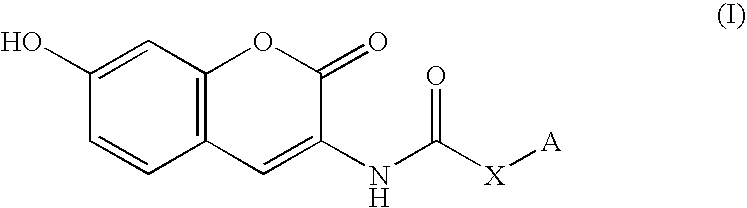
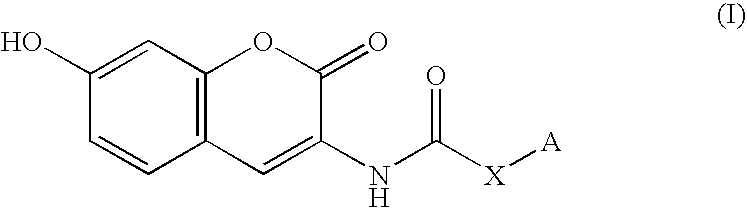
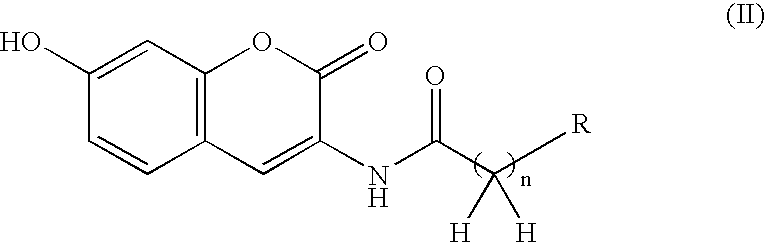
Coumarin derivative
a technology of coumarin and derivatives, applied in the field of medicaments, can solve the problems of significant deterioration of the qol (quality of life) of cancer patients, increase of doses, undesirable life-prolongation rate, etc., and achieve the effect of enhancing the effect of cancer therapy, reducing side effects, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 2-chloro-N-(7-hydroxy-2-oxo-2H-1-benzopyran-3-yl)benzamide (Compound 2)
(1) Preparation of 3-amino-7-hydroxy-2H-1-benzopyran-2-one.
A mixture of N-(7-hydroxy-2-oxo-2H-1-benzopyran-3-yl)benzamide (230 mg, 0.82 mmol), 1-propanol (6 ml) and concentrated hydrochloric acid (2 ml) was refluxed for 3 hours. Then, hydrochloric acid (2 ml) was added and the mixture was refluxed for further 7 hours. After cooling, the reaction mixture was poured into saturated aqueous sodium hydrogen carbonate and extracted with ethyl acetate. The ethyl acetate layer was washed successively with water and saturated brine, and after the layer was dried over anhydrous sodium sulfate, the residue obtained by evaporation of the solvent under reduced pressure was purified by column chromatography on silica gel (eluent: dichloromethane / ethyl acetate=2 / 1) to give the title compound as a yellow solid (127 mg, 87.7%).
1H-NMR(DMSO-d6, δ): 5.23(2H, s), 6.65-6.70(3H, m), 7.23(1H, d, J=8.4 Hz), 9.81(1H,
example 2
Preparation of 3-chloro-N-(7-hydroxy-2-oxo-2H-1-benzopyran-3-yl)benzamide (Compound 3)
Yield: 38.8%
1H-NMR(CDCl3+DMSO-d6, δ): [2.58(a signal of DMSO)], 6.78(1H, d, J=2.4 Hz), 6.84(1H, dd, J=8.7, 2.4 Hz), 7.41(1H, d, J=8.4 Hz), 7.50(1H, t, J=7.5 Hz), 7.57(1H, ddd, J=7.8, 1.8, 1.5 Hz), [7.85(a signal of CHCl3)], 7.86(1H, dt, J=7.5, 1.5 Hz), 7.95-7.96(1H, m), 8.61(1H, s), 9.23(1H, s), 10.12(1H, s).
example 3
Preparation of 3-bromo-N-(7-hydroxy-2-oxo-2H-1-benzopyran-3-yl)benzamide (Compound 4)
Yield: 67.9%
1H-NMR(CDCl3+DMSO-d6, δ): [2.59(a signal of DMSO)], 6.86-6.89(2H, m), [7.37(a signal of CHCl3)], 7.39(1H, d, J=6.0 Hz), 7.42(1H, d, J=8.1 Hz), 7.71(1H, ddd, J=7.8, 1.8, 1.2 Hz), 7.83(1H, ddd, J=7.8, 1.8, 1.2 Hz), 8.06(1H, t, J=1.8 Hz), 8.73(1H, s), 8.76(1H, s), 9.79(1H, s).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap