Lyotropic liquid crystal precursor as well as preparation method and application thereof
A technology of lyotropic liquid crystals and precursors, which is applied in the field of lyotropic liquid crystal precursors and their preparation, can solve the problems that drug therapy cannot effectively achieve the therapeutic purpose, lack of joint protection function, lack of joint cavity drug delivery system, etc., and achieve improved Joint protection function, improvement of treatment effect, effect of protecting structure and function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Glyceryl monooleate (GMO) was melted in a water bath at 45°C at 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9: The ratio of 1 (w / w) is uniformly mixed with N-methylpyrrolidone (NMP) respectively to obtain glycerol monooleate solutions with different concentrations;
[0055] Dispersing hyaluronic acid powder with a molecular weight of 50-100KDa in water to obtain hyaluronic acid particles with a water content of 10%;
[0056] The hyaluronic acid particles were uniformly dispersed in the glycerol monooleate solution at a concentration of 3.4 mg / mL to obtain precursor solutions with different formulation compositions.
[0057] The above precursor solution was mixed with water at a ratio of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1 (w / w) Mix the mixture evenly and place it at room temperature for 3 days. Observing the above sample with a hot stage polarizing microscope, photographing its polarized texture, identifying the crystal phase of the liquid crystal according to
Embodiment 2
[0060] Glyceryl monooleate (GMO) was melted in a water bath at 45°C and mixed uniformly with N-methylpyrrolidone (NMP) at a ratio of 8:2 (w / w) to obtain a glyceryl monooleate solution;
[0061] Dispersing hyaluronic acid powder with a molecular weight of 50-100KDa in water to obtain hyaluronic acid particles with a water content of 10%;
[0062] The hyaluronic acid particles were uniformly dispersed in the glycerol monooleate solution at a concentration of 3.4 mg / mL to obtain a lyotropic liquid crystal precursor solution.
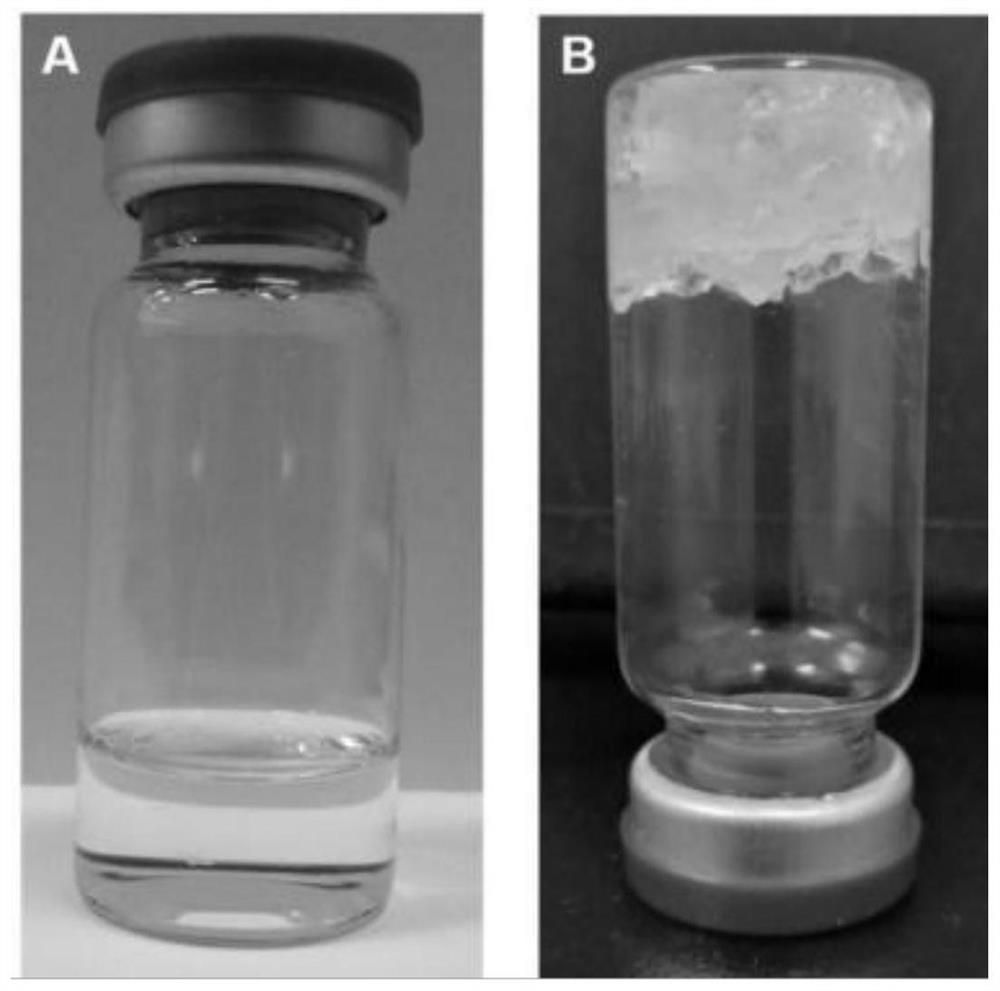
[0063] The ability of the lyotropic liquid crystal precursor solution in this embodiment to form a gel when it meets water is evaluated by using a Test-Tube experiment. Add 2mL of precursor solution into a 10mL vial, observe the morphological characteristics of the obtained precursor solution in upright and inverted states, then add deionized water drop by drop to the precursor solution until the precursor solution just forms a gel , and then observe the
Embodiment 3
[0066] 1. Glyceryl monooleate (GMO) was melted in a water bath at 45°C, and then mixed uniformly with N-methylpyrrolidone (NMP) at a ratio of 6:4, 7:3, and 8:2 (w / w) to obtain Different concentrations of glyceryl monooleate solution (recipe composition see Table 1).
[0067] 2. Glyceryl monooleate (GMO) was melted in a water bath at 45°C and mixed evenly with N-methylpyrrolidone (NMP) at a ratio of 8:2 (w / w) to obtain a glyceryl monooleate solution; the molecular weight The hyaluronic acid powder that is 50-100KDa is dispersed in water, obtains the hyaluronic acid particle (HA particle) that water content is respectively 5%, 10% and 15%; The concentration is uniformly dispersed in the glycerol monooleate solution to obtain a lyotropic liquid crystal precursor solution (refer to Table 1 for the composition of the recipe).
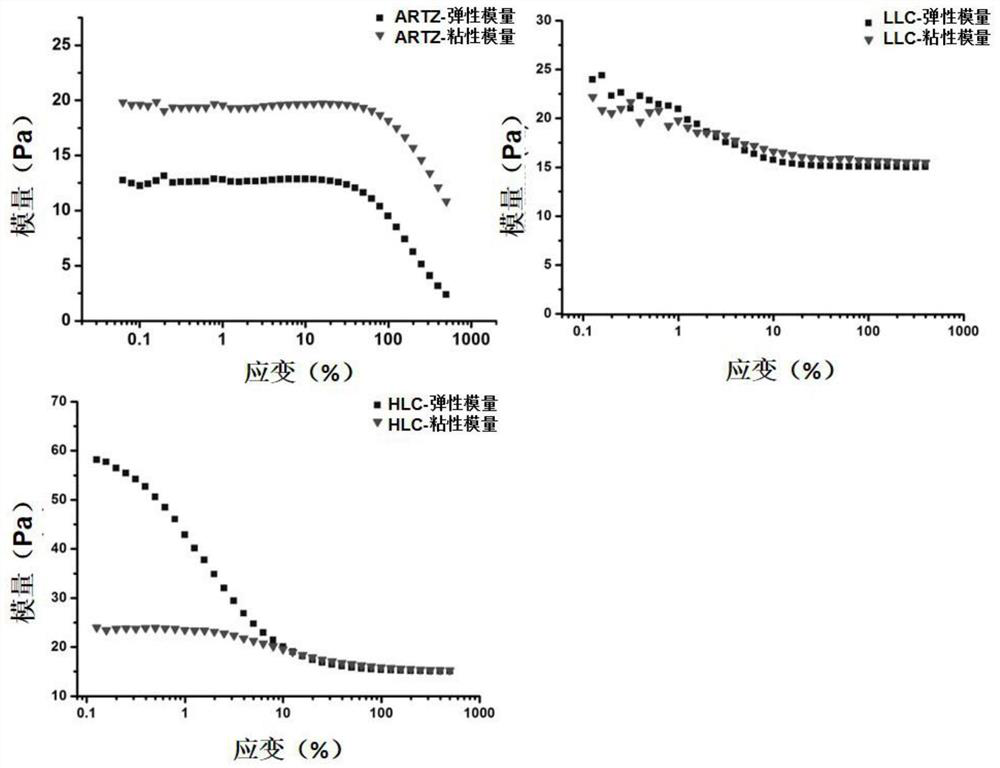
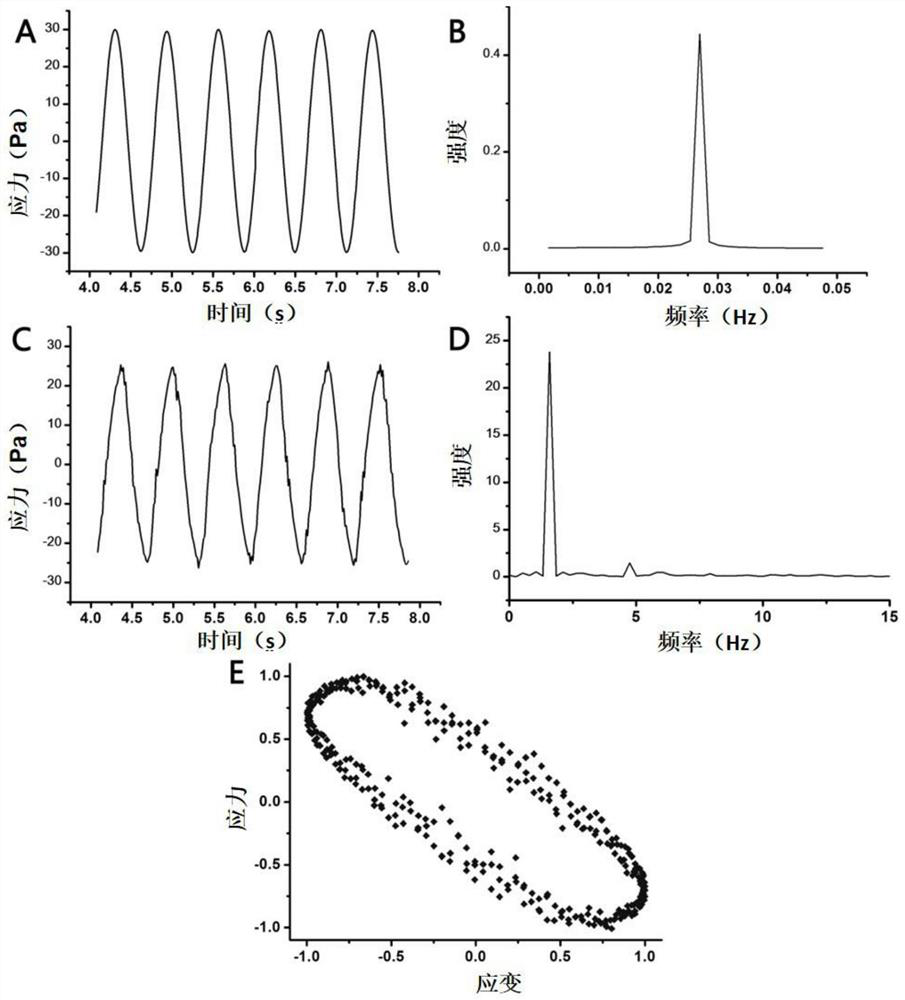
[0068] Viscosity test: using Kinexus lab + The viscosity test procedure of the rotational rheometer measures the viscosity change of the liquid crystal p
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Water content | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap