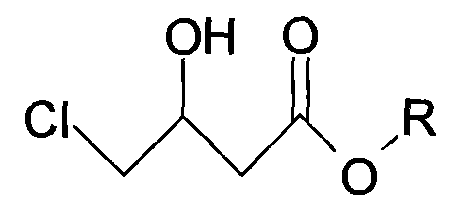
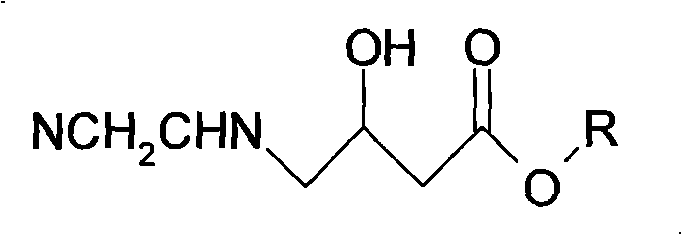
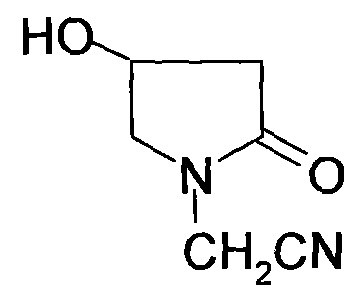
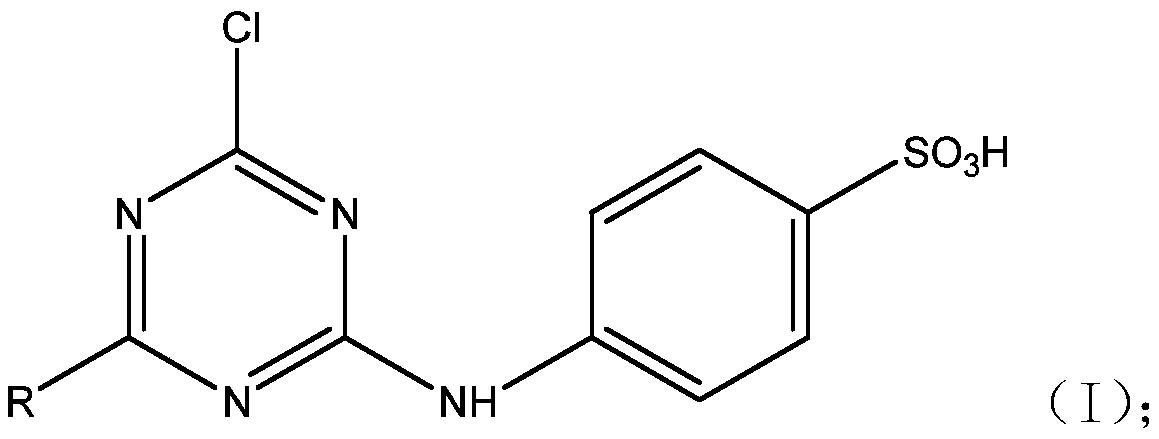
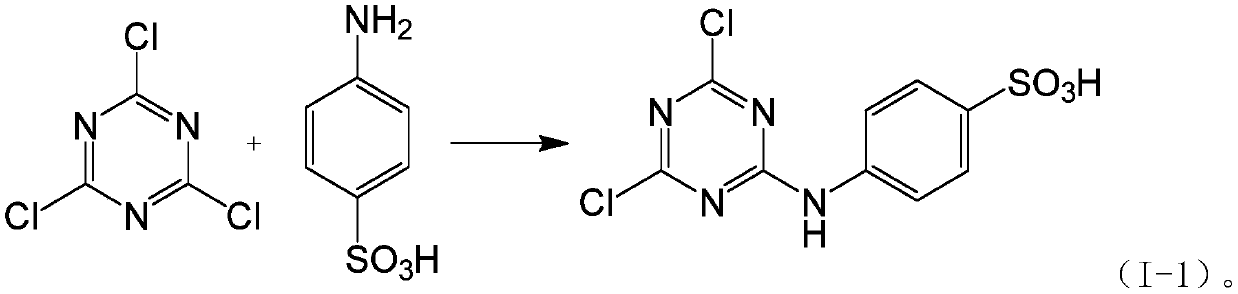
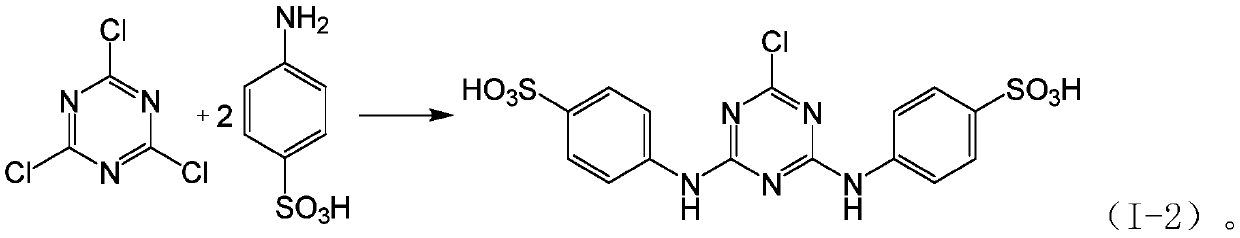
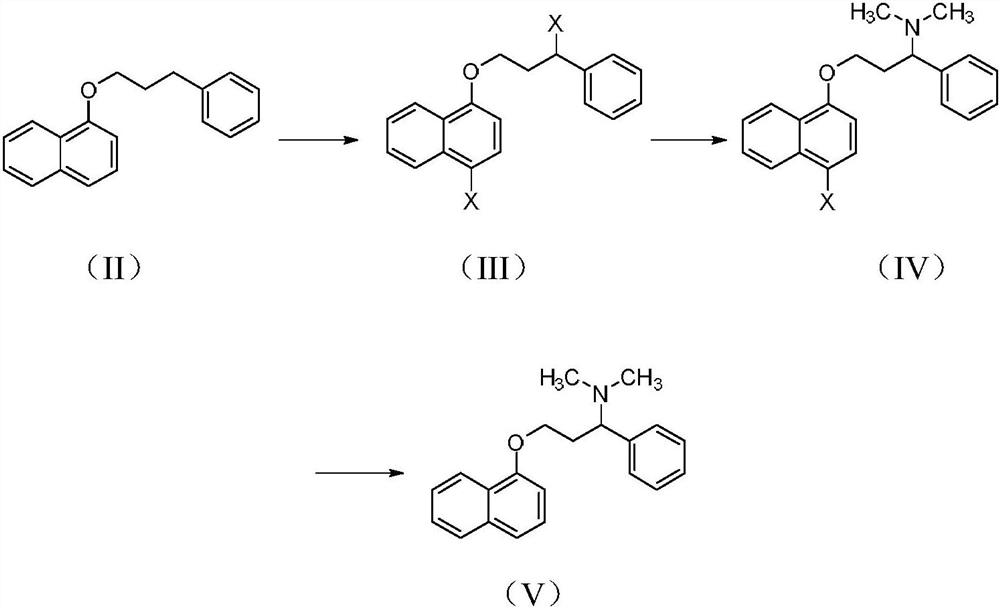
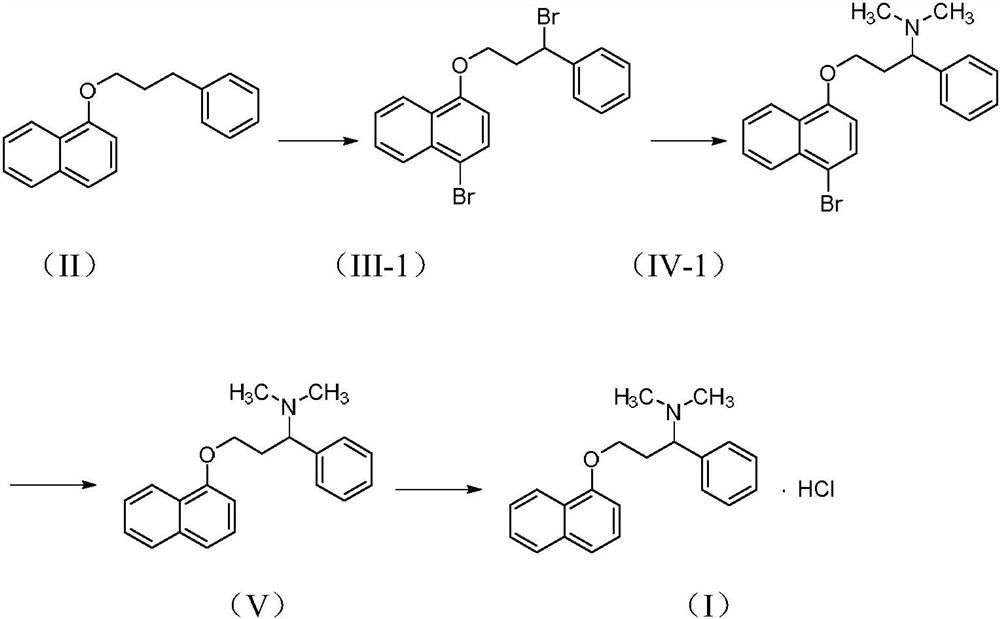
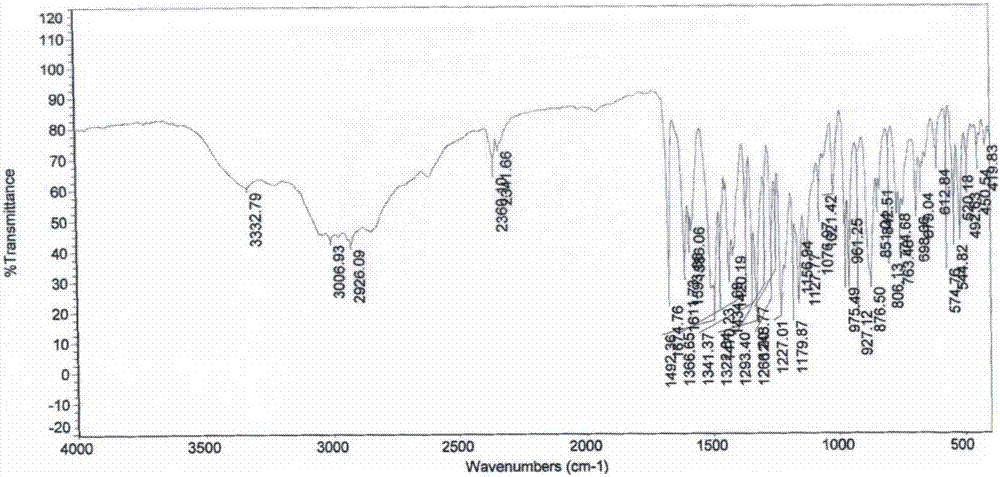
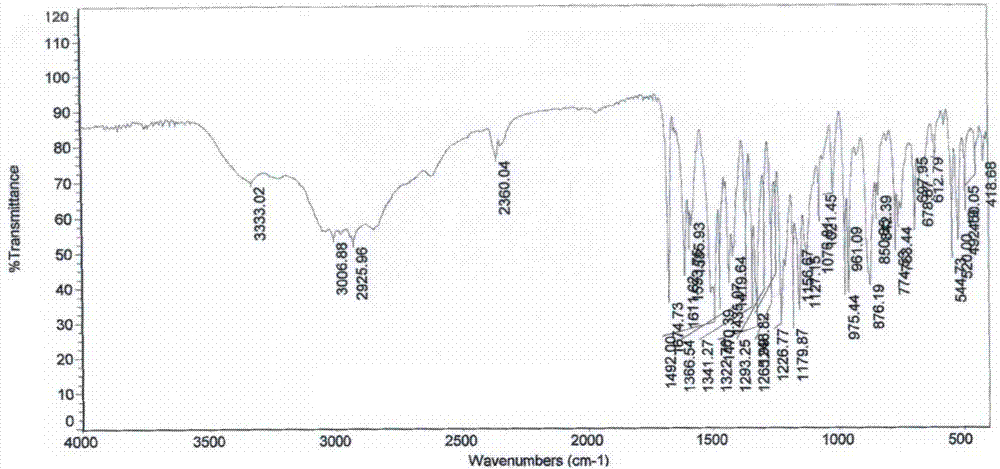
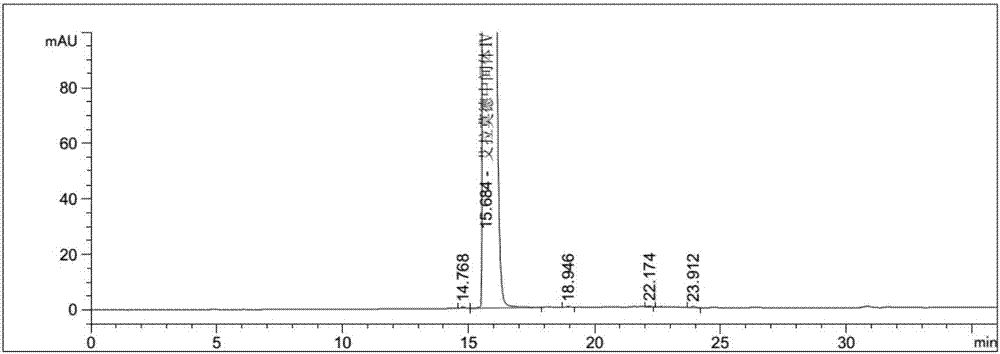
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
13 results about "Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative name is muriate, derived from hydrochloric acid's ancient name: muriatic acid.
PH-responsive polyethylene glycol-anticarcinogen conjugate, and synthetic method and application thereof
InactiveCN102475891APassive targetingBiocompatibleKetone active ingredientsPharmaceutical non-active ingredientsAnticarcinogenNano size
The invention discloses a pH-responsive polyethylene glycol-anticarcinogen conjugate, and a synthetic method and application thereof. After anticarcinogen molecules and polyethylene glycol are covalently linked through pH-sensitive phenylimide linkage, the conjugate is formed, wherein, anticarcinogen can be doxorubicin, epirubicin, daunorubicin, demethoxydaunorubicin or hydrochlorides of the above-mentioned anticarcinogens. The phenylimide linkage is stable under the condition of a normal physiological pH value but undergoes hydrolysis under the condition of a slightly acidic pH value, e.g., a solid tumor cell external pH value and a cell endosome and lysosome pH value; that is, the phenylimide linkage is sensitive to changes of pH values in a range of 7.4 to 4.5 or to below 4.5. The polyethylene glycol-anticarcinogen conjugate has amphiphilic nature and can form nanometer-scale micelle in a saline solution through self-assembly; the micelle can be used as a drug carrier to carry hydrophobic drugs and form a nanometer micelle preparation cladded with drugs, thereby realizing synergy therapy of a plurality of drugs.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Method for preparing 4-hydroxylethylpyrrolidone-2-acetamide
InactiveCN101693685ARaw materials are cheap and easy to getLow costOrganic chemistryAcetoacetatesAcetic acid
Owner:SUZHOU HOPE TECH
Method for preparing stimuli-responsive silicon dioxide nano particle
InactiveCN101792150AHigh stimulus responsivenessImprove stabilitySilicaSilicon dioxideMolecular recognition
The invention provides a method for preparing stimuli-responsive silicon dioxide nano particles. The method is that silanization treatment is conducted on the surface of the silicon dioxide nano particles to obtain nano particles with carboxyl functional groups at ends, self-assembly monomolecular layers are formed on the surfaces of the nano particles through a chemical covalent coupling method by using synthesized sulfo-alkyl ammonia-2-sulfur pyridine hydrochloride, the self-assembly monomolecular layers are mixed with reduced glutathione, mercaptopyridine micromolecules are produced through breaking sulfur-sulfur bonds, supernatant liquor is taken after centrifugation for ultraviolet detection, obvious absorption peaks can be observed at 343nm to prove the stimuli-responsiveness of the sulfur-sulfur bonds to sulfur compounds, and thereby the silicon dioxide nano particles with stimuli-responsiveness to sulfur-containing organic or biological molecules are prepared. The nano particles prepared by the method have the advantages of good molecular recognition function, high stimuli-responsiveness and high stability.
Owner:KUNMING UNIV OF SCI & TECH
Method for preparing test solution for quality detection of safe stagnation removing preparation
InactiveCN102539588AEliminate distractionsGood for qualitative and quantitative analysisComponent separationSolventAlkaloid
Owner:YUNNAN LIANGFANG PHARMA
Preparation method of telmisartan intermediate
The invention relates to a preparation method of a telmisartan intermediate, namely 2-n-propyl-4-methyl-6-Benzimidazolecarboxylic acid. The preparation method of the 2-n-propyl-4-methyl-6-Benzimidazolecarboxylic acid comprises the following steps: firstly, ethanol hydrochloride acts with butyronitrile and anhydrous hydrogen chloride within the range of 0-35 DEG C; the mixture obtained in the firststep reacts with 3-methyl-4-aminobenzoic acid and ice vinegar within the range of pH 5.0-11.0 at the controlled temperature of 10-40 DEG C, and an intermediate shown in the formula II is obtained; and then the intermediate shown in the formula II reacts with a sodium hypochlorite solution, and the 2-n-propyl-4-methyl-6-Benzimidazolecarboxylic acid is obtained. The intermediate preparation processis suitable for industrial production, and meanwhile the product quality is improved.
Owner:DIJIA PHARM CO LTD
Preparation method of sodium ibandronate
InactiveCN102898466AEasy to operateMeet the quality requirements of raw materialsGroup 5/15 element organic compoundsSkeletal disorderPhosphorous acidChlorobenzene
The invention relates to the technical field of pharmaceutical chemistry, particularly relates to a method of pharmaceutical synthesis, and specifically relates to a preparation method of sodium ibandronate. To overcome the disadvantages of high content of chlorides and phosphites in sodium ibandronate prepared by a conventional preparation method of sodium ibandronate, the preparation method of sodium ibandronate with extremely low content of chlorides and phosphites is provided. In the preparation method, 3-(N-methylpentylamino) propionic acid hydrochloride, phosphorus trichloride and phosphorous acid are employed as raw materials and reacted in a chlorobenzene solvent, so as to obtain sodium ibandronate with extremely low content of the chlorides and the phosphites. The obtained sodium ibandronate can not only meet impurity control standards of the chlorides and the phosphites in a sodium ibandronate crude drug, but also prevent low yield, long period and huge harm to human body and environment which are brought by a lot of refining steps.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Antibacterial finishing agent, antibacterial yarn and preparation method and application of antibacterial yarn
InactiveCN107217481AHas antibacterial propertiesPrevent mildewBiochemical fibre treatmentGrip property fibresYarnWater based
Owner:湖南莎丽袜业股份有限公司 +1
An antibacterial whitening mouthwash and a preparation method thereof
InactiveCN109172475AImprove securityHigh antibacterial activityCosmetic preparationsAntibacterial agentsManihotAdditive ingredient
Owner:上海怡竹生物科技有限公司
Preparation method of 5-amido-1,2,4-selenadiazole derivative
ActiveCN106220586AImprove universalityWide range of isonitrile structuresOrganic chemistryAir atmosphereAryl
The invention provides a preparation method of a 5-amido-1,2,4-selenadiazole derivative. The derivative comprises amidine hydrochloride shown in a formula (I) and an isonitrile compound shown in a formula (II), and selenium powder is subjected to a reaction on the condition of existence of an alkali compound to obtain the 5-amido-1,2,4-selenadiazole derivative. According to the preparation method, nontoxic selenium powder is adopted as a selenium source, no metal participation is needed, and only organic alkali and air atmosphere are needed; meanwhile, the adopted amidine hydrochloride and isonitrile are wide in structure range, aryl or alkyl substrates can be subjected to a reaction smoothly, and the high substrate universality is achieved; operation is easy, only simple heating is needed, the reaction can be completed within 4-6 h, the reaction time is short, and meanwhile the reaction yield can reach 80-90%.
Owner:SUZHOU UNIV
Cotton-fiber anionic modifier, preparation method thereof and dyeing process
InactiveCN110735331AColorfulVibrant color dyeing effectOrganic chemistryDyeing processSodium bicarbonateCotton cloth
Owner:ZHEJIANG YIDE CHEM
Preparation method of dapoxetine hydrochloride racemate
InactiveCN111646910AHigh yieldEasy post-processingOrganic compound preparationAmino-hyroxy compound preparationDapoxetine-N-oxideMedicinal chemistry
Owner:CHINA PHARM UNIV
New method for preparing an insect repellent agent
PendingUS20220248669A1Safe and efficientBiocidePreparation by isomerisationInsect repellentAldehyde formation
Owner:CENT NAT DE LA RECHERCHE SCI +1
Preparation method of iguratimod intermediate IV
InactiveCN107162942AReduce harmReduce pollutionSulfonic acid amide preparationChemical industryNitrobenzene
Owner:常州佳德医药科技有限公司
Popular searches
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap