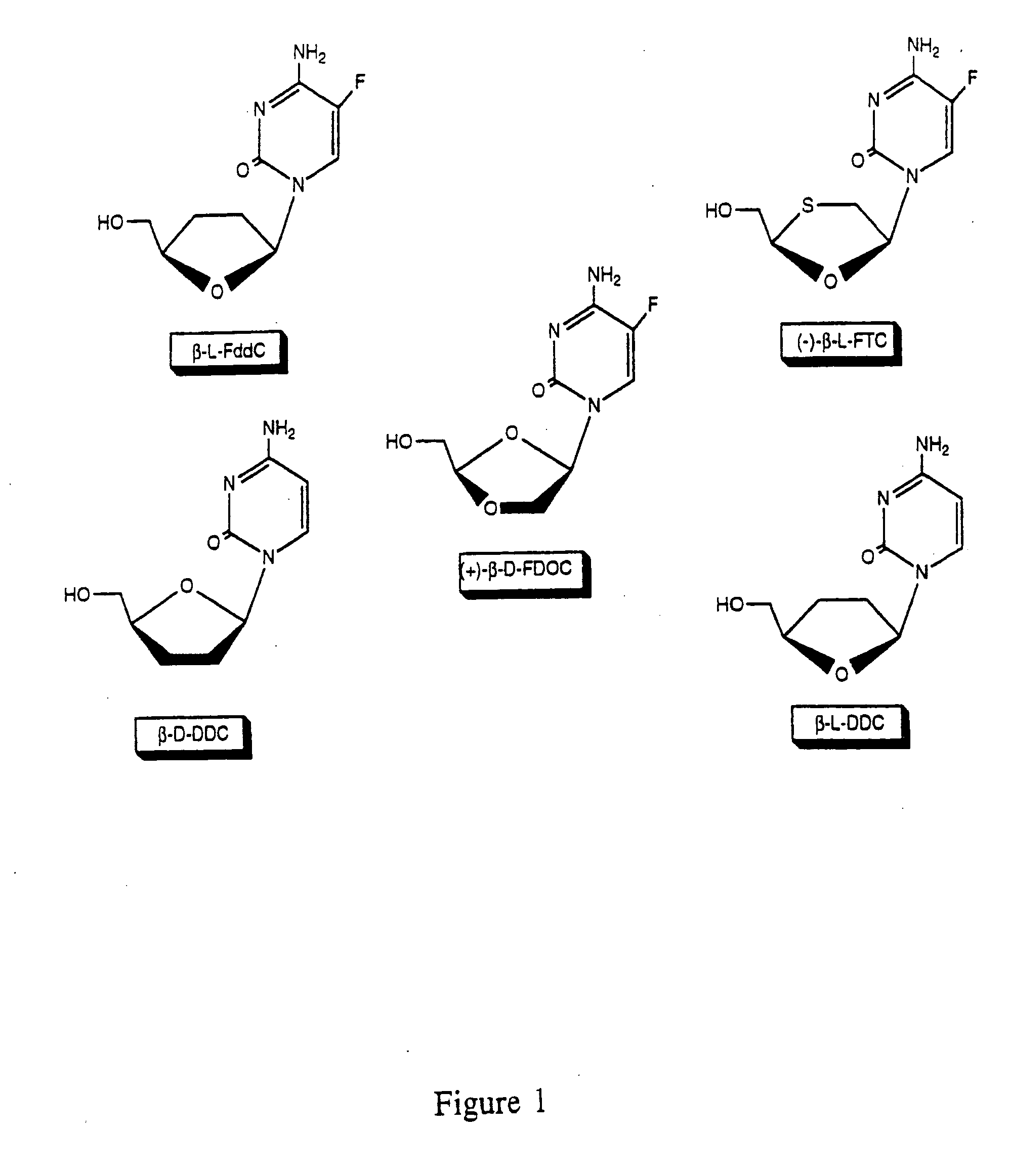
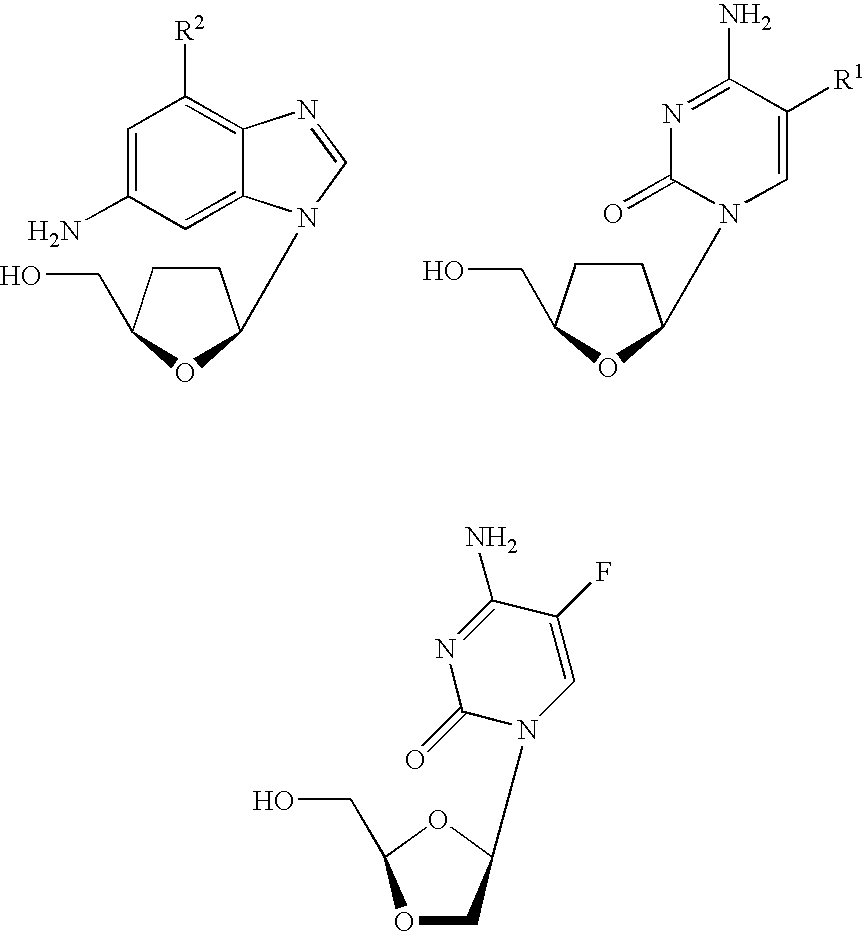
Thymine nucleosides with anti-hepatitis B virus activity
a technology of hepatitis b virus and nucleosides, which is applied in the direction of biocide, sugar derivatives, pharmaceutical non-active ingredients, etc., can solve the problems of fulminant hepatitis, rapid progression, and jaundice in the abdomen, so as to prevent or retard the progression of clinical illness and effective anti-hbv treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example = 1
EXAMPLE=1-(2-Fluoro-2,3-dideoxy-β-L-threo-pentofuranosyl)-5-fluorocytosine[2′-F-β-L β-L-FddC]
[0060]
[0061] Hitherto unknown 2′-F-β-L-FddC was synthesized in five steps from 1-(5-O-benzoyl-3-deoxy-β-L-erythro-pentofuranosyl)-5-fluorouracil 17 with an overall yield of 28% m.p. 209-210° C. (crystalized arom absolute ethanol); UV (Et OH) λmax 276 nm (ε, 9000), λmin 226nm (ε, 4000); 19F-NMR (DMSO-d6) δ ppm: −179.7 (m, F2′), −167.2 (dd, F5; JF.6=7.3 Hz, JF.1=1.5 Hz); 1H-NMR (DMSO-d6) δppm: 8.30 (d, 1H, H-6; J6,F=7.3 Hz), 7.8-7.5 (br s, 2H, NH2), 5.80 (d, 1H, H-1′ J1′,F=17.4 Hz), 5.34 (t, 1H, OH-5′; J=4.8 Hz), 5.10 (dd, 1H, H-2′; J2′,F=51.2 Hz; J2′,3′=3.4 Hz), 4.3 (m, 1H, H-4′), 3.8-3.6 (m, 2H, H-5′,5″), 2.2-2.0 (m, 2H, H-3′, H-3″); mass spectra (performed in: glycerol-thioglycerol, 1:1 υ / υ), FAB>O:248 (M+H)+, 130 (BH2)+; FAB−; [α]20D=−16.5 (−c 0.85, DMSO). Anal. Calc. for C9H11N3O3F2: C, 43.73; H, 9.49; N, 17.00; F, 15.37. Found: C, 43.56; H, 4.78; N, 16.75; F, 14.96.
II. Anti-HBV Activit
example 2
Toxicity of Compounds
[0072] The ability of the active compounds to inhibit the growth of virus in 2.2.15 cell cultures (HepG2 cells transformed with hepatitis virion) was evaluated. As illustrated in Table 1, no significant toxicity (greater than 50% depression of the dye untake levels observed in untreated cells) was observed for any of the test compounds at the concentrations 100 μM. The compounds were moderately toxic at 300 μM, however, all three compounds exhibited less toxicity at this concentration than β-D-ddC. It appears that the IC50 of β-L-ddC and β-L-FddC is approximately twice that of β-D-ddC.
[0073] Toxicity analyses were performed in 96-well flat bottomed tissue culture plates. Cells for the toxicity analyses were cultured and treated with test compounds with the same schedule as used for the antiviral evaluations. Each compound was tested at 4 concentrations, each in triplicate cultures. Uptake of neutral red dye was used to determine the relative level of toxicity. Th
example 3
Anti-Hepatitis B Virus Activity
[0074] The positive treatment control, β-D-2′,3′-dideoxycytosine [β-D-ddC], induced significant depressions of HBV DNA replication at the concentration used. Previous studies have indicated that at 9-12 μM of β-D-ddC, a 90% depression of HBV RI (relative to average levels in untreated cells) is typically observed in this assay system (Korba and Gerin, Antiviral Res. 19: 55-70, 1992). This is consistent with the data presented in Table 1.
[0075] The data presented in Table 1 indicates that all three test compounds ((β-L-FddC), (β-L-ddC), and β-D-FDOC)), were potent inhibitors of HBV replication, causing depression of HBV virion DNA and HBV RI to a degree comparable to, or greater than, that observed following treatment with β-D-ddC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap