A continuous manufacturing process for biologics manufacturing by integration of drug substance and drug product processes
a manufacturing process and biologic technology, applied in the field of biologic manufacturing process, can solve the problems of increased manufacturing cost and material waste, potential etc., and achieve the effect of reducing the manufacturing footprint of drug product production process and reducing loss of drug substance and/or destabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
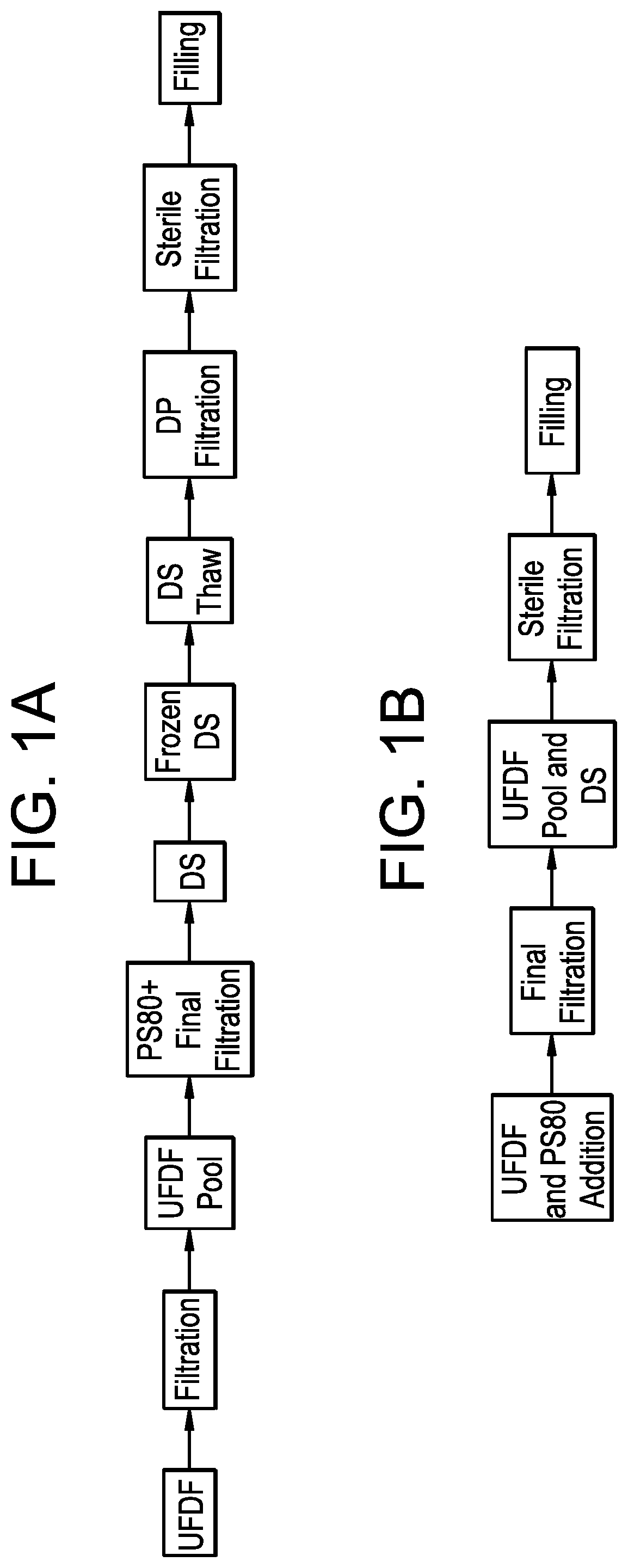
Connected DS-DP Operation
[0130]A 50 L bioreactor run was performed to produce a recombinant monoclonal antibody and forward-processed through a series of purification unit operations until the viral filter pool was obtained. An Akta flux 6 skid (GE Healthcare, Piscataway, N.J.) was used for UFDF. A Millipore Pellicon cassette holder was used to house the 30 kD UFDF membrane that totaled an area of 1.14 m2 (Millipore Sigma, Burlington, Mass.). Opticap XL 600 Sterile High Capacity filter (Millipore Sigma, Burlington, Mass.) was used as a bioburden-reduction filter. The final drug substance was collected in a Mobius Single-Use Mixing bag (Millipore Sigma, Burlington, Mass.).
[0131]The viral filter pool was used as the starting material for UFDF operation, Polysorbate 80 (PS80) addition and final 0.2-micron filtration. The skid and other product-contacting components were held in 0.2 N sodium hydroxide overnight prior to starting the operations. The formulation buffer used for diafiltration
example 3
High Membrane Loading and Increased Diavolumes for UFDF
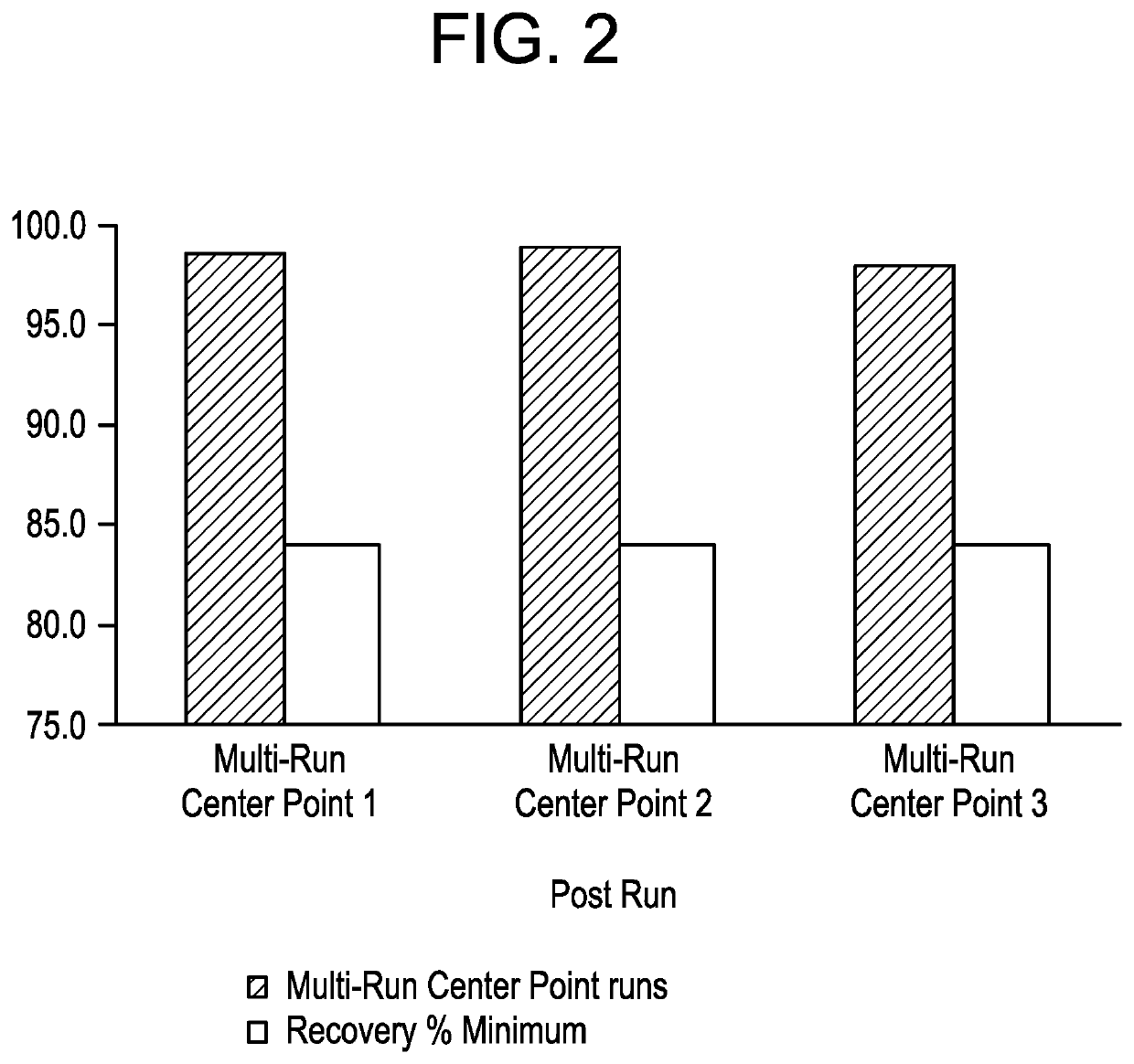
[0145]This experiment assessed ultrafiltration-diafiltration performance using a regenerated cellulose membrane specifically challenged with high membrane loading and increased number of diavolumes on product quality performance, using a half-life extended bispecific T cell engager molecule feed stream.
[0146]Frozen eluate pool material from a multimodal anionic (MMA) column comprising a half-life extended bispecific T cell engager, was thawed before processing. The eluate pool material was then loaded on to a regenerated cellulose membrane (Pellicon 3 (10 kD MWCO cutoff) (EDM Millipore, Danvers, Mass.)), with membrane area 0.0088 m2. The experimental conditions are summarized Table 3. All the experiments were performed using an AKTA crossflow UF / DF skid (GE Healthcare, Chicago, Ill.).
[0147]The filter was equilibrated with 100 mM Acetate, 180 mM NaCl, pH 5.0. Following membrane equilibration, the eluate pool material was concentra
example 4
Viral Filtration of a Formulated Bispecific T Cell Engager
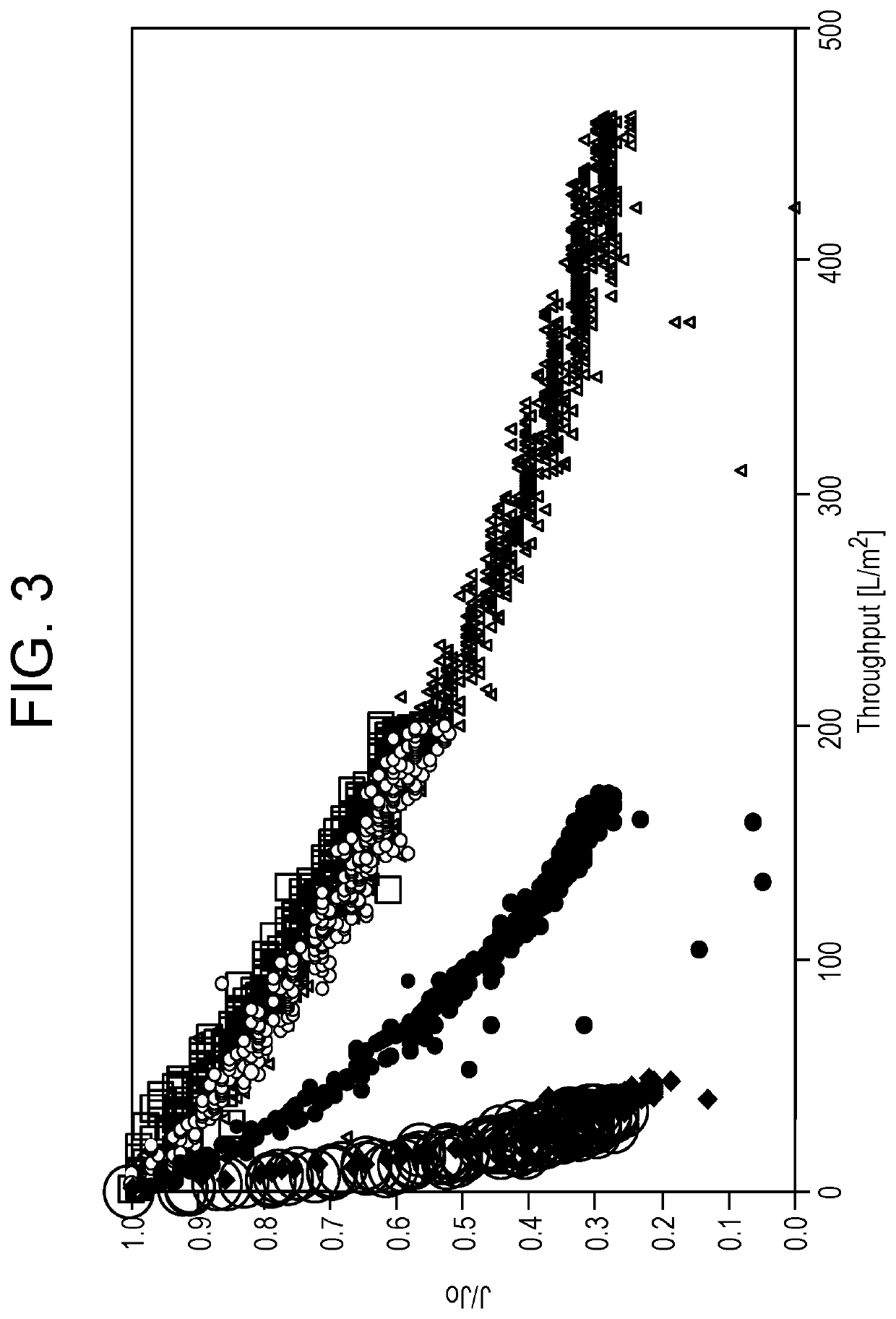
[0149]This experiment demonstrated the viral filtration of a half-life extended bispecific T cell engager (HLE BiTE®) in formulation buffer.
[0150]Half-life extended bispecific T cell engagers at various concentrations in a formulation buffer, 9% Sucrose, 10 Mm Glutamate, pH 4.2, was evaluated with respect to process and product quality performance using a hydrophilized polyvinylidene fluoride (PVDF) hollow fiber filter (Plavona™ BioEx) and a cuprammonium-regenerated cellulose hollow fiber filter (Planova 20N), 0.001 m2 virus removal filters (Asahi Kasei Bioprocesses, Glenville, Ill.) in a filter train at constant pressure. The filter train consisted of a pressure regulator connected to a pressure reservoir having a valve that was connected to the virus removal filter. The virus removal filter opened directly to a collection vessel attached to a balance. The filter train was connected to a computer for data collection and to a co
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Electrical conductivity | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap