; the raw material medicine powder is prepared by smashing raw material medicine of, by weight, 1-3 parts of girald daphne bark, 7-13 parts of rhizoma atractylodis macrocephalae, 2-3 parts of ganoderma, 5-10 parts of radix codonopsis, 5-10 parts of radix pseudostellariae, 5-10 parts of herbal artemisiae scopariae, 5-10 parts of herbal taraxaci, 3-7 parts of radix platycodonis, 3-7 parts of herbal pogostemonis and 2-4 parts of myristica fragrans to be of 200-500 mesh. The raw material medicine powder is prepared into solid dispersoids, the bioavailability of the drug is improved, and the anti-ulcer activity is obviously improved. However, the solid dispersoids have an excessively large specific surface area, absorb damp easily and are not suitable for storage and production, poor in fluidity and not convenient to produce and package. Thus, the solid dispersoids are prepared into the granules which facilitate storage, transportation and packaging of products and are convenient to use by patients.
Owner:门源县雪草木生物药业有限公司
Fipronil and methoprene dihydric alcohol plastid, and preparation method and application thereof
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Alkaloid compound, preparation method and application
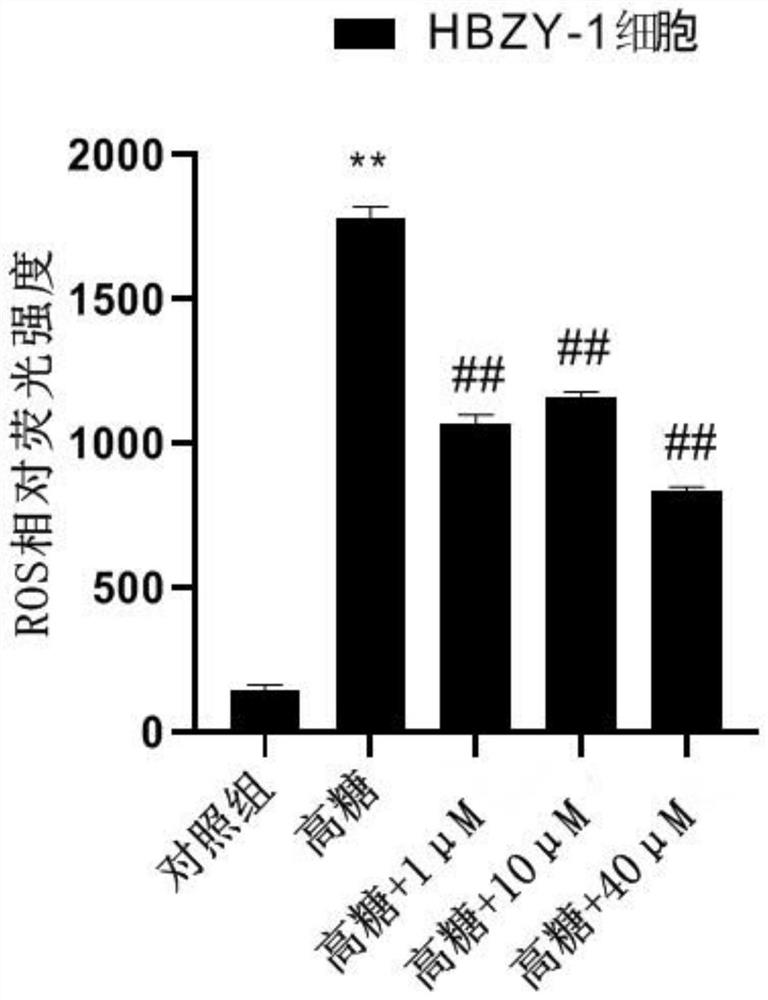
The invention belongs to a natural extraction compound as well as a preparation method and application thereof. In order to solve the technical problems that existing hypoglycemic drugs are more in variety, the side effect is still great, the curative effect on complications is poor and the drug resistance easily occurs with medication time extension, the invention provides an alkaloid compound aswell as a preparation method and application thereof. The molecular formula of the alkaloid compound is C46H63N3O23, and the compound is an indoline dimer alkaloid. The preparation method comprises the following steps: extracting total extract from calanthe discolor, extracting the total extract, and carrying out column chromatography separation on an n-butyl alcohol extraction layer. The preparation method is simple, convenient and effective, the alkaloid compound is obtained, the purity is high, the cost is low, and verification proves that the alkaloid compound can be applied to anti-inflammatory drugs and anti-diabetic drugs.
Owner:XI AN JIAOTONG UNIV
Double-drug skeleton polymer as well as preparation method and application thereof
ActiveCN111925517ARelease stabilityPrecision releaseKetone active ingredientsPharmaceutical non-active ingredientsCombinatorial chemistryDrug release
The invention discloses a double-drug skeleton polymer as well as a preparation method and application thereof. The double-drug skeleton polymer can be self-assembled in a water phase to form nano-micelles and is used as a double-drug transport carrier, specific response drug release can be realized on a reductive microenvironment of tumor tissues, and two drugs can be released in a stable and accurate proportion.
Owner:SOUTH CHINA UNIV OF TECH
Topical medicaments
Owner:ANKH LIFE SCI LTD
Drug mixing device
A drug mixing device is disclosed. The drug mixing device comprises a movable actuator, wherein movement of the actuator causes mixing of a drug within the drug mixing device; and an elastic member coupled to the actuator. The elastic member is configured upon release to transition from an extended state to a non-extended state, the elastic member storing energy in the extended state. The movementof the actuator is driven by the release of the stored energy as the elastic member transitions from the extended state towards the non-extended state.
Owner:JANSSEN BIOTECH INC
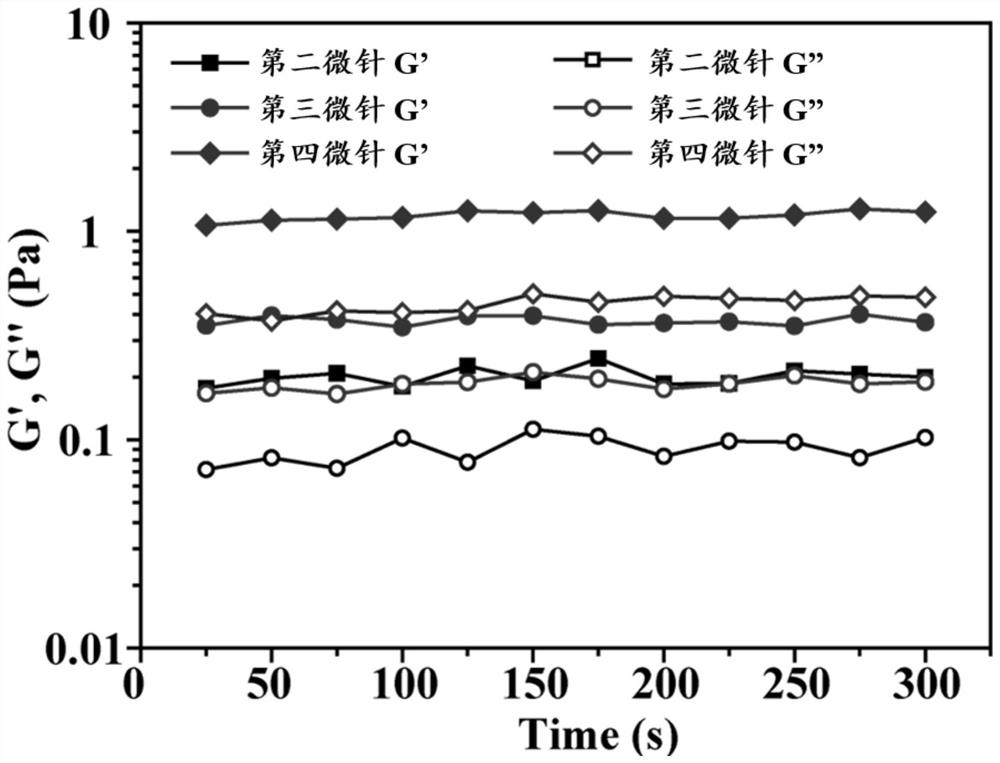
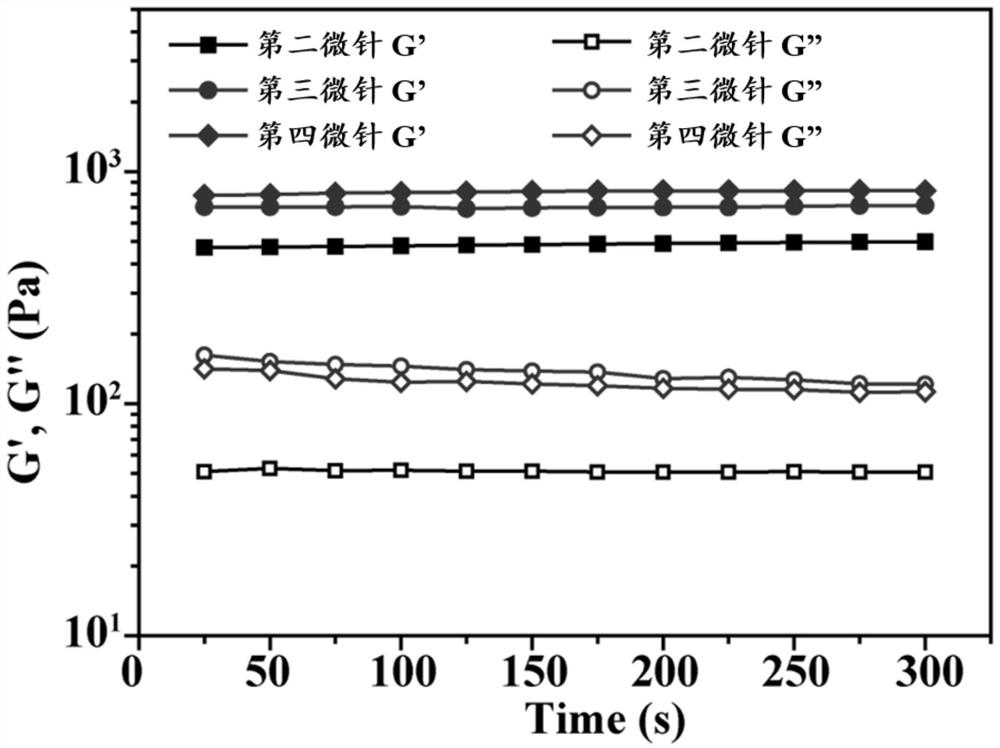
Microneedle for treating vitiligo as well as preparation method and application thereof
ActiveCN114796097AGood application effectReduce the number of dosesOrganic active ingredientsPeptide/protein ingredientsMelanophore-dispersing hormoneTofacitinib
Owner:广州市皮肤病防治所
Lyotropic liquid crystal precursor as well as preparation method and application thereof
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Chinese and western medicine composition for improving hypoglycemic ineffectiveness of oral metformin and preparation method and application thereof
InactiveCN112826854ASimple compositionRaise the ratioOrganic active ingredientsMetabolism disorderDiabrezideLicorice roots
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
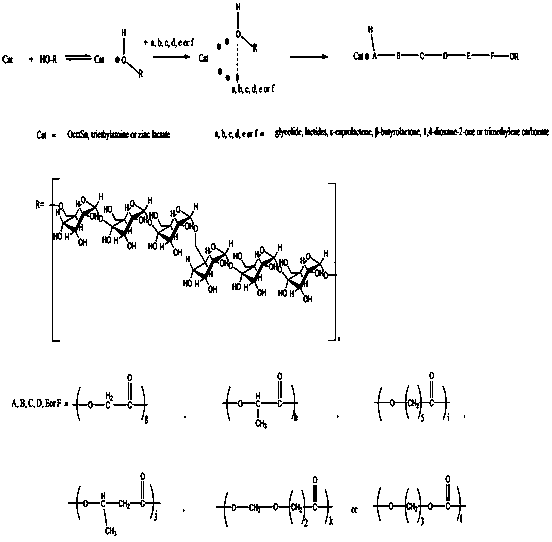
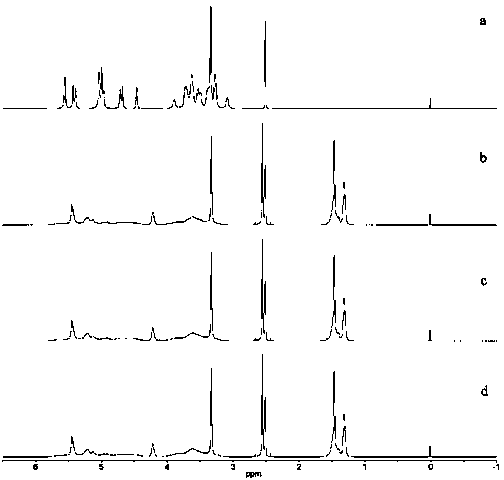
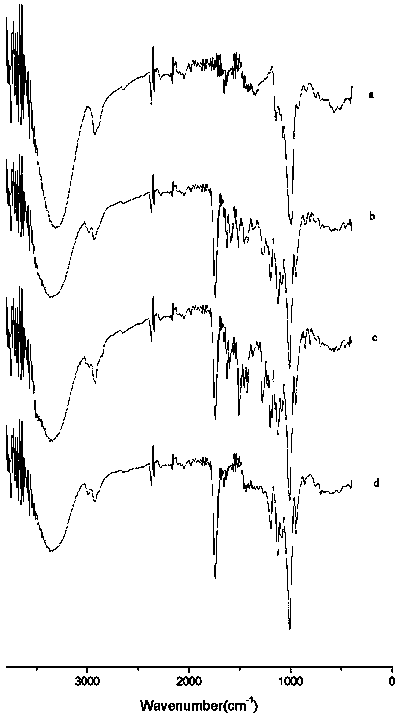
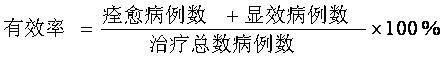
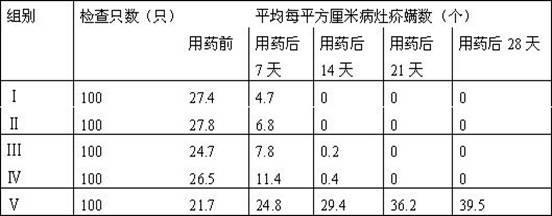
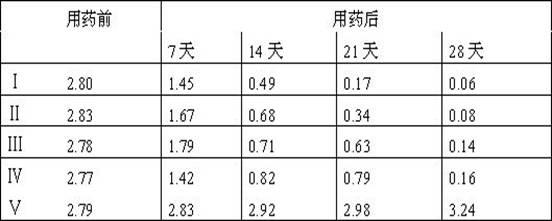
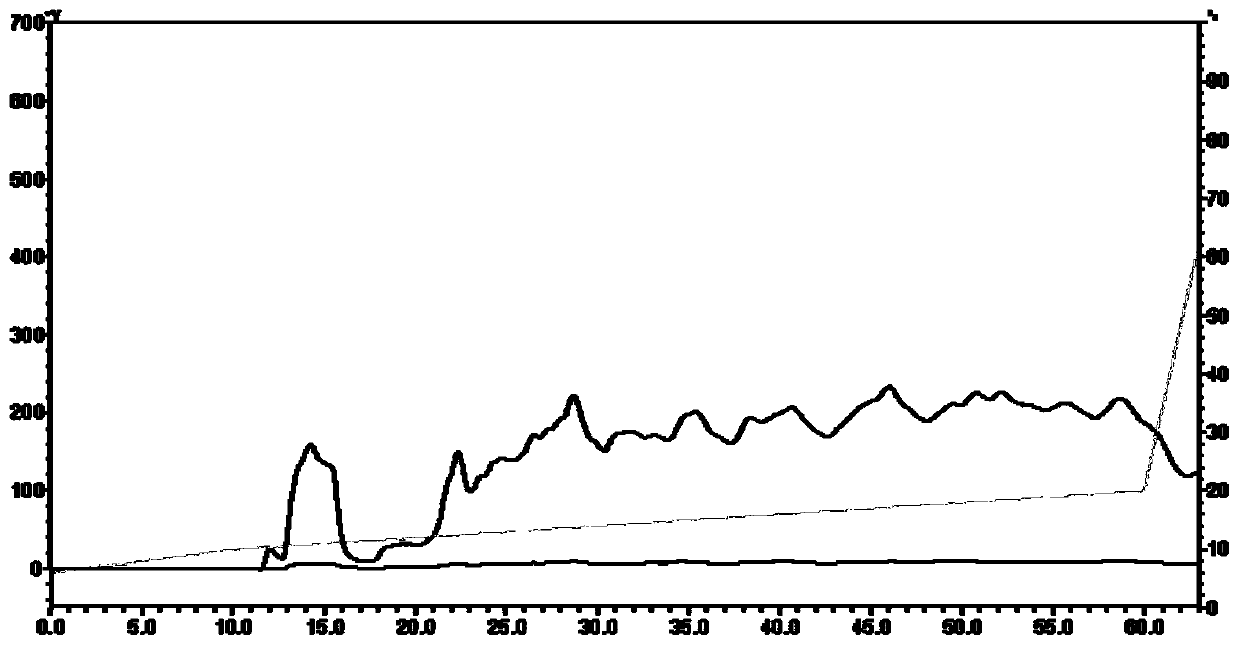
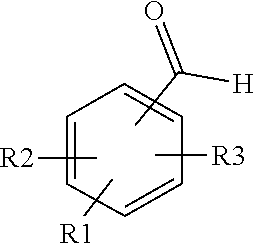
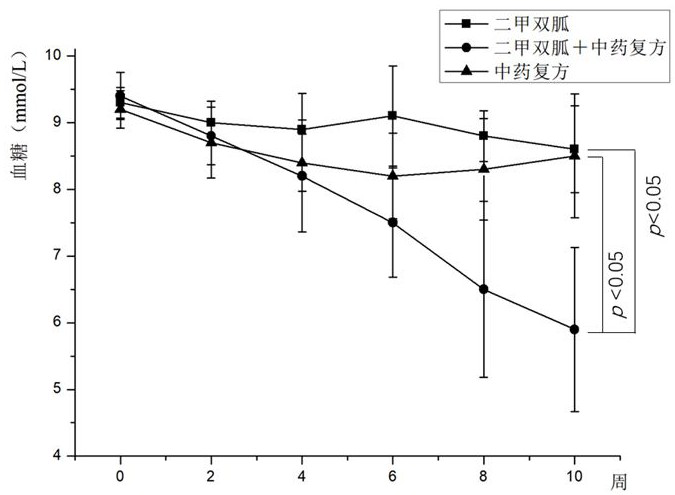
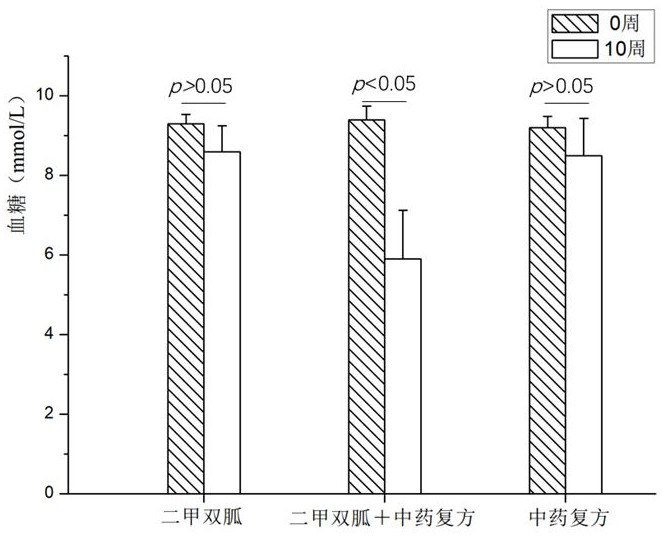
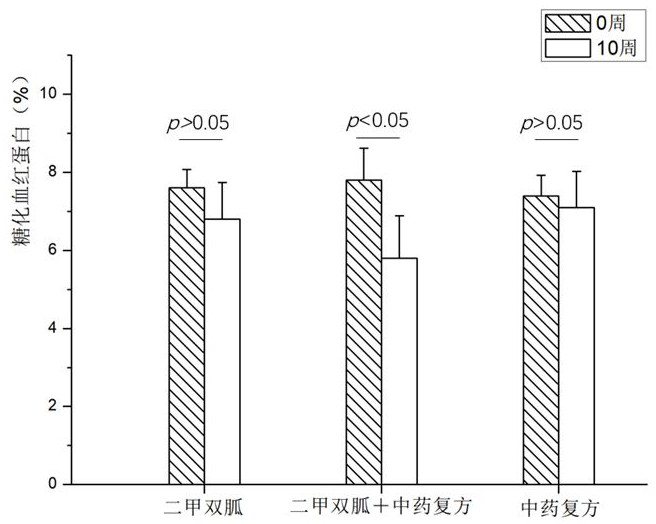
SUBSTITUTED 3-ISOBUTYL-9,10-DIMETHOXY-1,3,4,6,7,11B-HEXAHYDRO-2H-PYRIDO[2,1-a]ISOQUINOLIN-2-OL COMPOUNDS, THEIR SYNTHESIS, AND USE THEREOF
Owner:DISPERSOL TECH
Compound medicine containing Rosa laevigata Michx and ceftiofur sodium and used for livestock and poultry
InactiveCN106236879AGood synergyGood curative effectOrganic active ingredientsAntibacterial agentsBiotechnologyCeftiofur sodium
The invention relates to compound medicine containing Rosa laevigata Michx and ceftiofur sodium and used for livestock and poultry. The compound medicine comprises the Rosa laevigata Michx and the ceftiofur sodium, the weight ratio of the ceftiofur sodium to the Rosa laevigata Michx is (1:10)-(1:30) and is 1:10 preferably. The invention further relates to a compound preparation prepared by the compound medicine. The compound medicine has the advantages that experimental researches prove that when the ceftiofur sodium and the Rosa laevigata Michx are used in a combined manner according to a specific weight ratio, an evident synergic effect is achieved, and the compound medicine is quick in action, low in cost and the like.
Owner:GUANGXI UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap




![SUBSTITUTED 3-ISOBUTYL-9,10-DIMETHOXY-1,3,4,6,7,11B-HEXAHYDRO-2H-PYRIDO[2,1-a]ISOQUINOLIN-2-OL COMPOUNDS, THEIR SYNTHESIS, AND USE THEREOF SUBSTITUTED 3-ISOBUTYL-9,10-DIMETHOXY-1,3,4,6,7,11B-HEXAHYDRO-2H-PYRIDO[2,1-a]ISOQUINOLIN-2-OL COMPOUNDS, THEIR SYNTHESIS, AND USE THEREOF](https://images-eureka.patsnap.com/patent_img_release/d5b0f2ca-dbac-42a0-97c6-7b3364c5c7bb/US20210087191A1-C00001.png)
![SUBSTITUTED 3-ISOBUTYL-9,10-DIMETHOXY-1,3,4,6,7,11B-HEXAHYDRO-2H-PYRIDO[2,1-a]ISOQUINOLIN-2-OL COMPOUNDS, THEIR SYNTHESIS, AND USE THEREOF SUBSTITUTED 3-ISOBUTYL-9,10-DIMETHOXY-1,3,4,6,7,11B-HEXAHYDRO-2H-PYRIDO[2,1-a]ISOQUINOLIN-2-OL COMPOUNDS, THEIR SYNTHESIS, AND USE THEREOF](https://images-eureka.patsnap.com/patent_img_release/d5b0f2ca-dbac-42a0-97c6-7b3364c5c7bb/US20210087191A1-C00002.png)
![SUBSTITUTED 3-ISOBUTYL-9,10-DIMETHOXY-1,3,4,6,7,11B-HEXAHYDRO-2H-PYRIDO[2,1-a]ISOQUINOLIN-2-OL COMPOUNDS, THEIR SYNTHESIS, AND USE THEREOF SUBSTITUTED 3-ISOBUTYL-9,10-DIMETHOXY-1,3,4,6,7,11B-HEXAHYDRO-2H-PYRIDO[2,1-a]ISOQUINOLIN-2-OL COMPOUNDS, THEIR SYNTHESIS, AND USE THEREOF](https://images-eureka.patsnap.com/patent_img_release/d5b0f2ca-dbac-42a0-97c6-7b3364c5c7bb/US20210087191A1-C00003.png)