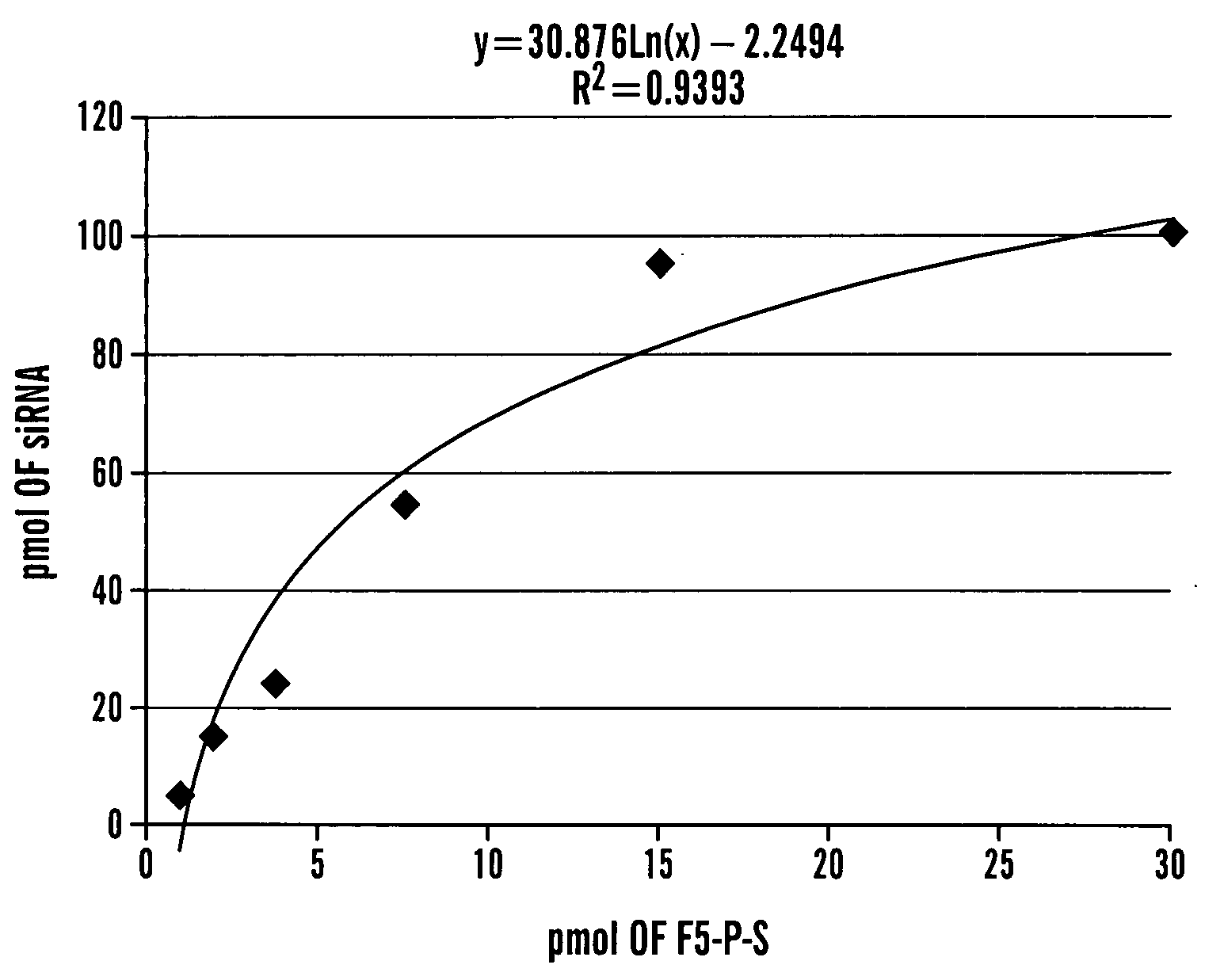
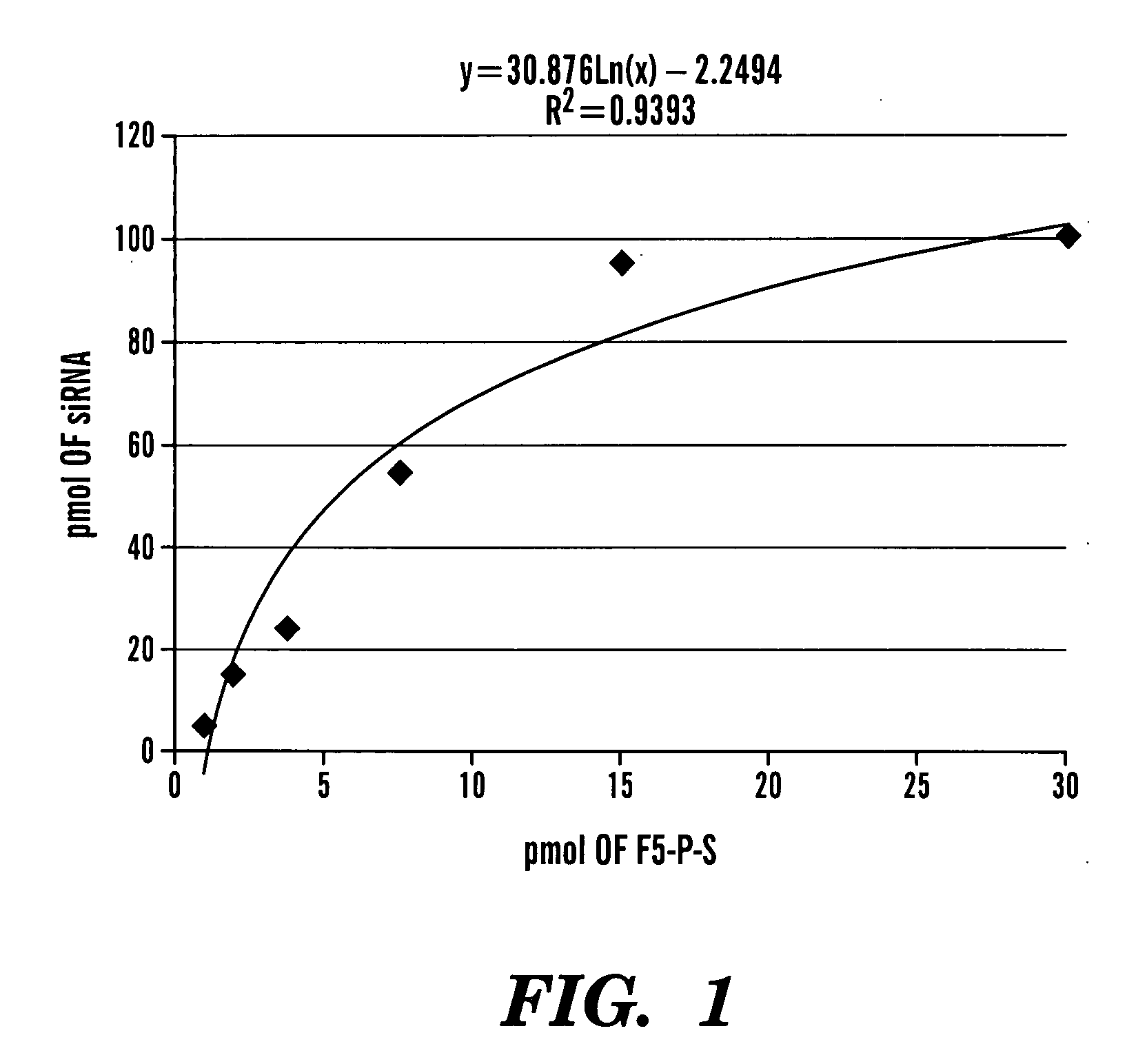
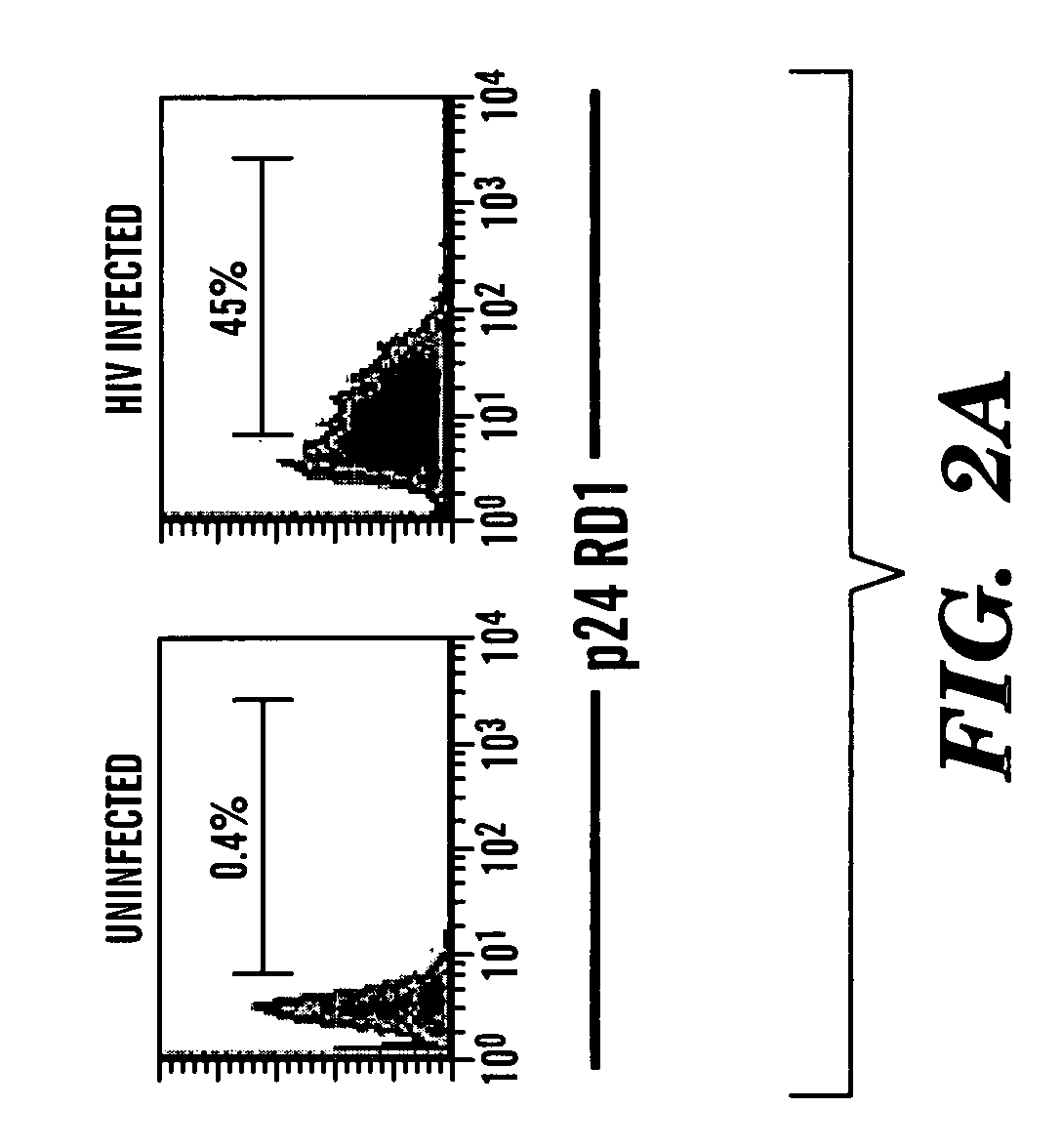
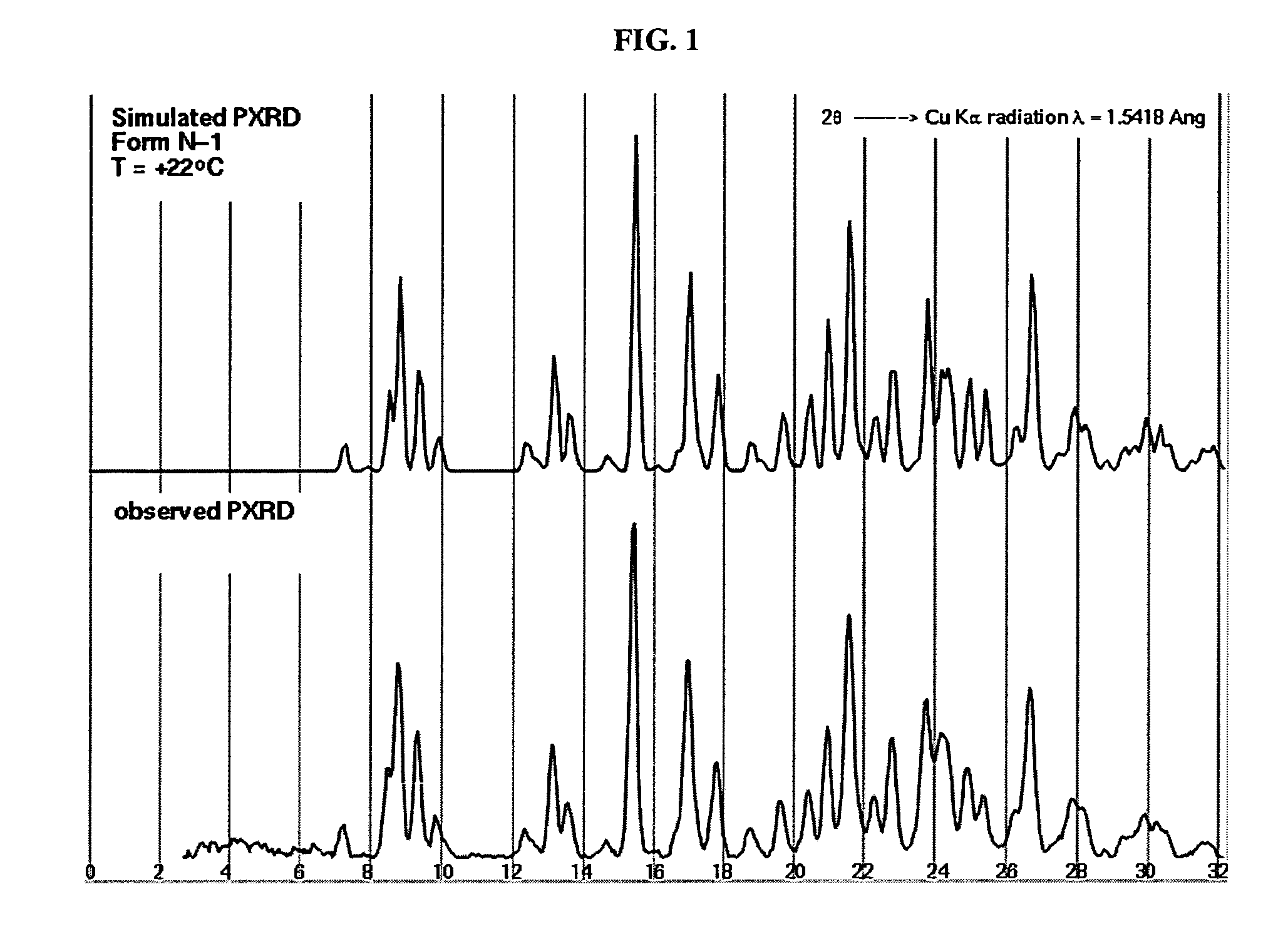
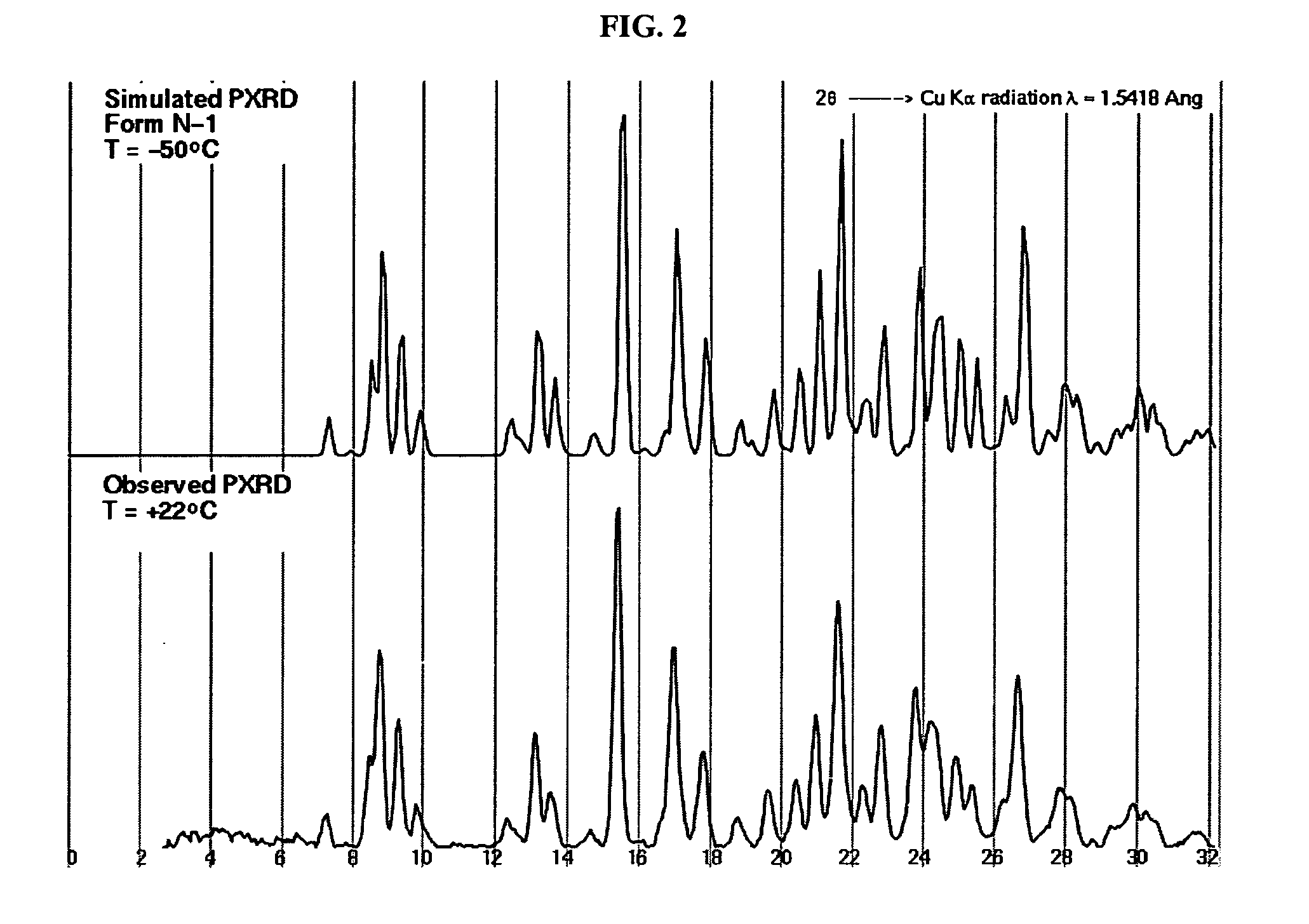
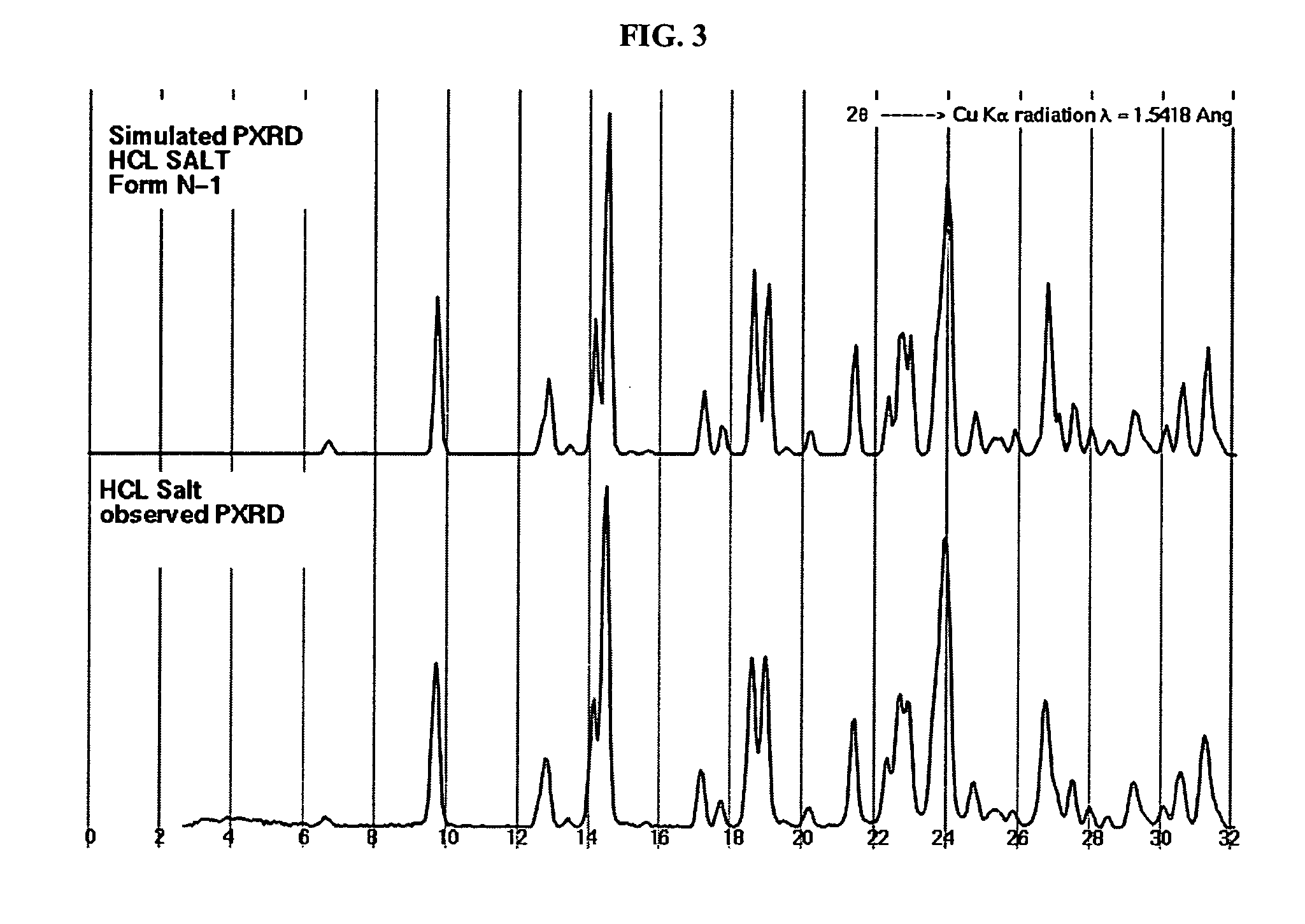
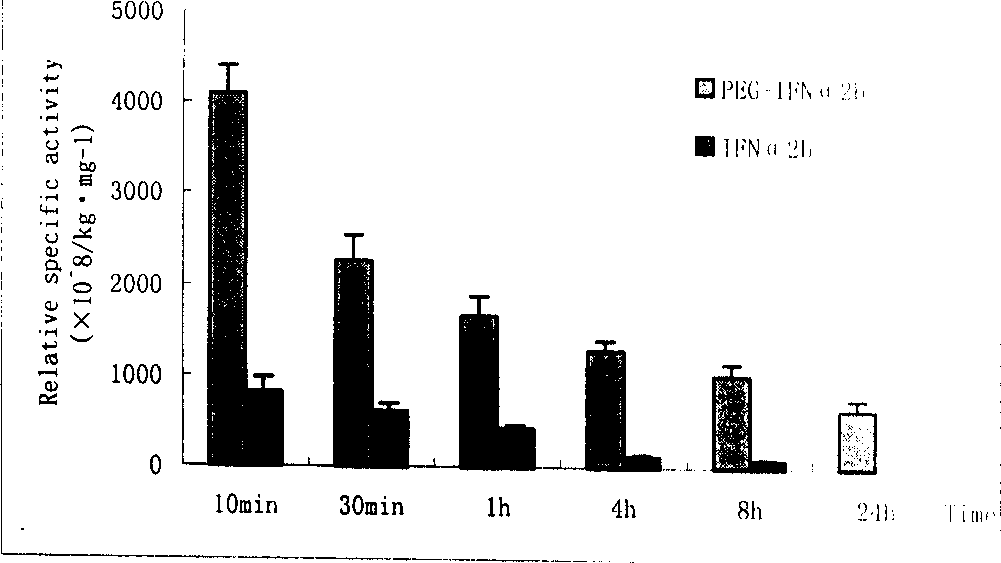
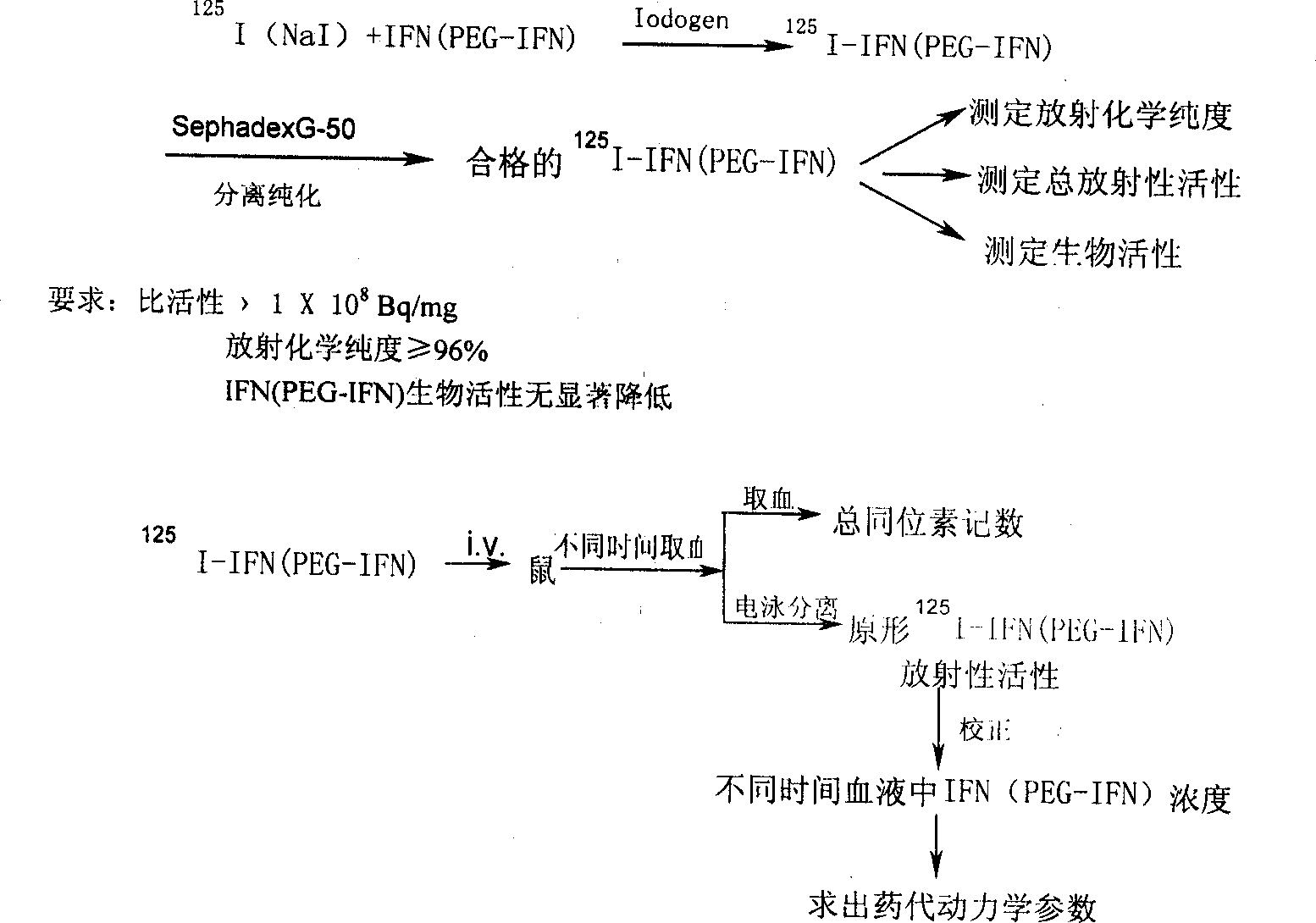
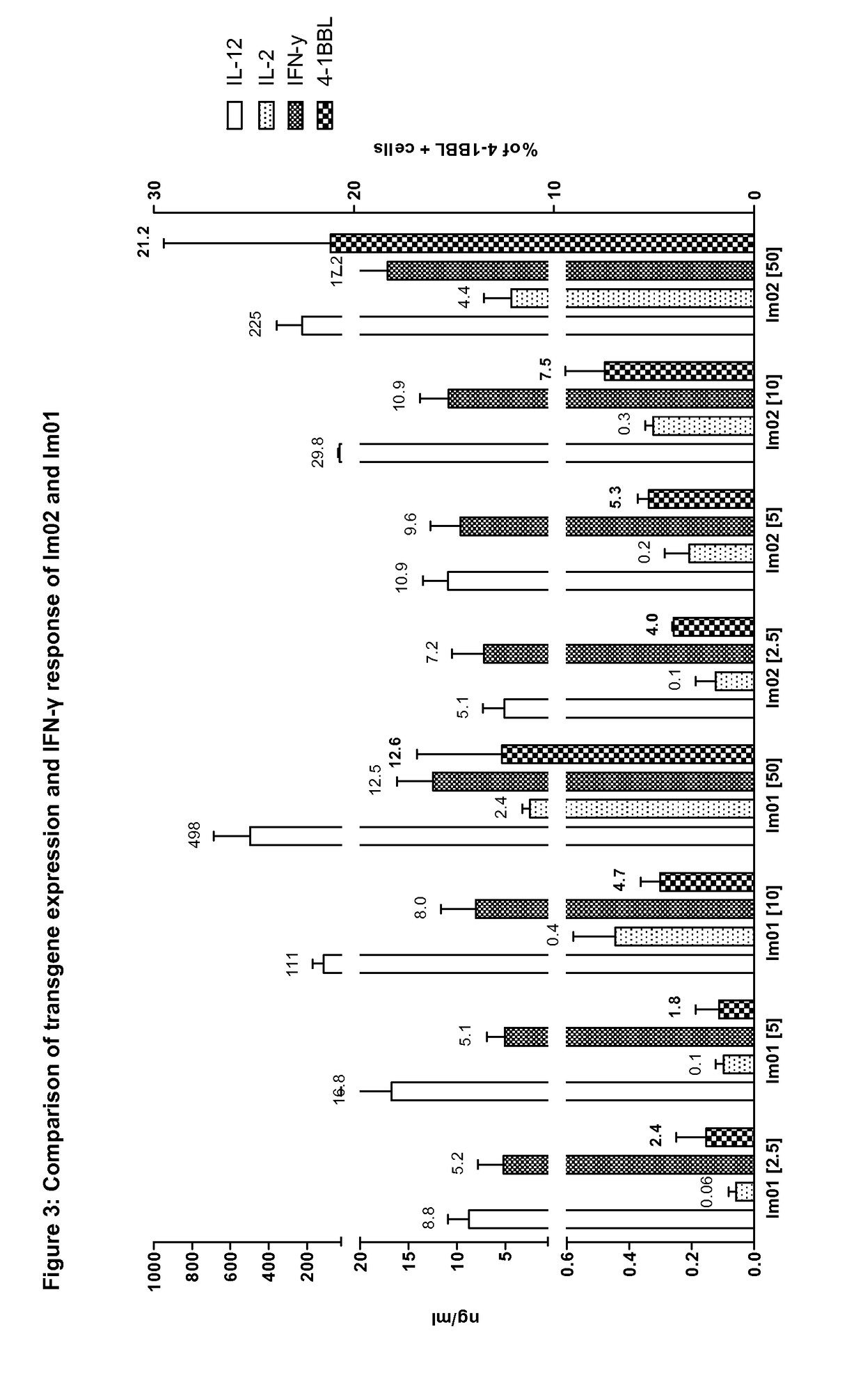
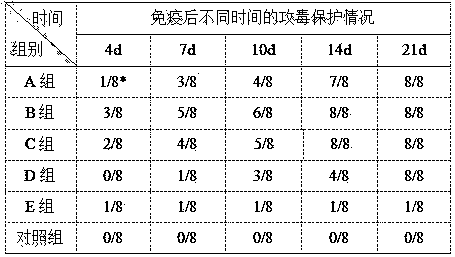
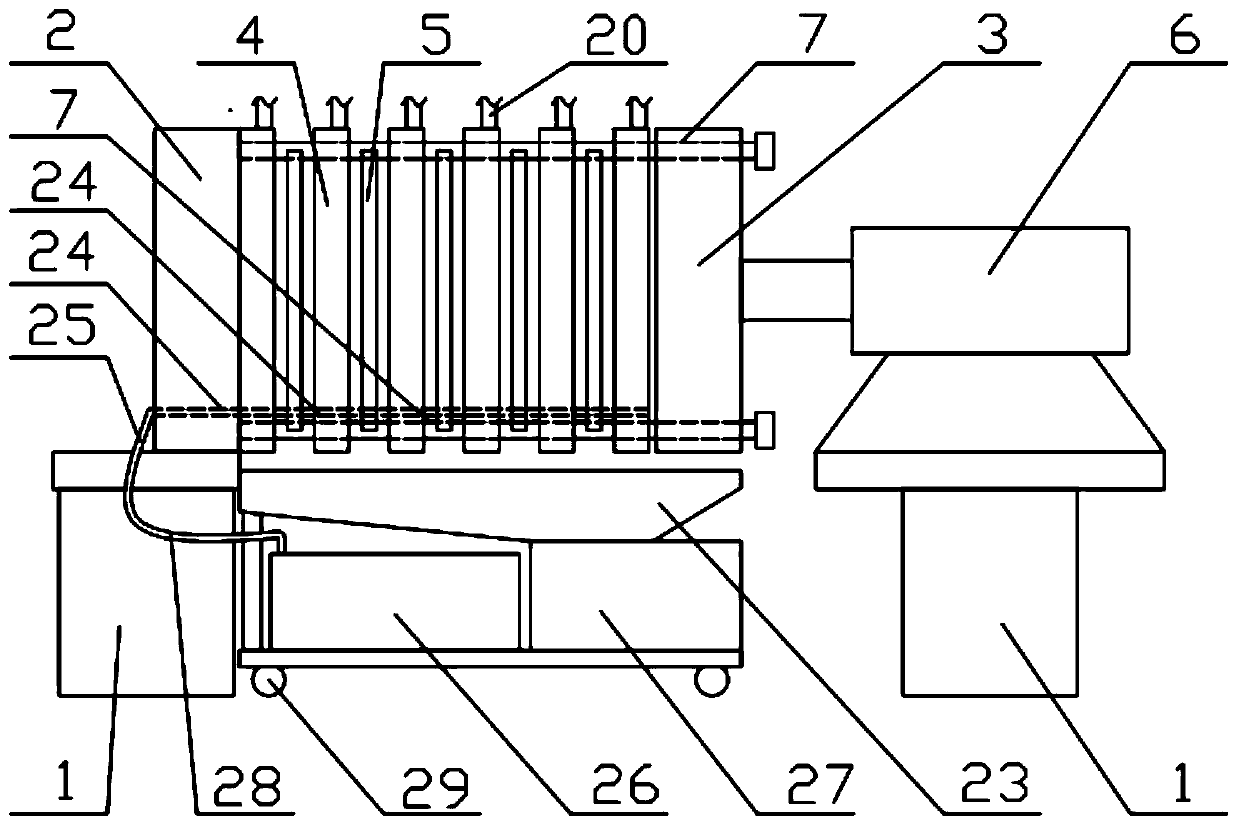
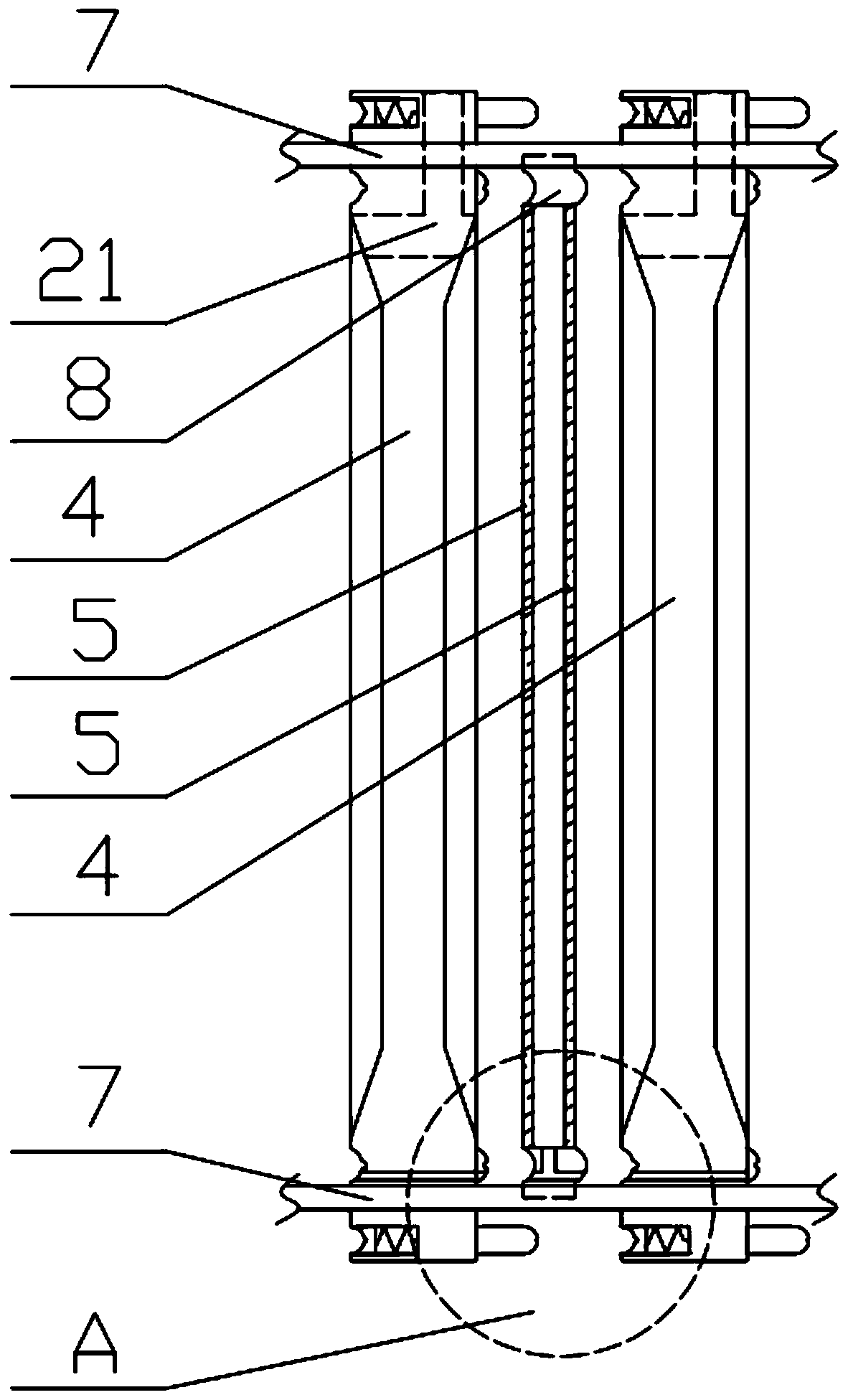
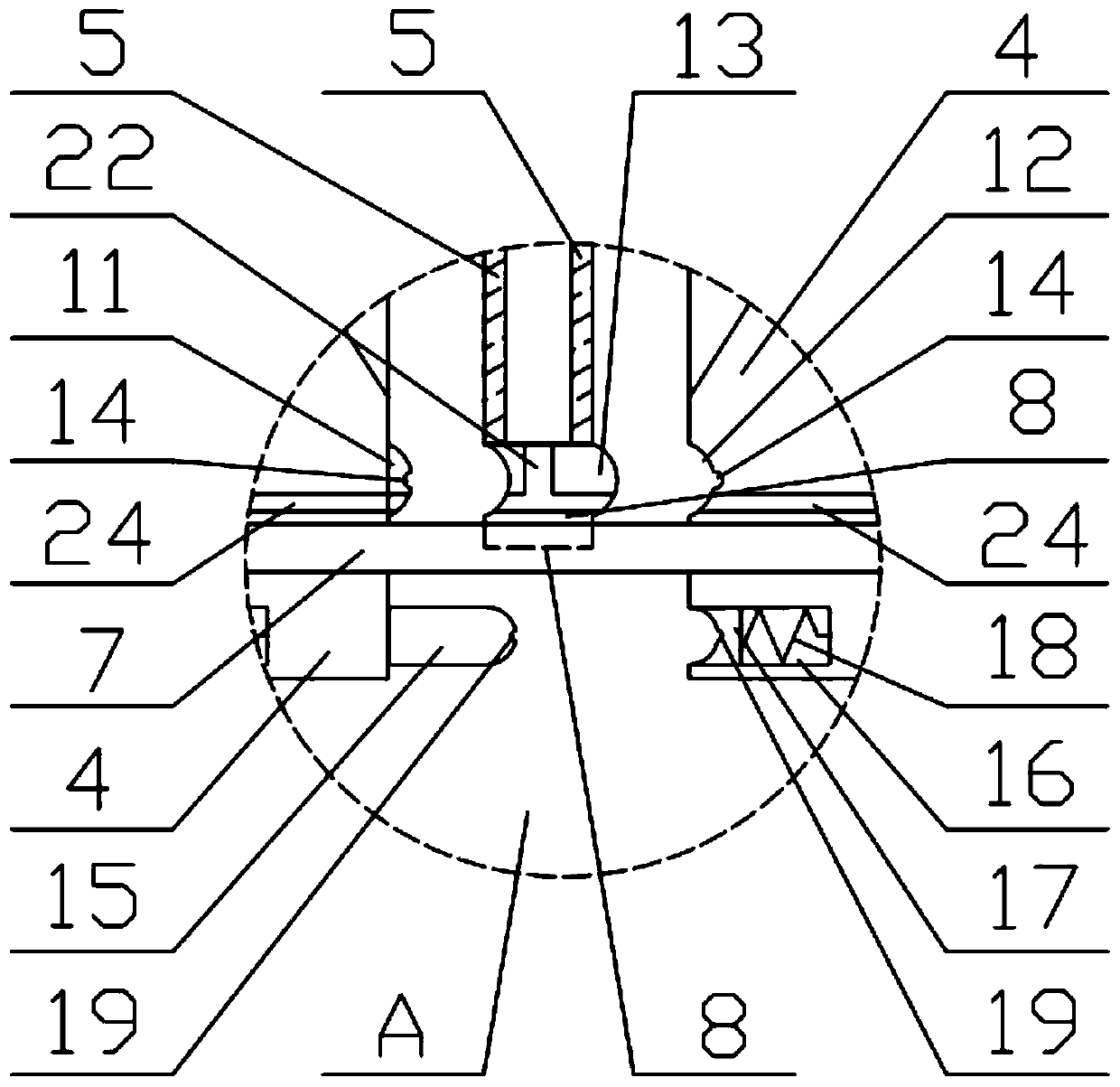
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
76results about "Antivirals" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions for sustrained release of nitric oxide, methods of preparing same and uses thereof
The invention provides compositions for releasing nitric oxide (NO) comprising a matrix that encapsulates nitric oxide. Nitric oxide is released when the composition is exposed to an aqueous environment. The invention further provides methods of preparing the compositions and uses of the compositions for treating infections and disorders.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Modified nucleosides for the treatment of viral infections and abnormal cellullar proliferation
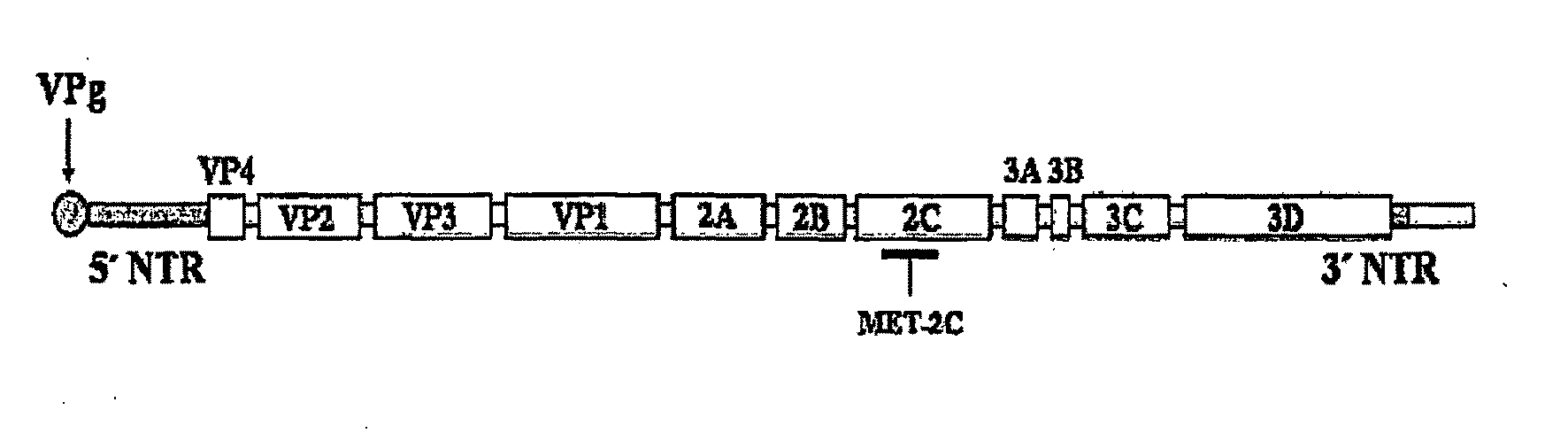
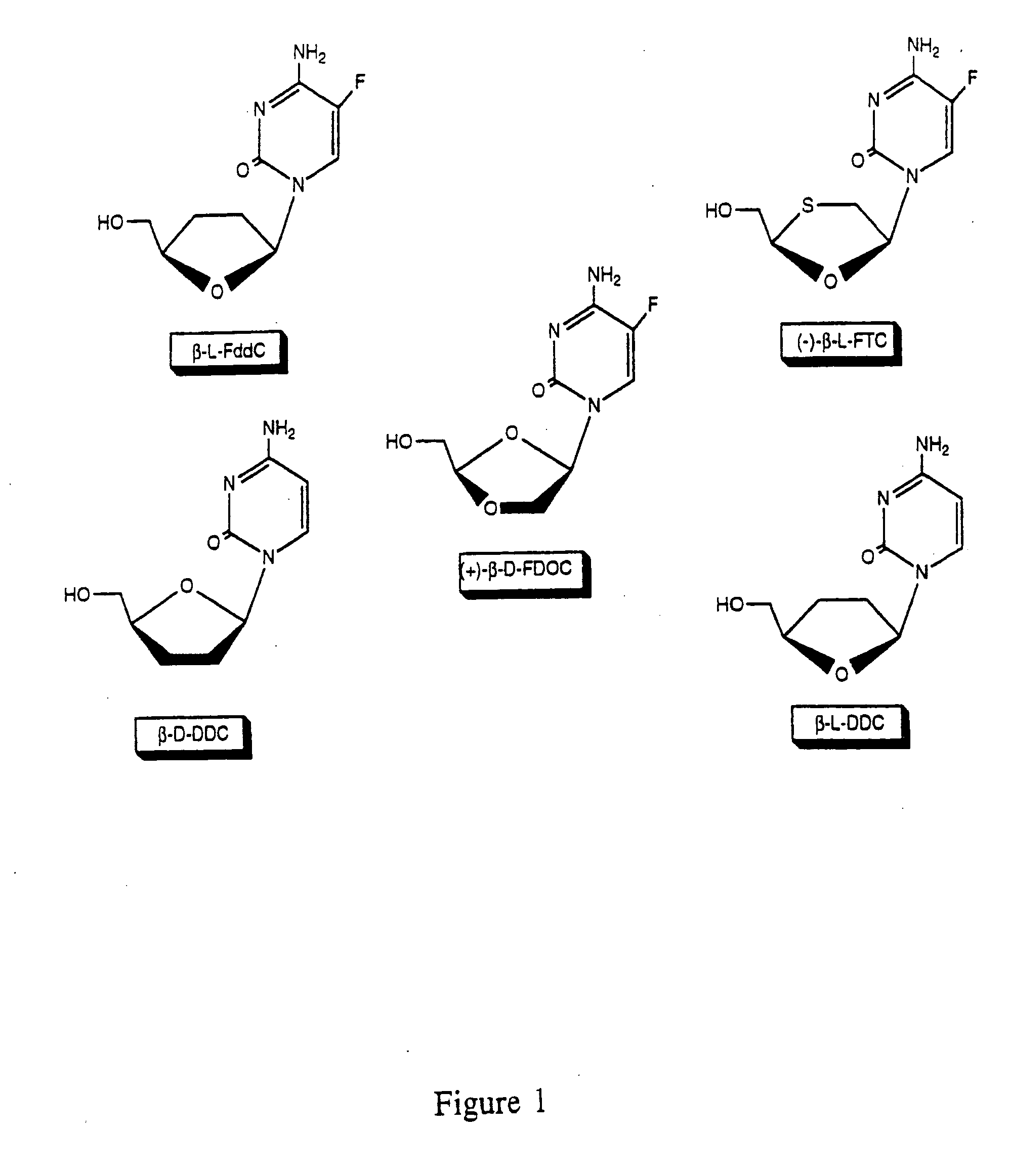
The disclosed invention is a composition for and a method of treating a Flaviviridae (including BVDV and HCV), Orthomyxoviridae (including Influenza A and B) or Paramyxoviridae (including RSV) infection, or conditions related to abnormal cellular proliferation, in a host, including animals, and especially humans, using a nucleoside of general formula (I)-(XXIII) or its pharmaceutically acceptable salt or prodrug.This invention also provides an effective process to quantify the viral load, and in particular BVDV, HCV or West Nile Virus load, in a host, using real-time polymerase chain reaction (“RT-PCR”). Additionally, the invention discloses probe molecules that can fluoresce proportionally to the amount of virus present in a sample.
Owner:GILEAD PHARMASSET LLC
Method of Delivering Rna Interference and Uses Thereof
InactiveUS20080153737A1Limiting potential side effectQuantity minimizationFusion with RNA-binding domainAntibacterial agentsGeneticsDouble strand
Owner:CHILDRENS MEDICAL CENT CORP
Crystalline forms and process for preparing spiro-hydantoin compounds
Owner:BRISTOL MYERS SQUIBB CO
Parenteral norovirus vaccine formulations
ActiveUS20130273102A1SsRNA viruses positive-senseViral antigen ingredientsVirus-like particleProtective immunity
The present invention relates to single dose parenteral vaccine compositions comprising mixtures of monovalent Norovirus virus-like particles. Methods of conferring protective immunity against Norovirus infections in a human subject by administering such compositions are also disclosed.
Owner:TAKEDA VACCINES INC
Mucosal Bioadhesive SLow Release Carrier for Delivering Active Principles
A mucosal bioadhesive slow release carrier comprising an active principle and devoid of starch, lactose, which can release the active principal for a duration of longer than 20 hours. This bioadhesive carrier contains a diluent, an alkali metal alkylsulfate, a binding agent, at least one bioadhesive polymer and at least one sustained release polymer, as well as a method for its preparation.
Owner:ONXEO SA
Arylalkyl and Heteroarylalkyl Derivaties of Cyclosporine a for the Treatment and Prevention of Viral Infection
This invention provides compounds of general formula (T): (I) wherein A, B, R1, R2, and X are as defined in this specification, and pharmaceutical compositions prepared from the same, for use in treatment of hepatitis C virus and / or human immunodeficiency virus.
Owner:SCYNEXIS INC
Polyglycol modified recombinant human interferon
InactiveCN1375502AMaintain antiviral activitySimple manufacturing methodPeptide/protein ingredientsAntiviralsMedicineHalf-life
Owner:CHINA PHARM UNIV
Traditional Chinese medicine microbial ecological agent for preventing and treating infectious bursal disease of chicken and preparation method thereof
InactiveCN103961418AIncrease the number ofImprove disease resistanceAntiviralsPlant ingredientsBiotechnologyDisease
Owner:广州中冠动物药业有限公司
O type foot and mouth disease virus-like particle vaccine as well as preparation method and application thereof
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
Nucleoside prodrug and application thereof
ActiveCN113999237AImprove oral bioavailabilityImprove performanceOrganic chemistryAntiviralsAnimal virusOral treatment
The invention relates to a nucleoside prodrug capable of being orally taken for treating mammalian virus infection, and especially relates to a compound shown as a formula (I) or pharmaceutically acceptable salt or stereoisomer thereof, or a pharmaceutical composition thereof, and application of the compound or the composition in preparation of drugs for treating, inhibiting or preventing diseases caused by virus infection.
Owner:RISEN SUZHOU PHARMA TECH CO LTD
Novel immunostimulating vector system
ActiveUS20190046664A1High expressionGenetic therapy composition manufactureNGF/TNF-superfamilyVector system4-1BB ligand
Owner:PROVECS MEDICAL
Recombinant vector and use in gene therapy
InactiveUS20080226675A1Preventing HIV infectionHydrolasesGenetic material ingredientsWild typeHuman cell
Owner:XU HONGZHAN
Immunopotentiator and vaccine preparation for emergency vaccination of Newcastle disease
InactiveCN104069480AProduced fastEasy to operateViral antigen ingredientsPeptide/protein ingredientsBiotechnologyHemagglutinin
Owner:GUANGZHOU SOUTH CHINA BIOLOGICAL MEDICINE
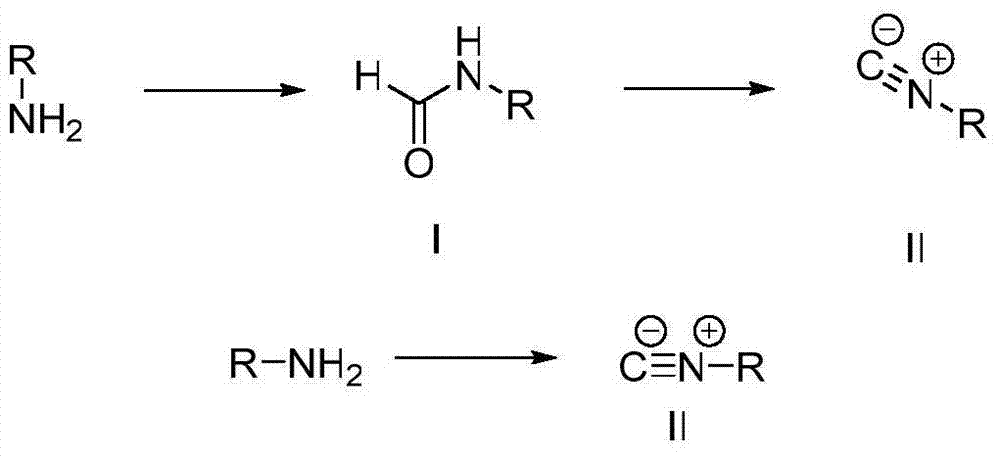
Formamide and isonitrile compounds serving as influenza A virus inhibitors and preparation and application thereof
InactiveCN103922966AAvoid drug resistanceHigh activityOrganic compound preparationAntiviralsFormamideStructural formula
Owner:WUHAN UNIV
Viral capsid assembly intermediates and methods of production
InactiveUS7638269B2Amenable to a wide variety of manipulationsReduce concentrationVirusesPeptide/protein ingredientsCell freeScreening method
A cell-free method for translation and assembly of viral capsid and capsid intermediates is disclosed. Also disclosed are novel capsid assembly intermediates and novel host proteins which bind to such assembly intermediates. The invention also includes a screening method for compounds that alter viral capsid assembly, and a method of treating viral infection using compounds which inhibit the capsid assembly pathway.
Owner:UNIV OF WASHINGTON
Long-acting HIV fusion inhibitor and application thereof
ActiveCN105646717APromote research and developmentExtended half-lifePeptide/protein ingredientsGenetic material ingredientsHalf-lifeTherapy HIV
Owner:FUDAN UNIV
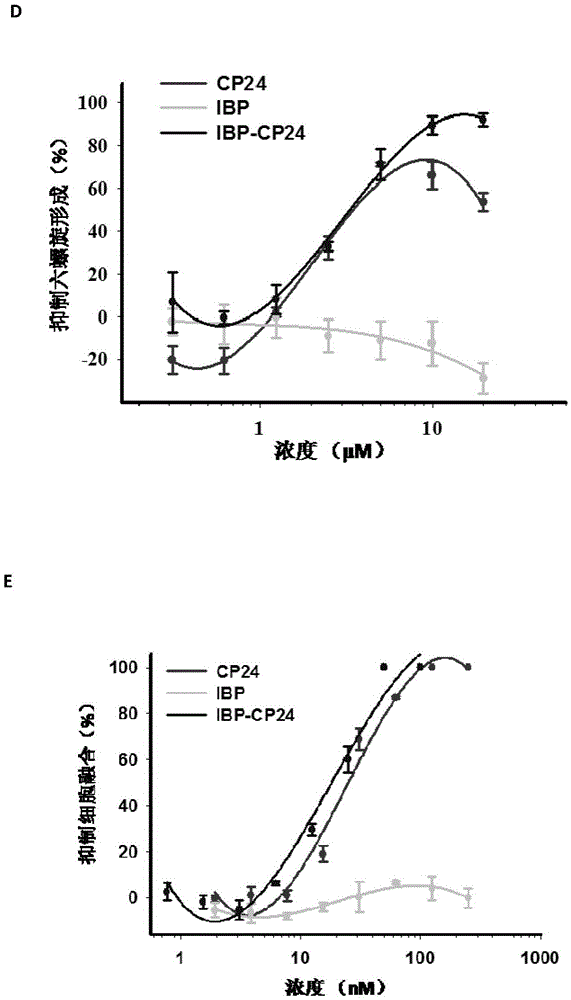
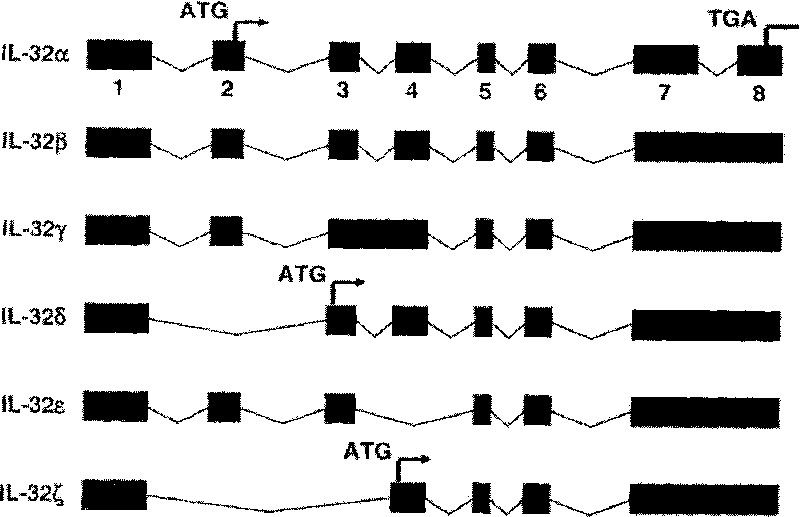
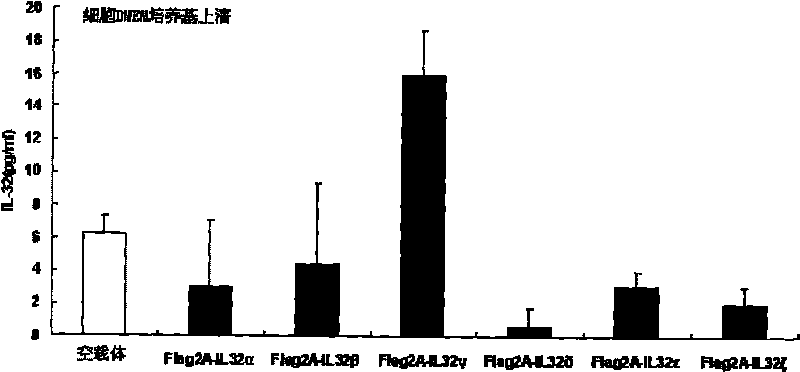
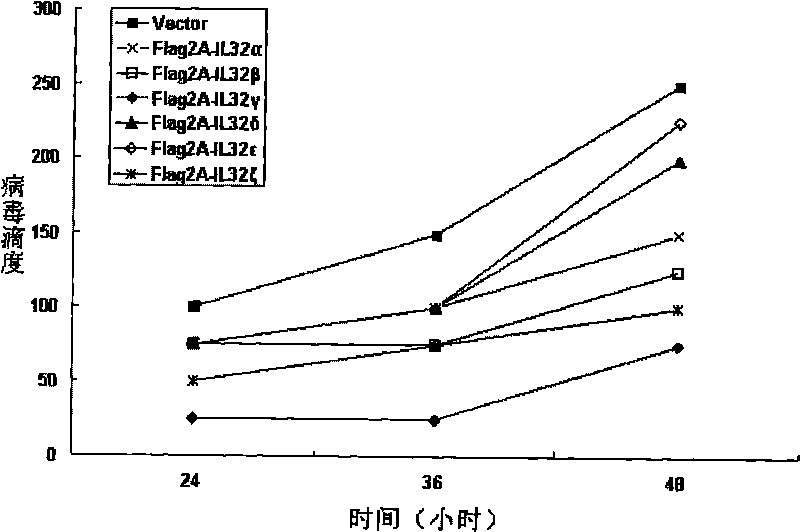
Use of human interleukin 32 in preparation of medicament for treating or preventing influenza A virus infection
Owner:WUHAN UNIV
Preparation method of high-purity gynostemma pentaphylla total saponin for veterinary drug
ActiveCN103520256AHigh purityMeet the protection requirementsAntibacterial agentsAntimycoticsReflux extractionMicrofiltration
Owner:江西嘉博生物工程有限公司 +1
Recombinant adenovirus rAd-ORF2-TCE and application thereof
InactiveCN103805573AEnhance humoral immunityEnhance cellular immunityGenetic material ingredientsMicroorganism based processesPorcine circovirusOrganism
The invention relates to a recombinant adenovirus rAd-ORF2-TCE. The recombinant adenovirus rAd-ORF2-TCE is obtained through fusing and connecting an immunogenic protein gene ORF2 of PCV2 (Porcine Circovirus Type 2) and three T lymphocyte epitope (TCE) series genes, and then, recombining to an adenovirus vector. The invention further relates to application of the recombinant adenovirus rAd-ORF2-TCE in porcine viral immunization vaccines. According to the recombinant adenovirus rAd-ORF2-TCE disclosed by the invention, an organism can be effectively stimulated to generate a high-level specific antibody and generate T lymphocyte proliferation, and both the concentration of IFN-gamma in blood serum and the concentration of IL-2 in the blood serum are increased remarkably; compared with ORF2 of separate PCV2, the recombinant adenovirus rAd-ORF2-TCE has the advantage that both humoral immunity level and cellular immunity level of an animal body are increased remarkably.
Owner:GUANGDONG WENS DAHUANONG BIOTECH +1
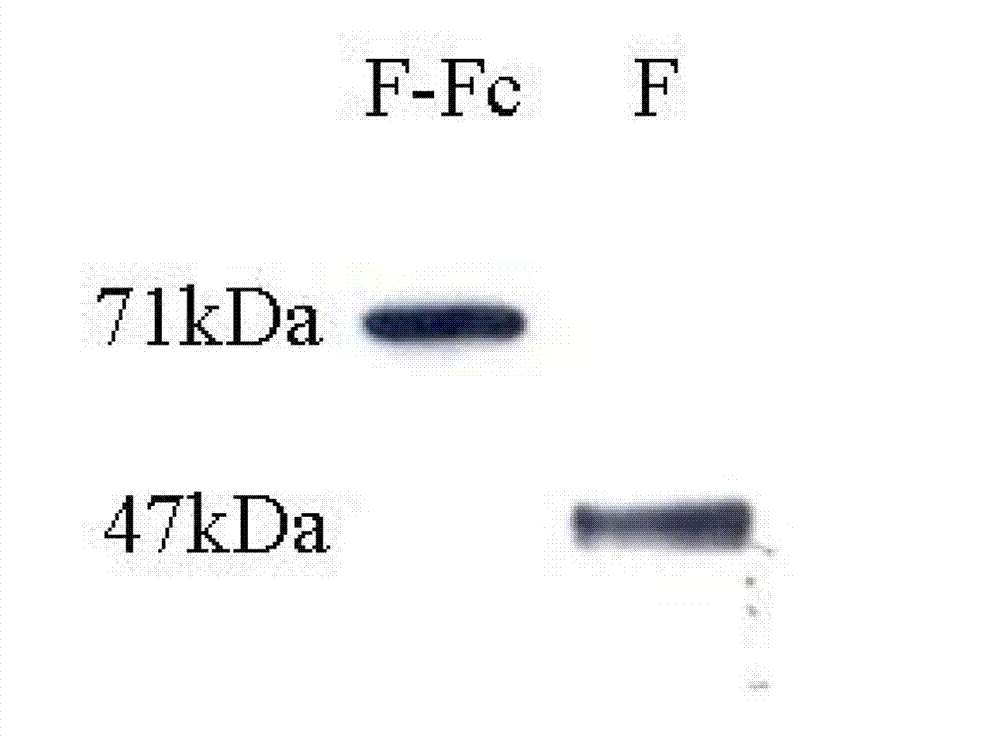
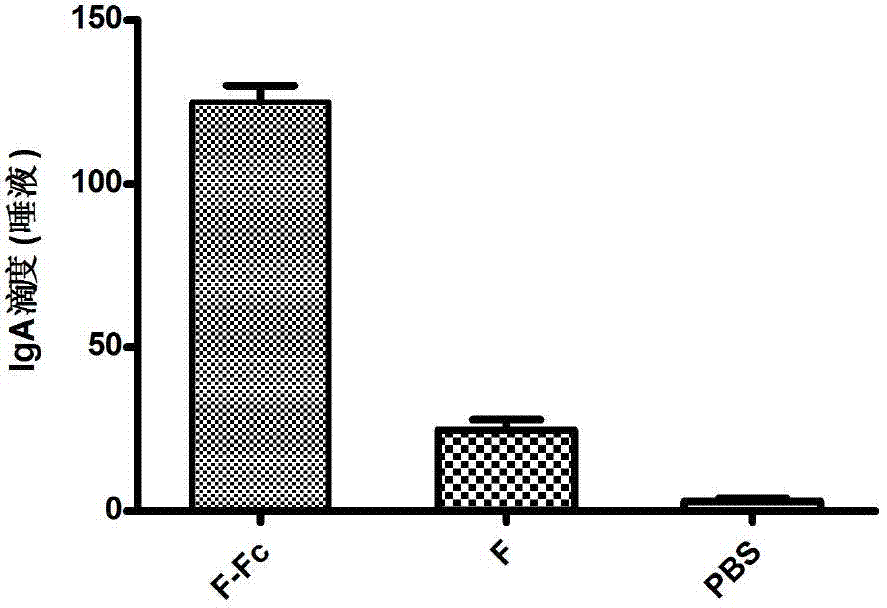
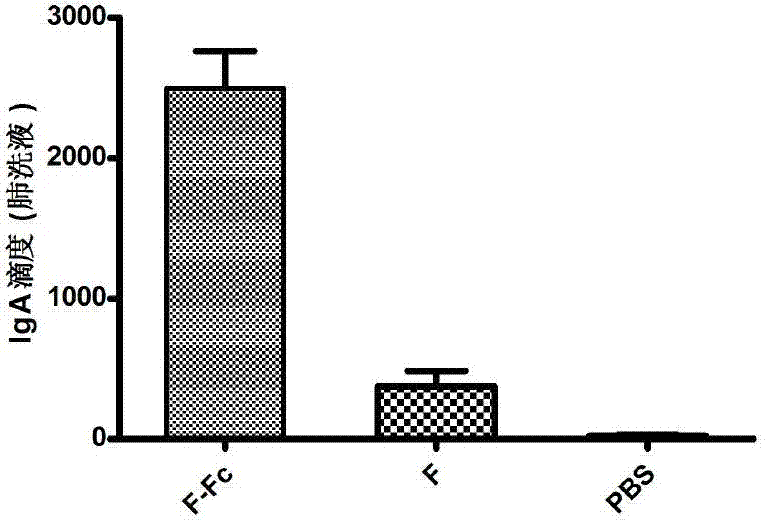
Fusion protein of RSV (respiratory syncytial virus) protein F and Fc, and application thereof
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Use of effective part of traditional Chinese medicine in HIV latency-resistant treatment
The invention provides a use of an effective part of a traditional Chinese medicine in HIV latency-resistant treatment. Through an ingenious activity screening method, it is found that a traditional Chinese medicine rhizoma atractylodis macrocephalae has effects of resisting AIDS. Through a solvent extraction method, a gradient extraction method, a silica gel column chromatography and the combination with activity screening, an effective part of rhizoma atractylodis macrocephalae is determined and is prepared. The prepared effective part of rhizoma atractylodis macrocephalae can be used as an active component for resisting AIDS. The effective part of rhizoma atractylodis macrocephalae has an effect of HIV latency intervention, can be combined with a drug for resisting retroviruses, can accelerate the removal of latent virus reservoirs, and provides a novel approach for thorough curing of AIDS.
Owner:SHANGHAI XINHAO BIOLOGICAL TECH
Sirna having antiviral activity against nonpolio enterovirus
Owner:UNIV OF ULSAN FOUND FOR IND COOPERATION
Application and preparation method of candidate strain of human type-3 adenovirus expressed human type-14 adenovirus neutralization epitope vaccine
InactiveCN105238766AAvoid infectionRetain major antigenic activityMicroorganism based processesAntiviralsAntigenHuman type
Owner:东莞市第八人民医院
Traditional Chinese medicine composition for treating rubella
InactiveCN103550376AEasy to prepareWide range of medicinesAntiviralsDermatological disorderLedebouriella seseloidesSide effect
The invention relates to a traditional Chinese medicine composition for treating rubella. The traditional Chinese medicine composition is composed of the following traditional Chinese medicine raw materials in parts by weight: 5-25 parts of lithospermum, 5-25 parts of kochia scoparia, 5-20 parts of fructus cnidii, 10-30 parts of angelica, 5-25 parts of ledebouriella seseloides, 5-20 parts of gentiana macrophylla, 5-25 parts of dangshen, 5-25 parts of radix sophorae flavescentis, 5-25 parts of indigo naturalis, 5-20 parts of folium artemisiae argyi, 5-20 parts of eucommia, 5-25 parts of purple perilla, 5-25 parts of kudzu root, 5-20 parts of notopterygium, 5-25 parts of radix clematidis, 5-20 parts of radix angelicae, 5-25 parts of prepared rhizome of rehmannia, 5-20 parts of radix paeoniae alba, 10-30 parts of radix rehmanniae root of rehmannia, 5-20 parts of corktree and 5-15 parts of licorice. The traditional Chinese medicine composition provided by the invention has the effects of soothing liver, strengthening spleen, clearing heat, cooling blood, dispelling wind and dampness, and detoxifying and promoting eruption, and the traditional Chinese medicine composition is significant in curative effect on acne, reliable in function, wide in component medicine source, simple and convenient to prepare and free of toxic and side effect.
Owner:孙志军
Application of EGCG palmitate to drugs for treating or preventing hepatitis C virus infection
InactiveCN104138371ANon-cytotoxicGood prevention effectOrganic active ingredientsAntiviralsPositive controlPalmitates
The invention relates to the technical field of the new application of drugs and particularly discloses the application of EGCG palmitate to the drugs for treating or preventing hepatitis C virus infection. The application verifies that the EGCG palmitate has the good antiviral effect in the test of in-vitro hepatitis C virus infection cells, and can prevent and treat the hepatitis C virus infection, the effect of the EGCG palmitate is equal to the that of positive control drugs IFN-alpha1, and the EGCG palmitate has the prospect for developing the drugs resisting hepatitis C viruses.
Owner:WUHAN SHENGDAKANG BIOTECHNOLOGY CO LTD
Compositions and methods for the treatment of influenza infection
ActiveUS20070166801A1Inhibit expressionInhibition is effectiveSpecial deliveryPeptide/protein ingredientsAnimal geneOligonucleotide
Owner:LAKEWOOD AMEDEX
Thymine nucleosides with anti-hepatitis B virus activity
InactiveUS20050277616A1Prevent and retard progressionReduce usageBiocideSugar derivativesPhosphateThymine
Owner:OWENS HOWARD JR
Novel coronavirus vaccine antigen presentation system of attenuated salmonella secretory expression NTD structural domain protein and application of novel coronavirus vaccine antigen presentation system
PendingCN114480462AEnables activation/regulationPromote secretionSsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationSecretion expression
Owner:NANJING JIRUIKANG BIOTECHNOLOGY RES INST CO LTD +1
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap