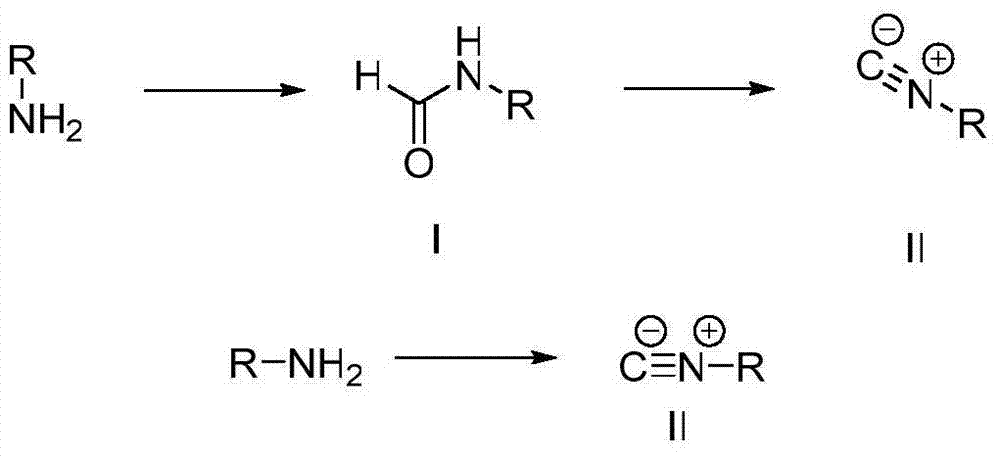
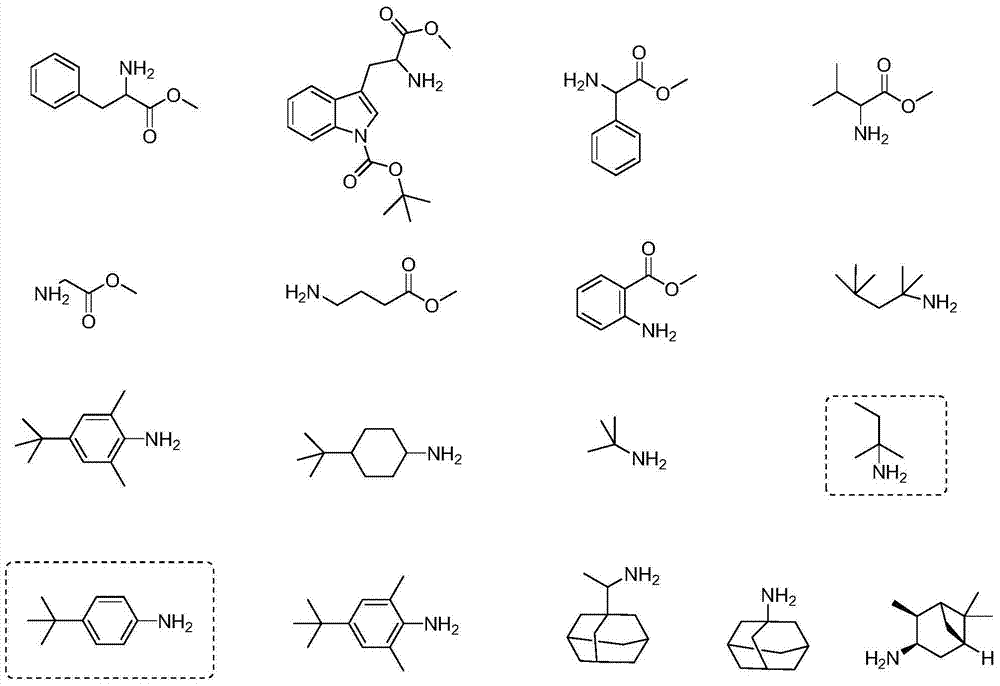
Formamide and isonitrile compounds serving as influenza A virus inhibitors and preparation and application thereof
A type of influenza A virus and formamide technology, applied in the field of formamide and isonitrile compounds, can solve the problem of lack of literature reports on the antiviral activity of isonitrile, and achieve the effect of overcoming drug resistance and avoiding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Phenylalanine methyl ester isonitrile
[0034]
[0035] (1) Preparation of phenylalanine methyl ester formamide
[0036]
[0037] Add acetonitrile solution, phenylalanine (8g, 45mmol) and ammonium formate (5.61g, 90mmol) into a 150mL round bottom flask, heat to reflux at 90°C, a large amount of white solid dissolves slowly and a small amount of white solid appears, and the reaction is complete after 24 hours . Filtrate, concentrate the supernatant by rotary evaporation in vacuum, extract three times with water and ethyl acetate to combine the organic phases, dry with anhydrous sodium sulfate, filter, and concentrate the supernatant by rotary evaporation in vacuum to obtain a reddish-brown liquid with a yield of 85%.
[0038] 1 H NMR (400MHz, CDCl 3)δ8.07(s,1H),7.26(ddd,J=12.3,7.7,4.4Hz,3H),7.13–7.09(m,2H),4.92(dd,J=13.5,6.4Hz,1H),3.70 (s,3H),3.10(ddd,J=33.5,13.9,6.1Hz,2H),2.01(s,1H).
[0039] 13 C NMR (101MHz, CDCl 3 )δ171.02, 160.31, 136.04, 129
Embodiment 2
[0044] Example 2: Tryptophan methyl ester isonitrile
[0045]
[0046] (1) Preparation of tryptophan methyl ester formamide
[0047]
[0048] Add acetonitrile solution, tryptophan (14g, 45mmol) and ammonium formate (5.61g, 90mmol) into a 150mL round bottom flask, heat to reflux at 90°C, a large amount of white solid slowly dissolves and a small amount of white solid appears, and the reaction is complete after 24 hours. Filtrate, concentrate the supernatant by rotary evaporation in vacuum, extract three times with water and ethyl acetate to combine the organic phases, dry with anhydrous sodium sulfate, filter, and concentrate the supernatant by rotary evaporation in vacuum to obtain a brown liquid with a yield of 85%.
[0049] 1 H NMR (400MHz, CDCl 3 )δ8.19(s,1H),8.10(d,J=7.8Hz,1H),7.48(d,J=7.6Hz,1H),7.40(s,1H),7.33–7.29(m,1H), 7.26–7.21(m,1H),5.04-4.99(m,1H),3.71(s,3H),3.31-3.23(m,1H),1.67(s,3H).
[0050] 13 C NMR (101MHz, CDCl 3 )δ171.67, 160.92, 149.54, 135.24, 130.
Embodiment 3
[0055] Example 3: Methyl 2-isocyano-2-phenylacetate
[0056]
[0057] (1) Preparation of phenylglycine methyl ester formamide
[0058]
[0059] Phenylglycine methyl ester (1.4g, 8.5mmol) was dissolved in 6mL methyl formate, triethylamine (1.8mL, 13mmol) was added at room temperature, stirred at room temperature for 5-25 hours to complete the reaction, the reaction solution was concentrated by rotary evaporation in vacuo, Extracted three times with dichloromethane, combined the organic phases, washed three times with saturated sodium chloride solution, dried with anhydrous sodium sulfate, filtered, and concentrated by rotary evaporation in vacuum to obtain a light yellow liquid with a yield of 43%.
[0060] 1 H NMR (400MHz, DMSO-d6) δ8.15(s, 1H), 7.65–7.14(m, 5H), 5.56(d, J=7.5Hz, 1H), 3.64(s, 3H).
[0061] 13 C NMR (101MHz, CDCl 3 )δ171.05, 160.73, 136.03, 129.00, 128.66, 127.26, 55.08, 52.89.
[0062] (2) Preparation of 2-isocyano-2-phenylacetic acid methyl ester
[
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap