Cariprazine tartrate, preparation method therefor and medical use thereof
A technology of cariprazine and tartaric acid, applied in the field of medicine, can solve the problems of inconvenient research of cariprazine hydrochloride, water solubility, poor stability and fluidity of hydrochloride, and achieve the effects of good solubility and not easy to absorb moisture.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
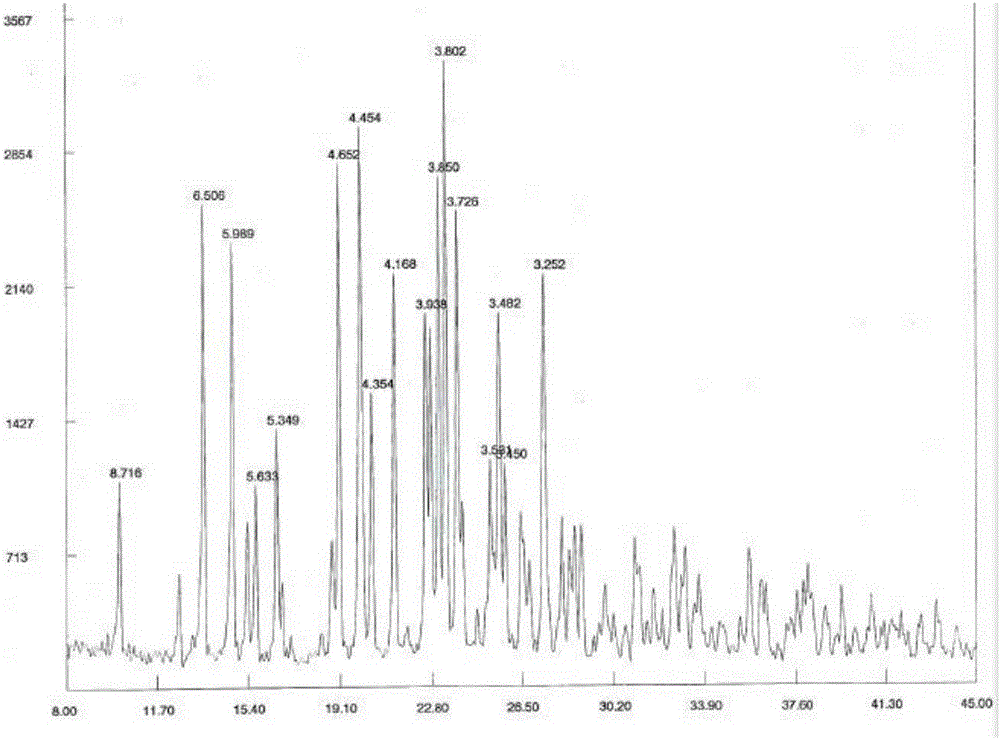
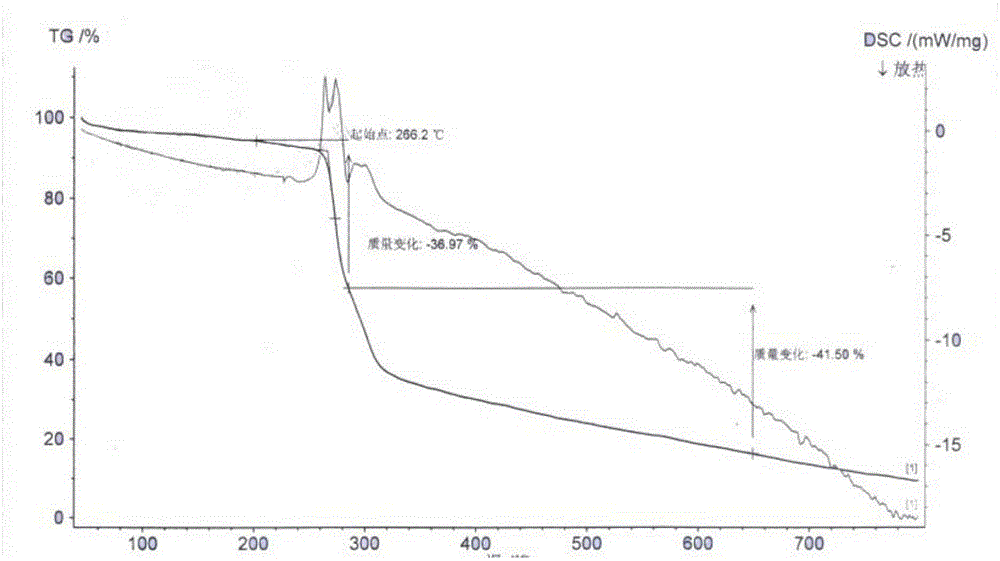
[0028] Embodiment 1: the preparation of cariprazine tartrate
[0029] Dissolve 30g of cariprazine in 650ml of absolute ethanol, stir and heat to 60-65°C, add 10.5g of tartaric acid, keep stirring for 2h, cool to 0-5°C, let stand for 5h, filter, and use proper amount of absolute ethanol for solid After washing, vacuum drying at 65-70°C for 6 hours, 35.8 g of cariprazine tartrate was obtained, with a yield of 74%, mp: 266-268°C (decomposition). HPLC content 99.2%.
[0030]
[0031] 1 H—NMR (400MHz, CDCl 3 / TMS,ppm):
[0032] δ: 6.91~7.15(m, 3H, Ar- H ), 1.08~1.53(m, 7H, cyclohexane- H ), 2.55~2.62(m, 4H, piperazine-7 H ), 3.11~3.26(m, 4H, piperazine- H ), 1.79~1.82 (m, 2H, -NC H 2 CH 2 -), 2.07~2.12(t, 2H, J=7.6Hz, -NCH 2 C H 2 -), 3.76~3.79(m, 1H, piperazine-1 H ), 6.54~6.57 (m, 1H, N H ), 2.83~2.95(m, 6H, N(C H 3 ) 2 ).
[0033] MS: m / z (M + ) 428 (M-C 4 h 6 o 6 +H).
Embodiment 2
[0034] Embodiment 2: the preparation of cariprazine hydrochloride
[0035] Dissolve 15g of cariprazine in 150ml of N-methylpyrrolidone, stir and heat to 30-35°C, drop in 12ml of isopropanol solution of 25-35% hydrogen chloride, keep stirring for 1h, then add 300ml of isopropanol, and stir for 10min , cooled to 0-5°C, let stand for 2h, filtered, washed the solid with an appropriate amount of isopropanol, and dried in vacuum at 65-70°C for 6h to obtain 12.3g of cariprazine hydrochloride.
Embodiment 3
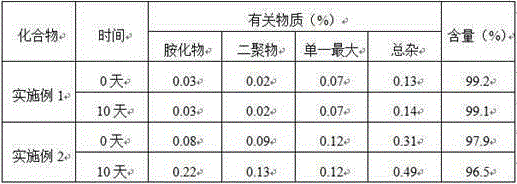
[0036] Embodiment 3: physicochemical property comparison
[0037] According to the four routine tests of the Chinese Pharmacopoeia 2015 edition.
[0038] Conclusion: Cariprazine tartrate of the present invention has good solubility and fluidity, and is not hygroscopic.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap