Compound, curable composition, cured product, optical member, and lens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
application example
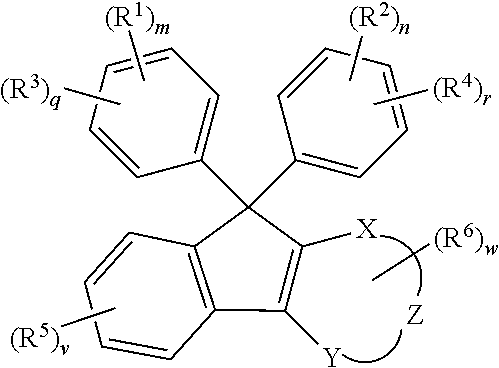
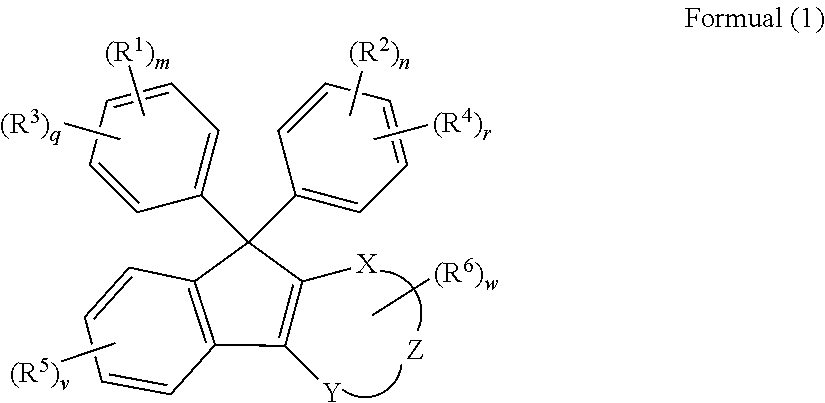
[0210]The optical member using the cured product of the present invention is preferably used particularly in a lens substrate. The lens substrate manufactured by using the curable composition of the present invention has the low Abbe number, preferably has a high refractive index, light transmittance, and light weight, and has excellent optical properties. It is possible to optionally adjust the refractive index of the lens substrate by suitably adjusting the types of the monomer for forming the curable composition.
[0211]In the present specification, a “lens substrate” means a single member that may exhibit a lens function. A film or a member may be provided on the surface or the periphery of the lens substrate according to the use environment and application of the lens. For example, a protective film, an antireflection film, a hard coat film, and the like may be formed on the surface of the lens substrate. A composite lens obtained by laminating a glass lens substrate or a plastic le
examples
[0214]Hereinafter, characteristics of the present invention are more specifically described with reference to the examples and comparative examples. A material, an amount used, a treatment detail, a treatment order, and the like provided in the following examples can be suitably changed without departing from the gist of the present invention. Accordingly, the scope of the present invention should not be construed in a limited manner by the following specific examples.
Synthesis of Compound A
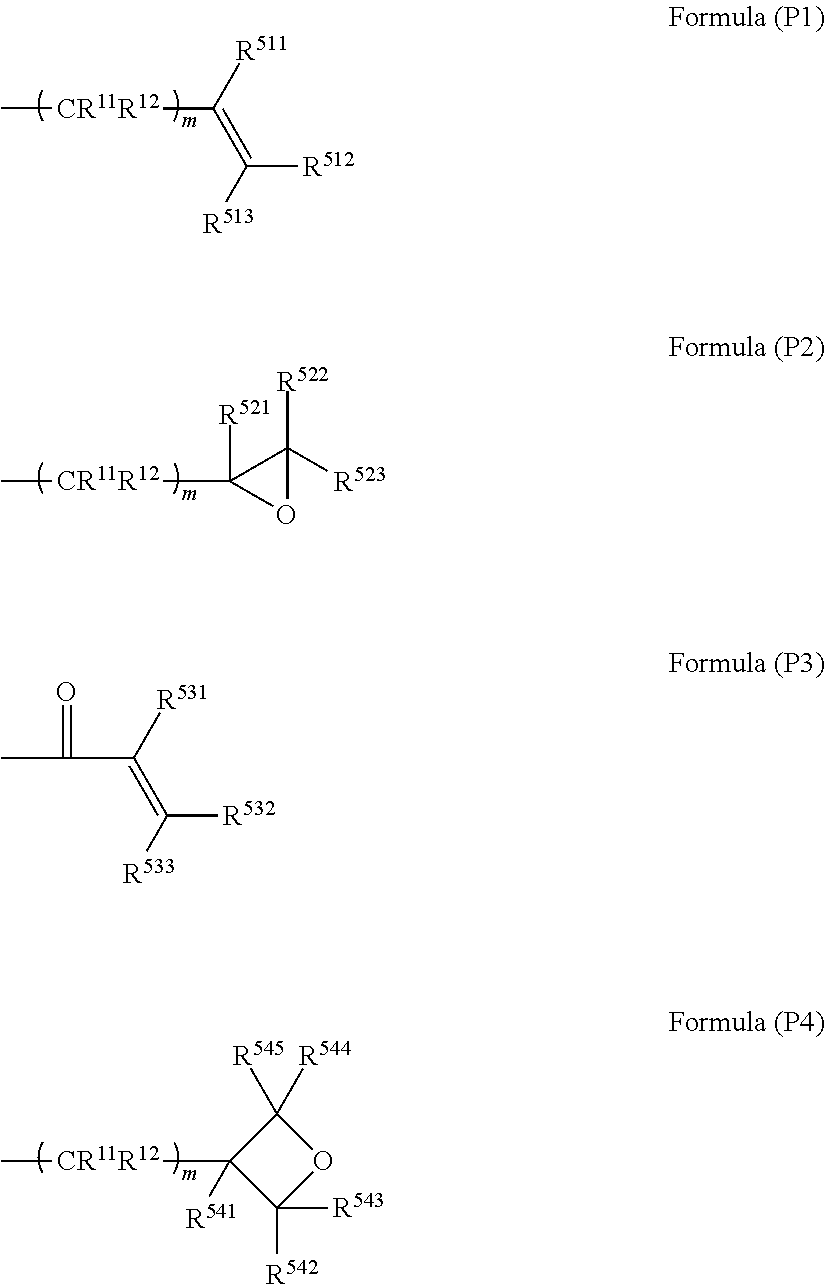
[0215]132 mL of triethylamine and 650 mL of butyl acetate were added to 100 g of 2-hydroxyethyl acrylate and stirred. While the reaction solution was maintained at 5° C., 70 mL of methanesulfonic acid chloride was added dropwise over one hour. After stirring was performed for one hour, 500 mL of water was added to the reaction solution and stirred, and an operation of removing a water layer was repeated three times. After 30 mg of dibutylhydroxytoluene was added, butyl acetate was distilled off unde
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap