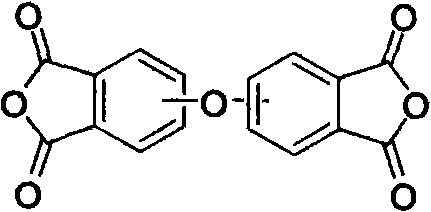
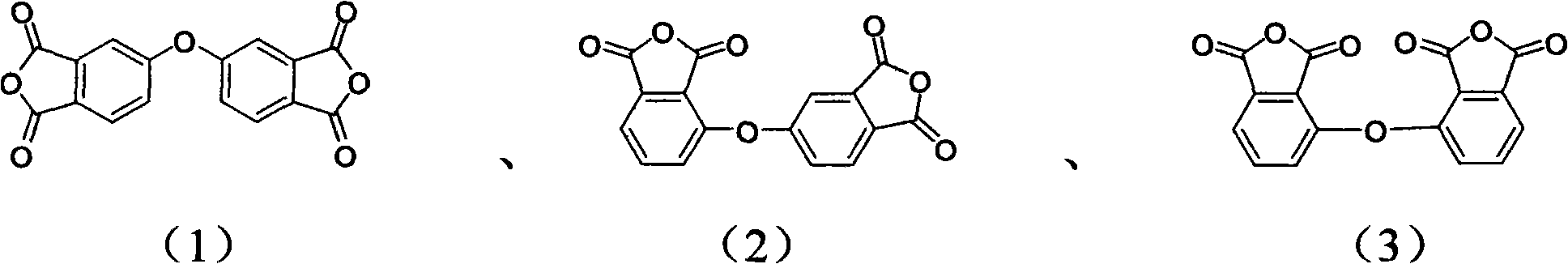
Preparation method of diphenyl ether dianhydride and isomer thereof
A technology of diphenyl ether dianhydride and isomers, which is applied in the preparation of diphenyl ether dianhydride and the field of dianhydride preparation, can solve the problems of difficult separation and purification, low yield, and many reaction by-products, etc. Simple process, high product purity and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add trichlorotoluene 200ml, 4-chlorophthalic anhydride 35g in the reactor that 500ml has drying and distillation unit, in N 2 In the case of protection, heat up and reflux for 20min, add K 2 CO 3 13.5 g and 3.4 g of triphenoxyphosphine were refluxed for 6 hours, cooled and filtered to obtain 23.85 g of crude product with a yield of 80%.
[0033] Add 100ml of benzonitrile and the crude product prepared by the above reaction in a clean reactor with a drying and distillation unit, in N 2 Under protection, heat up and reflux for 1 hour, cool down to -10°C, filter, and dry to obtain 22.4 g of diphenyl ether dianhydride and its isomers. The yield was 75.1%.
Embodiment 2
[0035] Add o-dichlorobenzene 180ml, 4-chlorophthalic anhydride 30.5g in the reactor that has drying and distillation device in 500ml, in N 2 In the case of protection, heat up and reflux for 35min, add K 2 CO 3 12.7 g and 4.2 g of hexaethylguanidine chloride were reacted under reflux for 5.5 hours, cooled and filtered to obtain 21.5 g of crude product with a yield of 82.7%.
[0036] Add the mixed solution of 120ml benzonitrile and trichlorotoluene and the crude product prepared by the above-mentioned reaction in clean reactor with drying and distillation unit, in N 2 Under protection, heat up and reflux for 40 minutes, cool down to -12°C, filter, and dry to obtain 20.8 g of diphenyl ether dianhydride and its isomers. The yield was 80.1%.
Embodiment 3
[0038] Add o-dichlorobenzene 230ml, trichlorotoluene 190ml, 4-chlorophthalic anhydride 92g in 1000ml reactor that has drying and distillation device, in N 2 In the case of protection, heat up and reflux for 60min, add K 2 CO 3 36g, 5.2g of hexabutylguanidine chloride and 2.1g of triphenylphosphine bromide were refluxed for 7 hours, cooled and filtered to obtain 69.7g of crude product with a yield of 89%.
[0039] Add the crude product prepared by 190ml benzonitrile and above-mentioned reaction in clean reactor with drying and distillation unit, in N 2 Under the condition of protection, heat up and reflux for 35 minutes, cool down to -15°C, filter, and dry to obtain 68 g of diphenyl ether dianhydride and its isomers. The yield was 86.8%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap