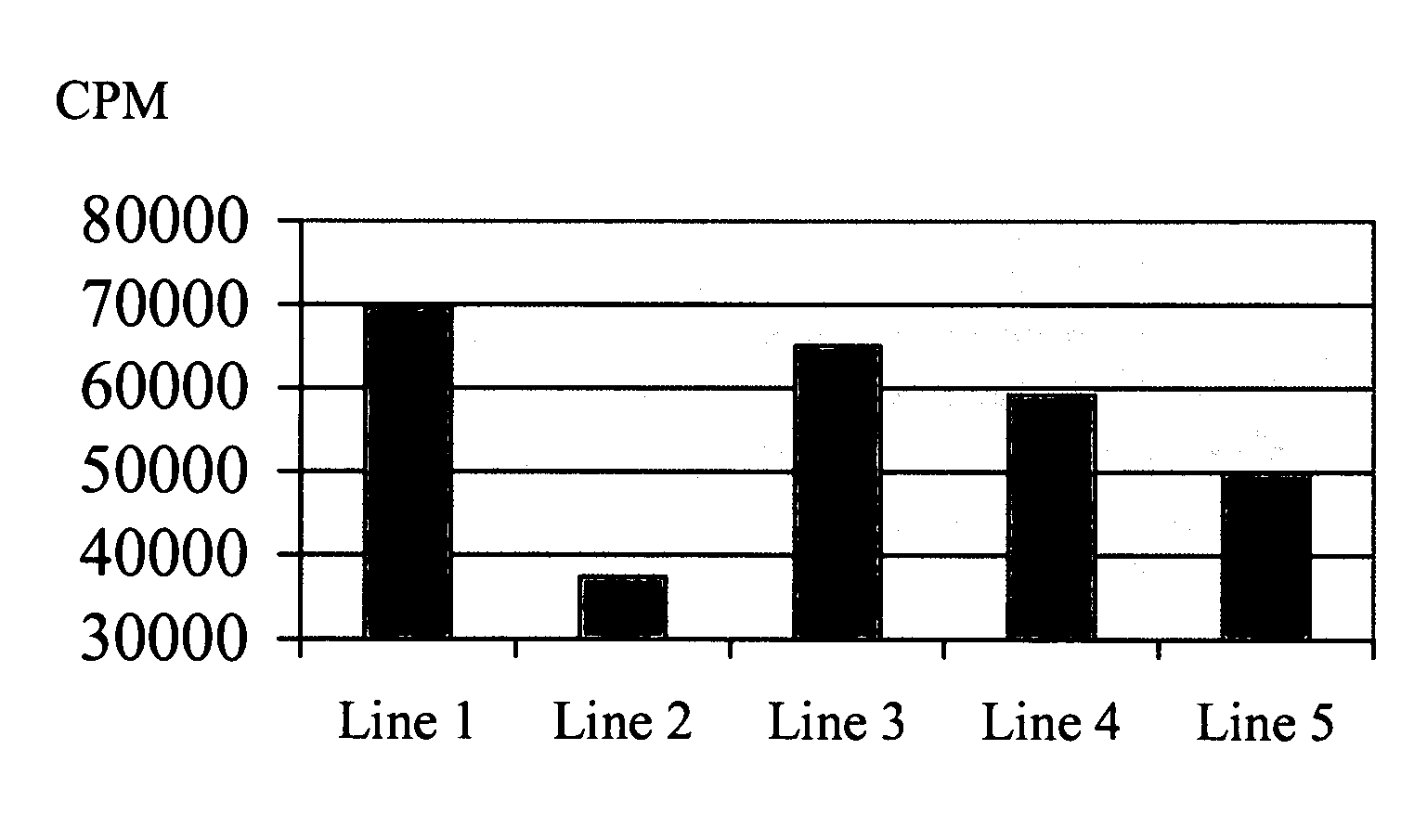
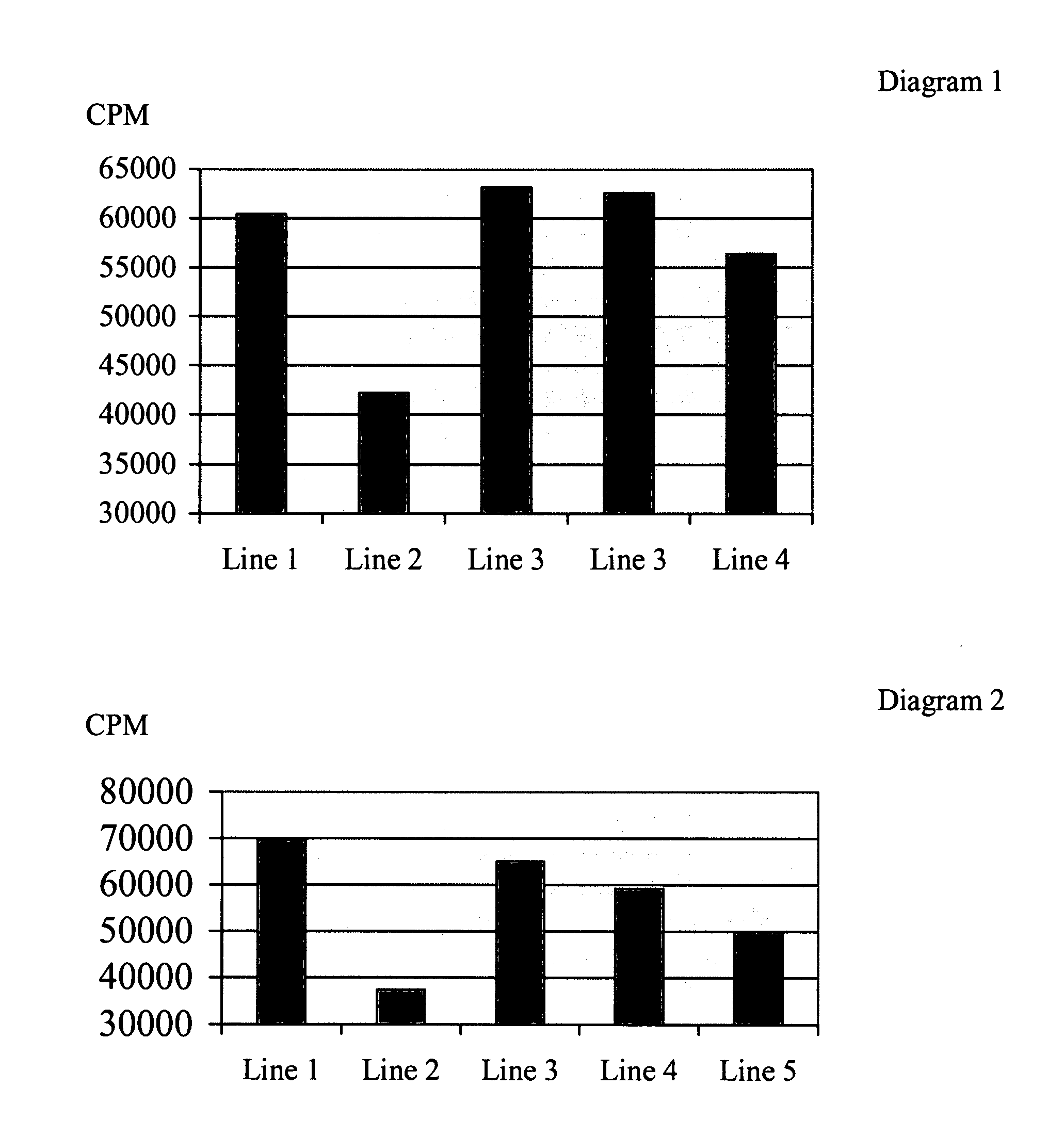
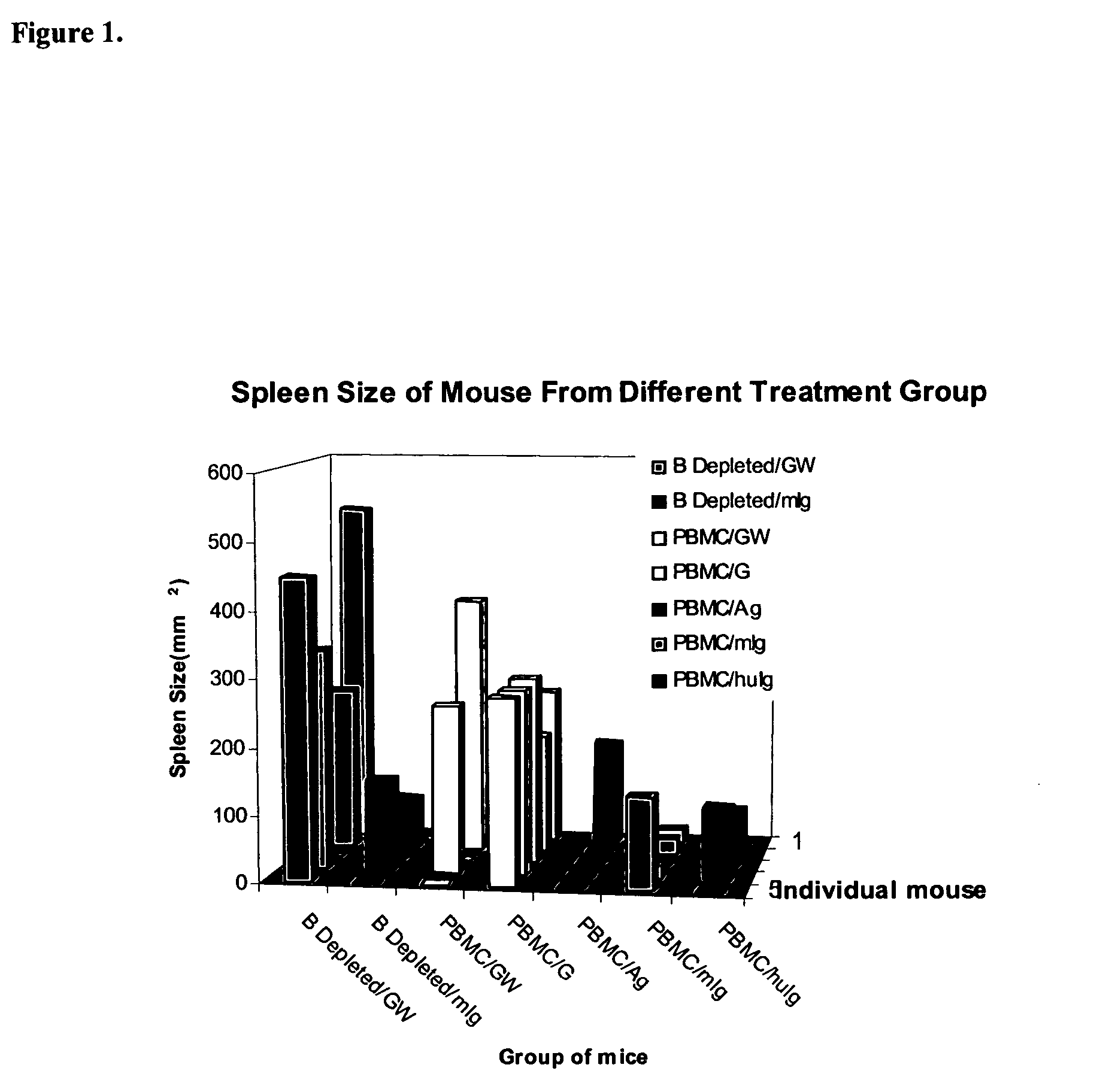
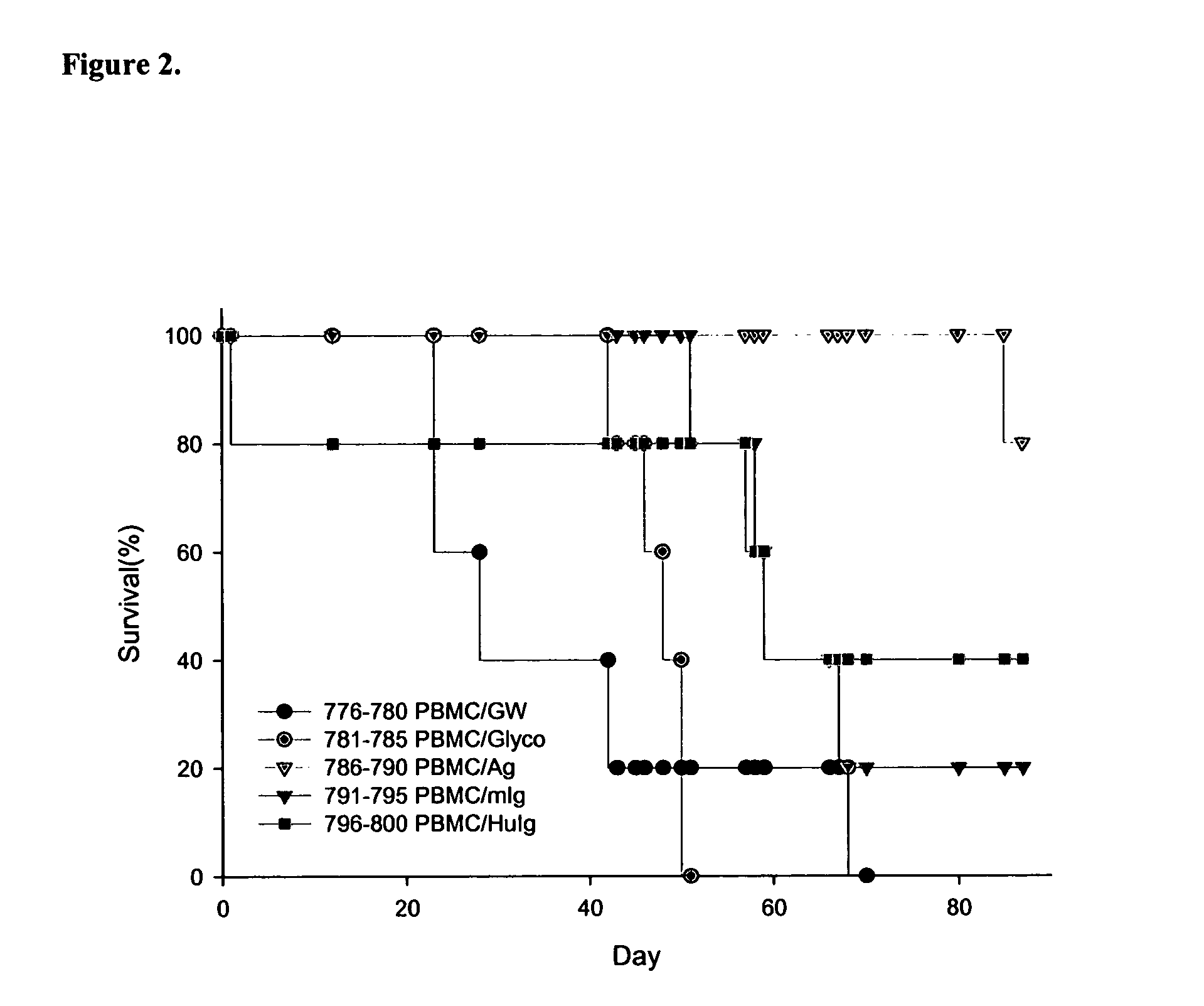
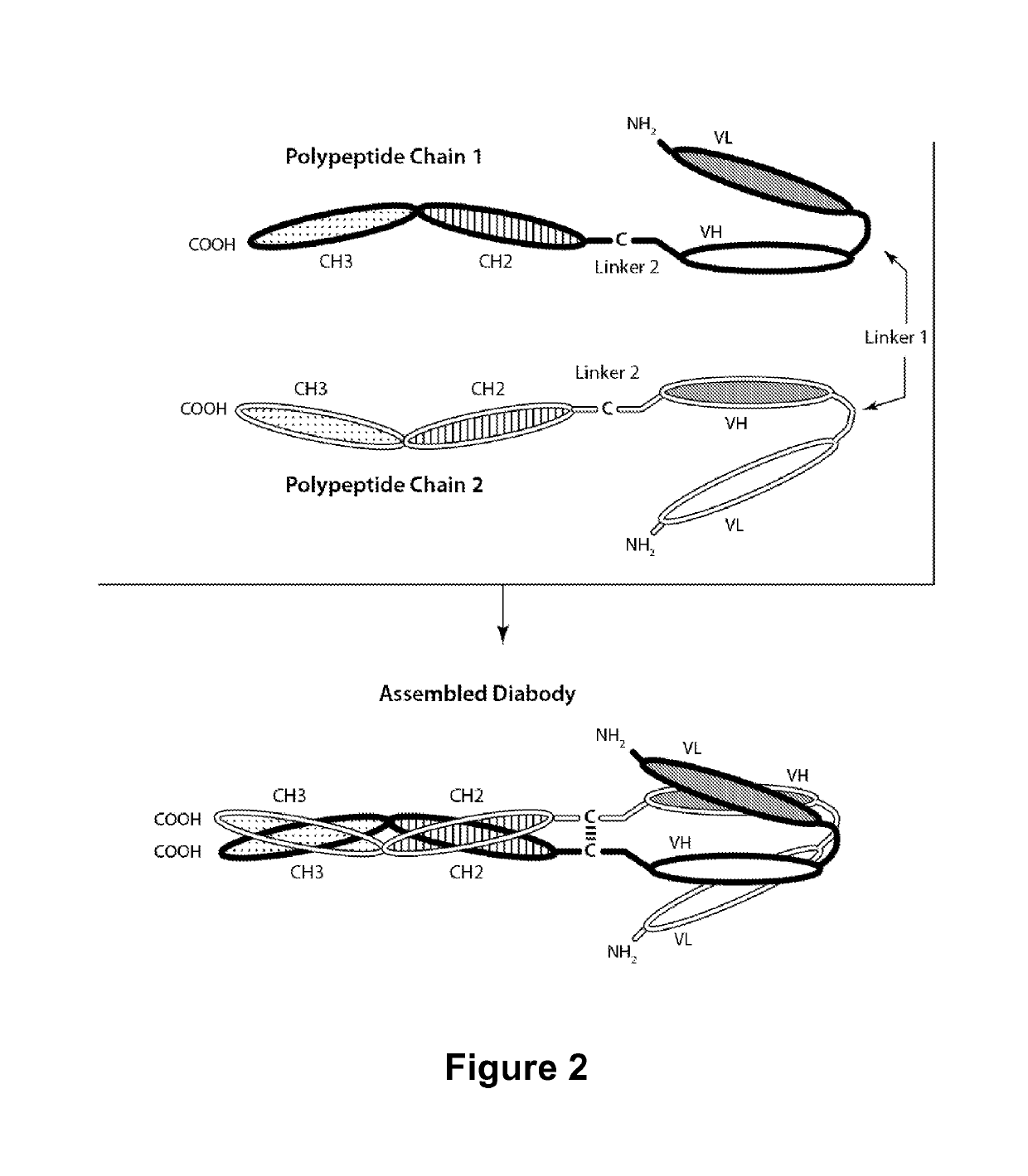
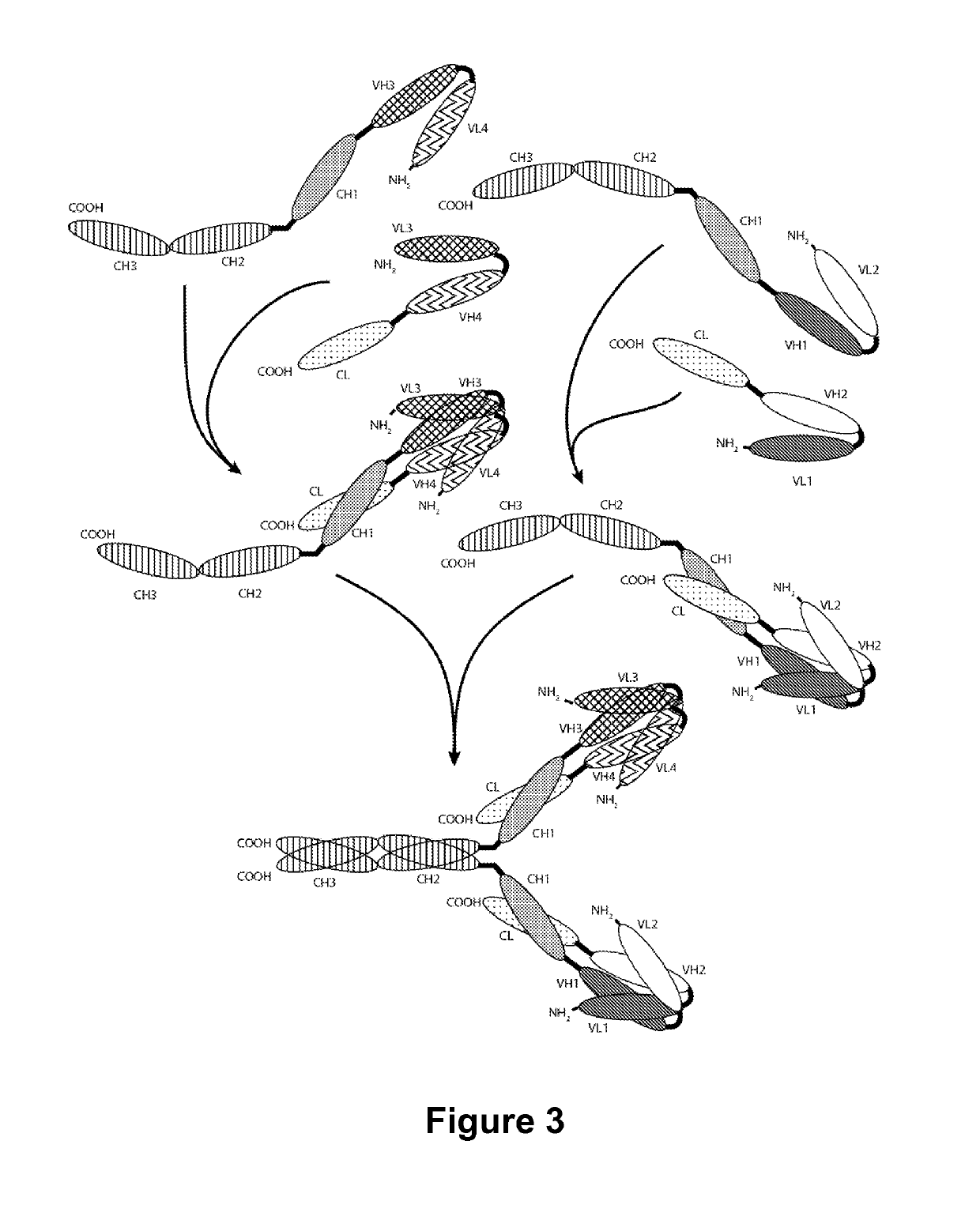
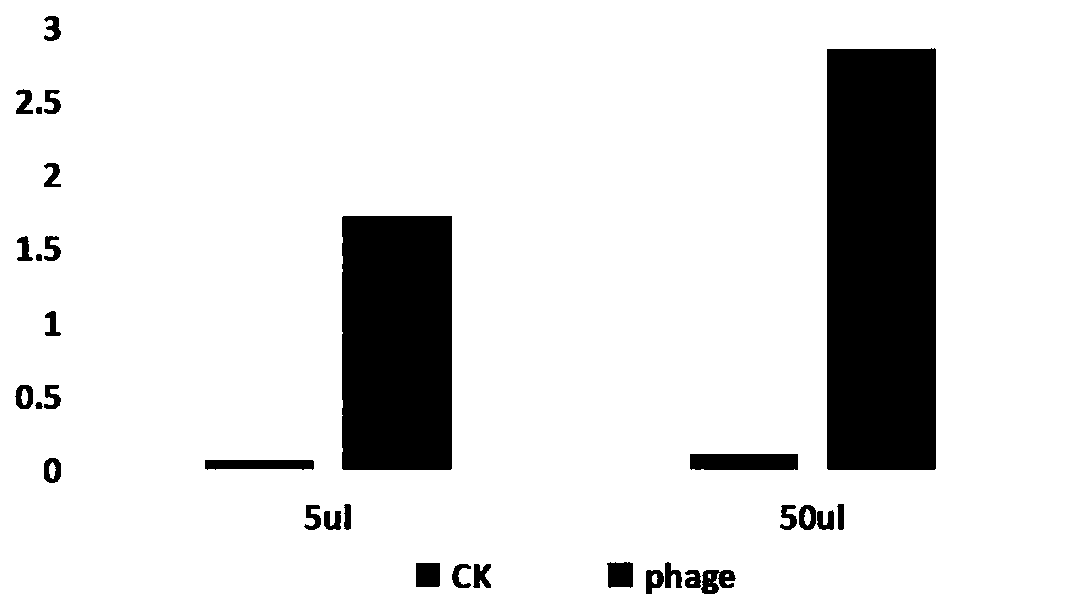
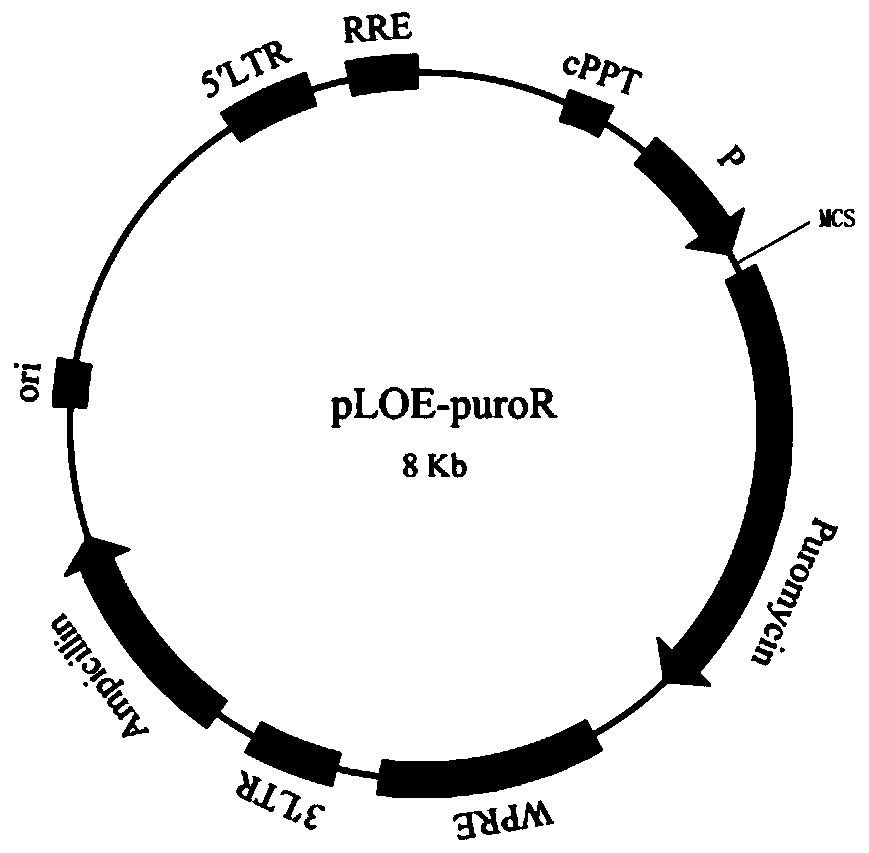
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38results about "Antibody ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
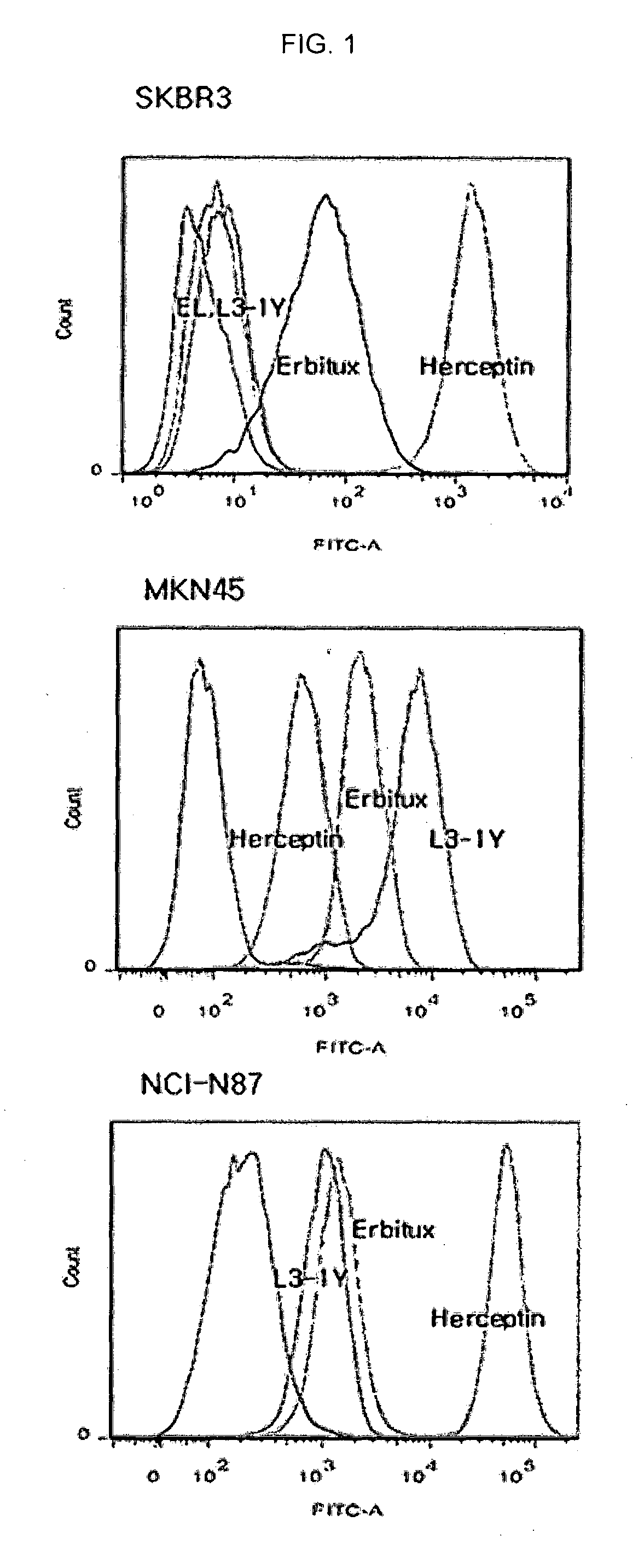
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS20070036799A1Good curative effectEnhanced ADCC activityAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsTherapeutic antibodyWild type
Owner:MACROGENICS INC
Bispecific Anti-vegf/Anti-ang-2 antibodies
Owner:F HOFFMANN LA ROCHE INC
Methods for the diagnosis, prognosis and treatment of metabolic syndrome
InactiveUS20060211020A1Sugar derivativesPeptide/protein ingredientsPhosphatidate cytidylyltransferaseGlycerol kinase
The present invention provides methods for detecting susceptibility to metabolic syndrome. In particular, the presence of differences in at least one of the following genes; microsomal triglyceride transfer protein (MTP), fatty acid binding protein 2 (FABP2), annexin A5 (ANXA5), pyruvate dehydrogenase (lipoamide) alpha 2 (PDHA2), CDP-diacylglycerol synthase (phosphatidate cytidylyltransferase) 1 (CDS 1), and glycerol kinase 2 (GK2) serves as a prognostic and diagnostic indicator of metabolic syndrome. Furthermore, metabolic syndrome can be treated by regulating the levels of MTP, FABP2, ANXA5, PDHA2, CDS1, and GK2.
Owner:TRUSTEES OF BOSTON UNIV
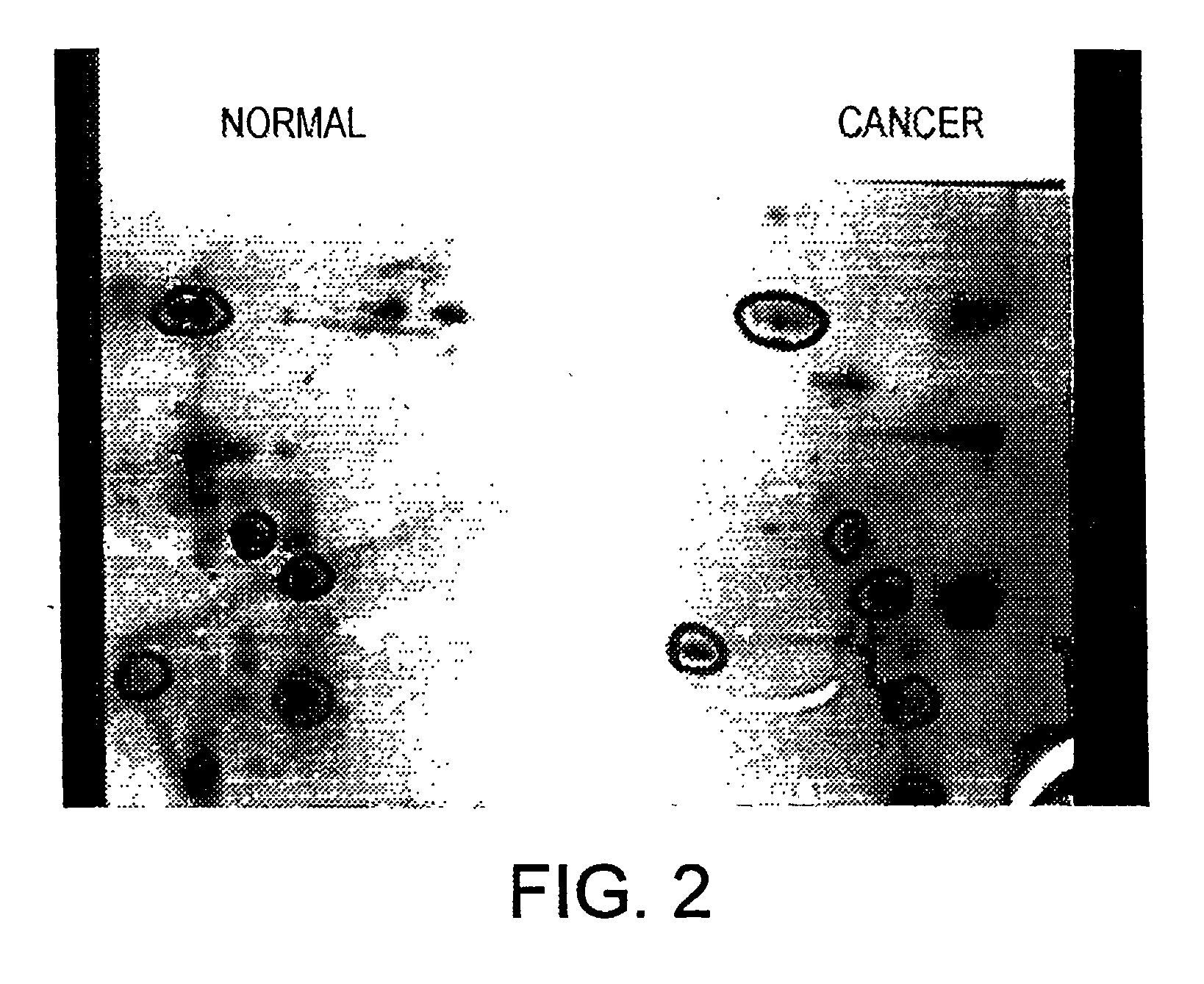
Genes differentially expressed in cancer cells to design cancer vaccines
Owner:GENZYME CORP
Uses for and article of manufacture including her2 dimerization inhibitor pertuzumab
InactiveUS20130095172A1Prolong progression-free survivalReduce riskOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMetastatic gastric cancerHER2 Positive Breast Cancer
The present application describes uses for and articles of manufacture including Pertuzumab, a first-in-class HER2 dimerization inhibitor. In particular, the application describes methods for extending progression free survival in a HER2-positive breast cancer patient population; combining two HER2 antibodies to treat HER2-positive cancer without increasing cardiac toxicity; treating early-stage HER2-positive breast cancer; treating HER2-positive cancer by co-administering a mixture of Pertuzumab and Trastuzumab from the same intravenous bag; treating HER2-positive metastatic gastric cancer; treating HER2-positive breast cancer with Pertuzumab, Trastuzumab and Vinorelbine; treating HER2-positive breast cancer with Pertuzumab, Trastuzumab and aromatase inhibitor; and treating low HER3 ovarian, primary peritoneal, or fallopian tube cancer. It also describes an article of manufacture comprising a vial with Pertuzumab therein and a package insert providing safety and / or efficacy data thereon; a method of making the article of manufacture; and a method of ensuring safe and effective use of Pertuzumab related thereto. In addition the application describes an intravenous (IV) bag containing a stable mixture of Pertuzumab and Trastuzumab suitable for administration to a cancer patient.
Owner:GENENTECH INC
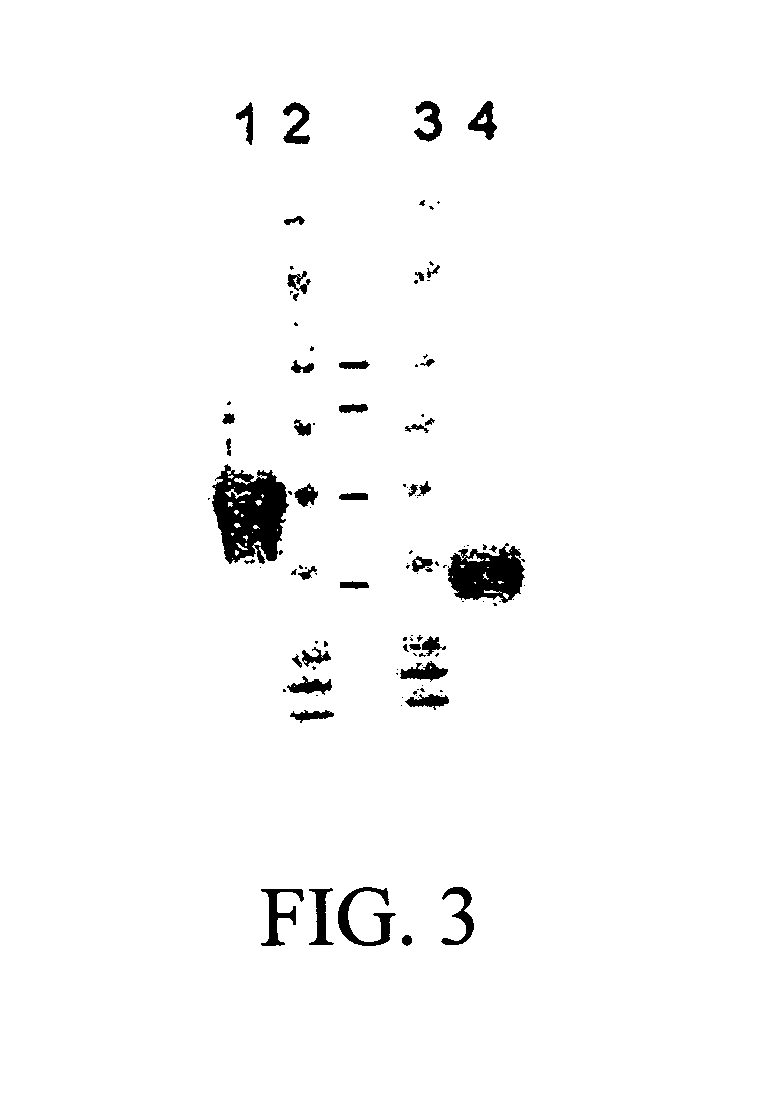
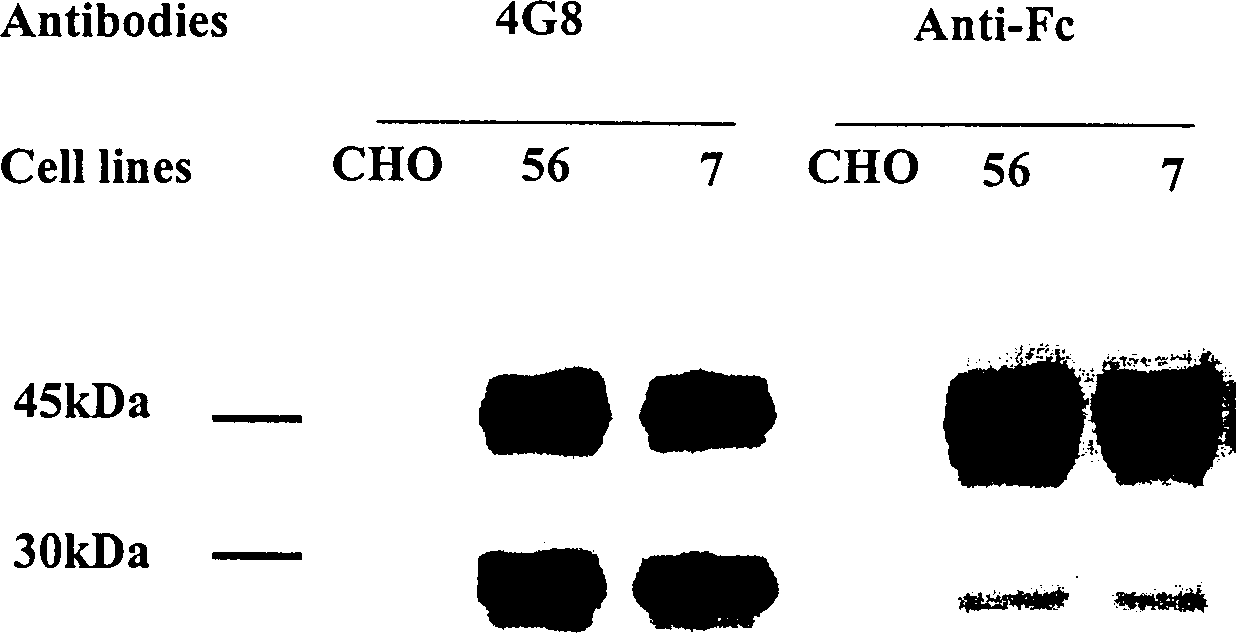
Human fusion antibody for reducing cerebral amyloid fibers associated with senile dementia
Owner:张小如 +1
Combination therapy
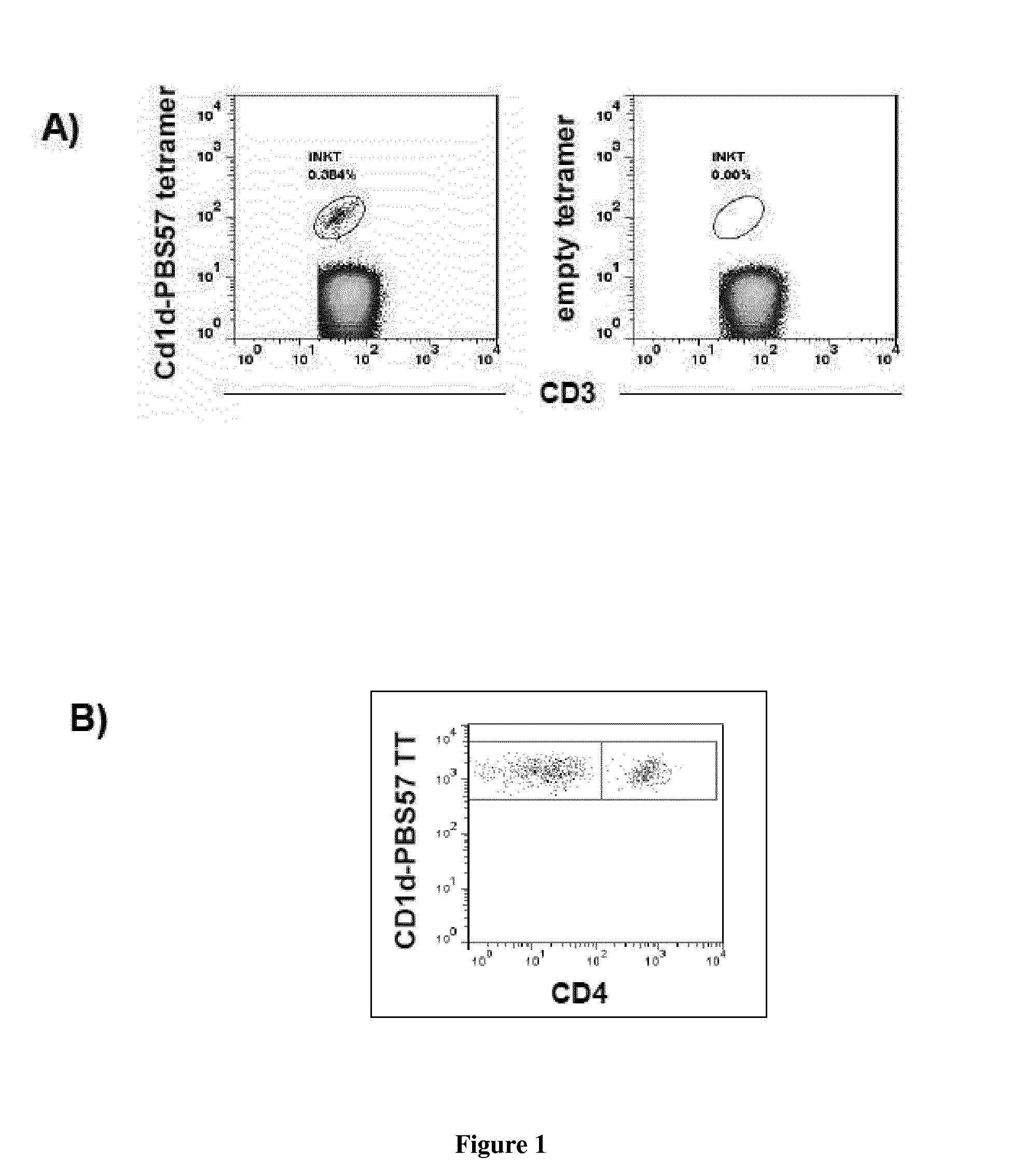
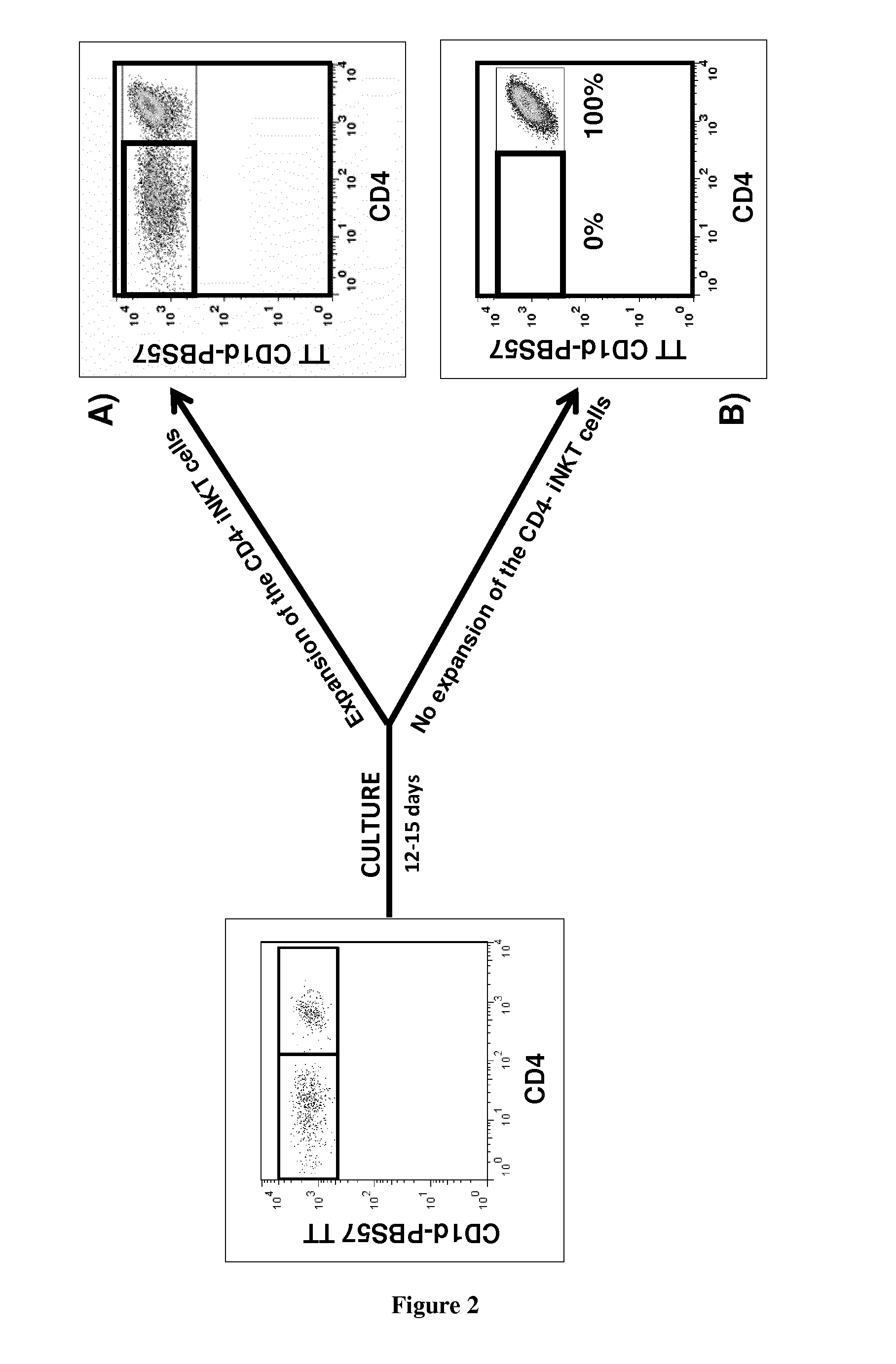
InactiveUS20150165021A1Enhance immune responseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsPharmaceutical drugINKT Cells
Owner:NKT THERAPEUTICS
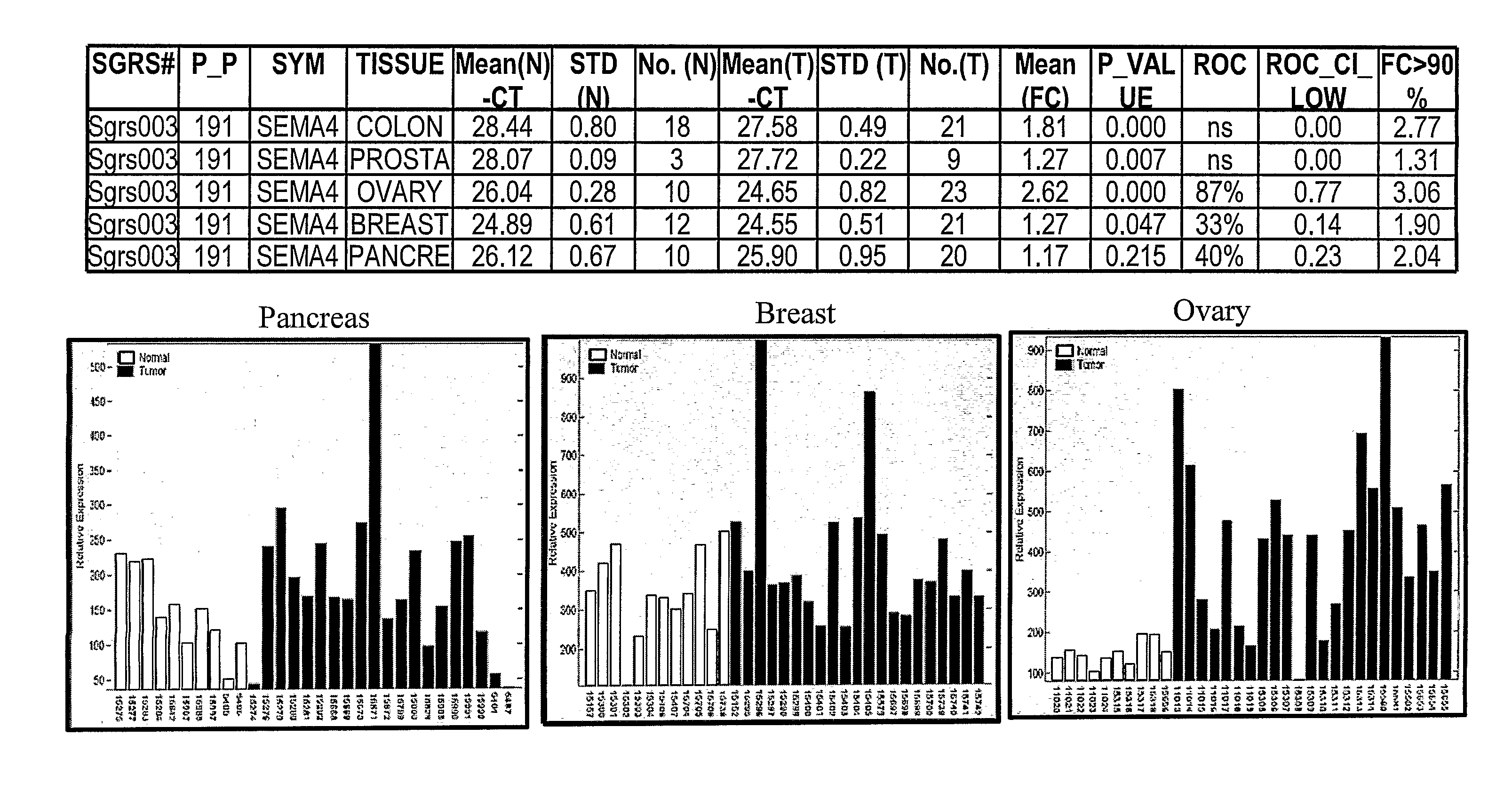
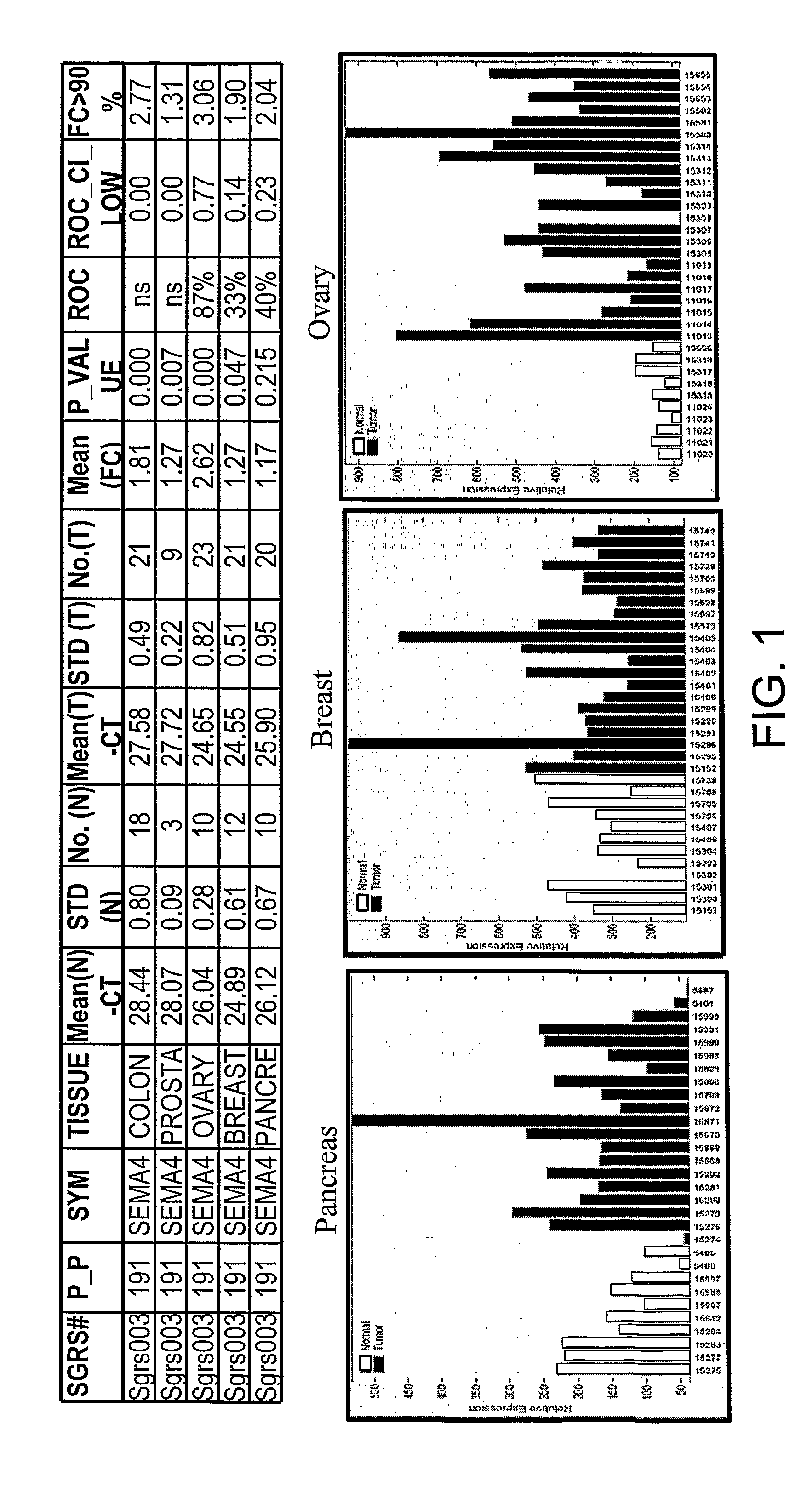
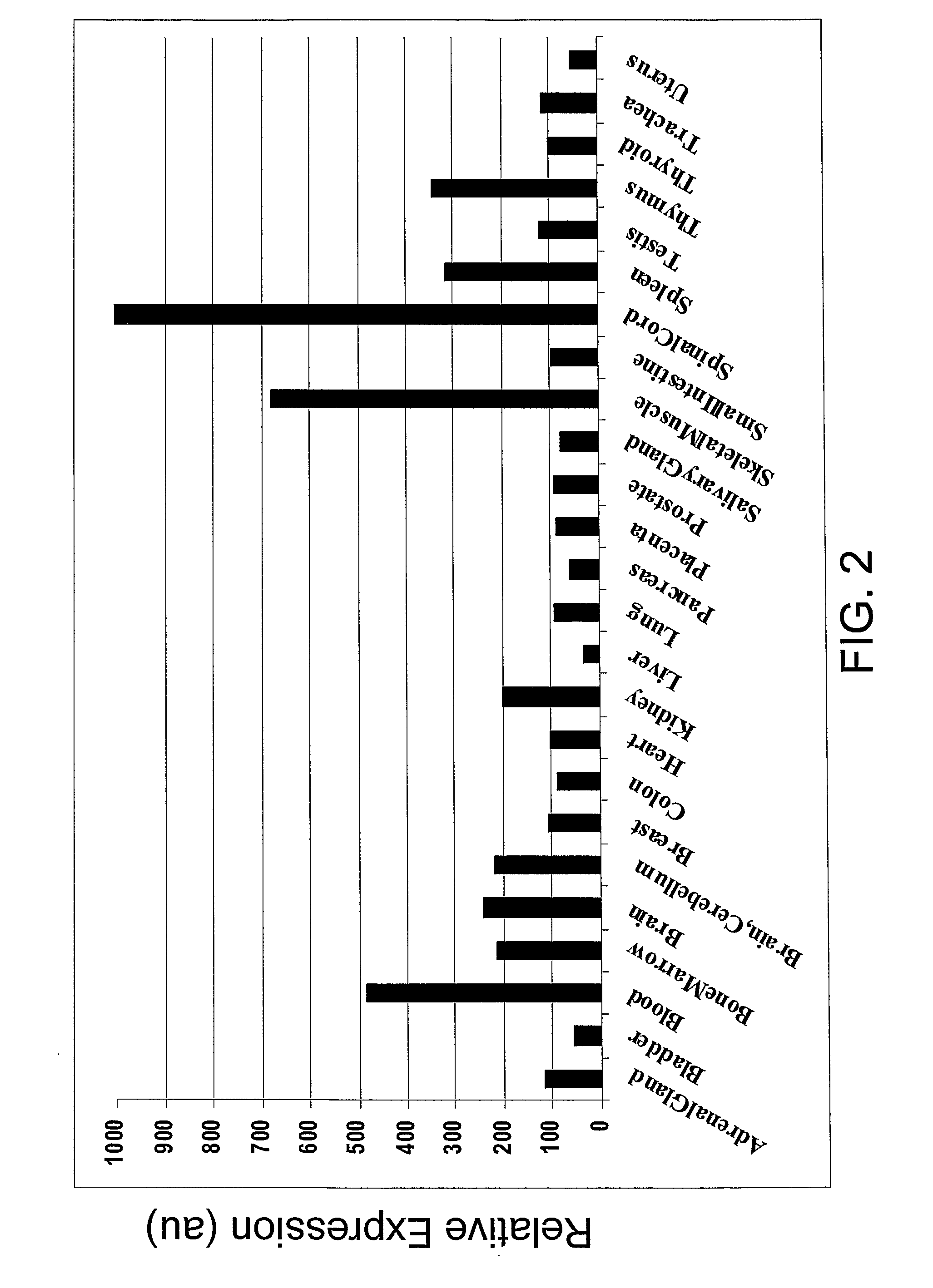
SEMA4D in Cancer Diagnosis, Detection and Treatment
InactiveUS20090104193A1Organic active ingredientsPeptide/protein ingredientsCancers diagnosisCancer research
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC +1
Methods for the treatment of lada and other adult- onset autoimmune using immunosuppressive monoclonal antibodies with reduced toxicity
InactiveUS20100015142A1Low toxicityDamage autoimmunityMetabolism disorderAntibody ingredientsDiseaseAutoimmune responses
The present invention provides methods of treating, preventing or ameliorating the symptoms of Latent Autoimmune Diabetes in Adults (LADA) and adult-onset type 1 diabetes through the use of anti-human CD3 antibodies. In particular, in invention provides methods of preventing or delaying insulin requirement in patients diagnosed with LADA. The methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the invention provides for modification of the anti-human CD3 antibodies such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-human CD3 antibodies.
Owner:MACROGENICS INC
Method for retarding unhealth manifestations brought by ageing of human beings
InactiveUS20090053200A1Reduce functionReduced stress resistancePeptide/protein ingredientsHydrolasesDna antibodyBlood plasma
Owner:CLS THERAPEUTICS
Anti-CD137 antibody as an agent in the treatment of cancer and glycosylation variants thereof
InactiveUS20060182744A1Reduce and prevent inactivationEasy to manufactureImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAnticarcinogenCD137
Owner:GTC BIOTHERAPEUTICS INC
Method for treating melanoma using a herpes simplex virus and an immune checkpoint inhibitor
Owner:AMGEN INC
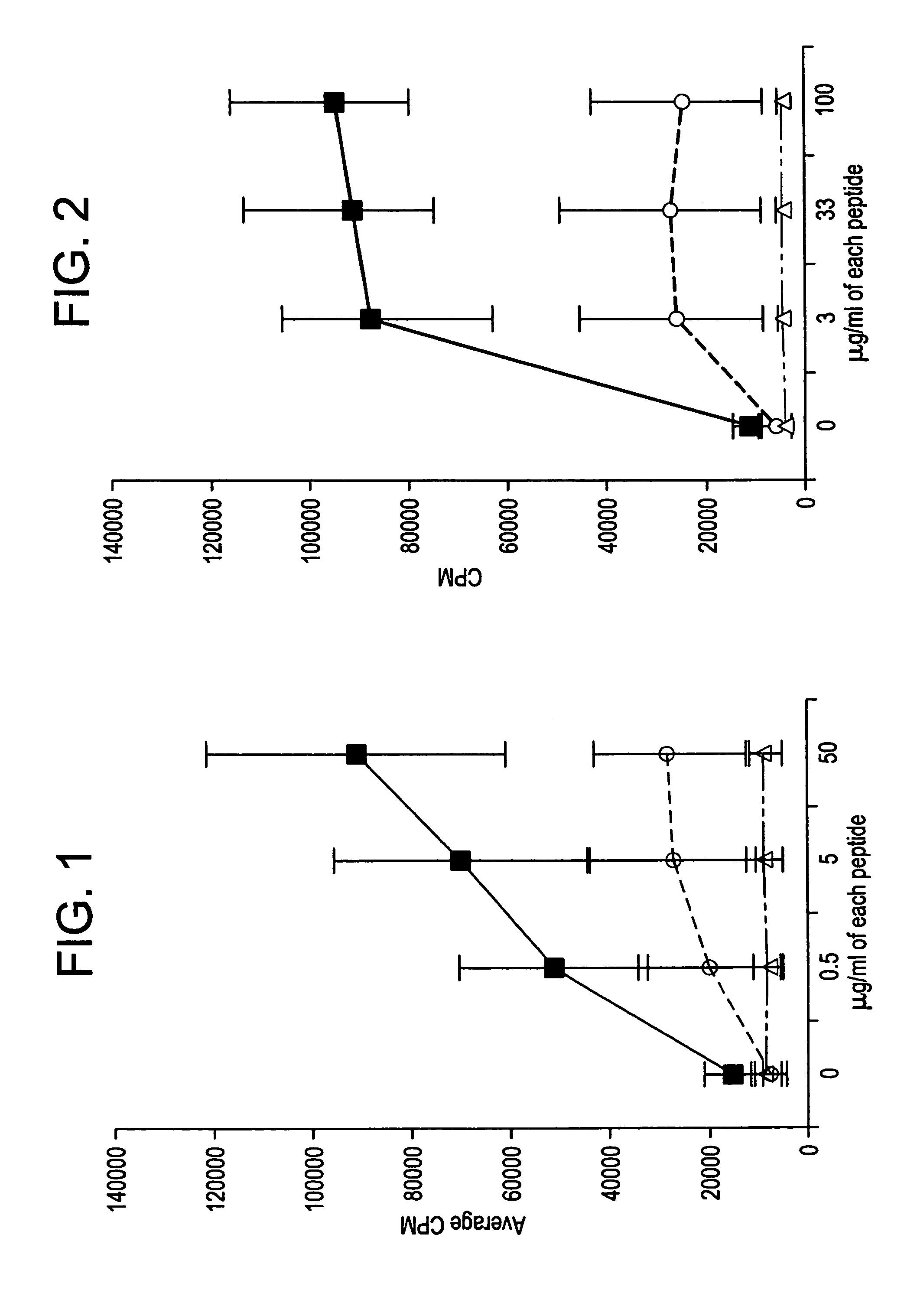
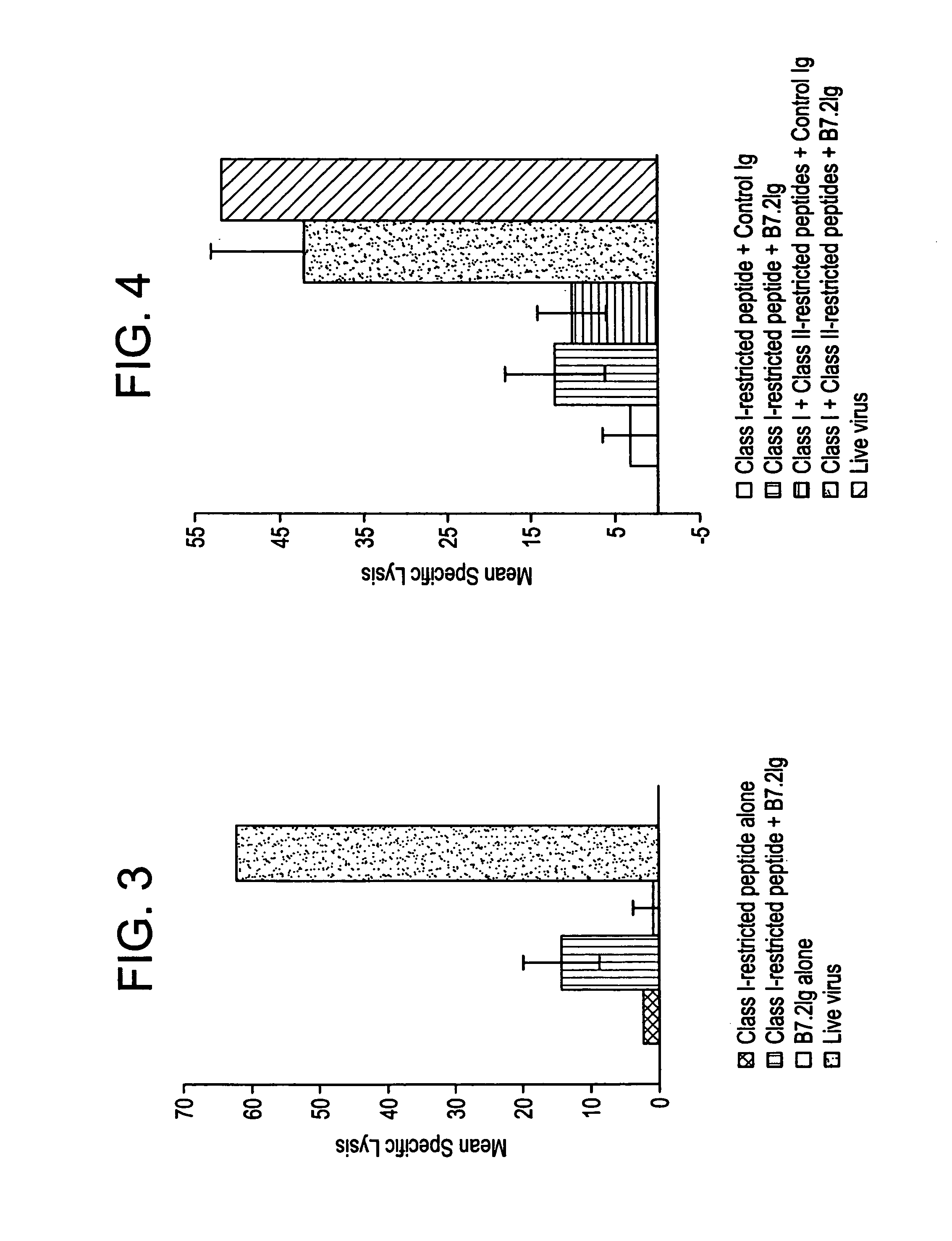
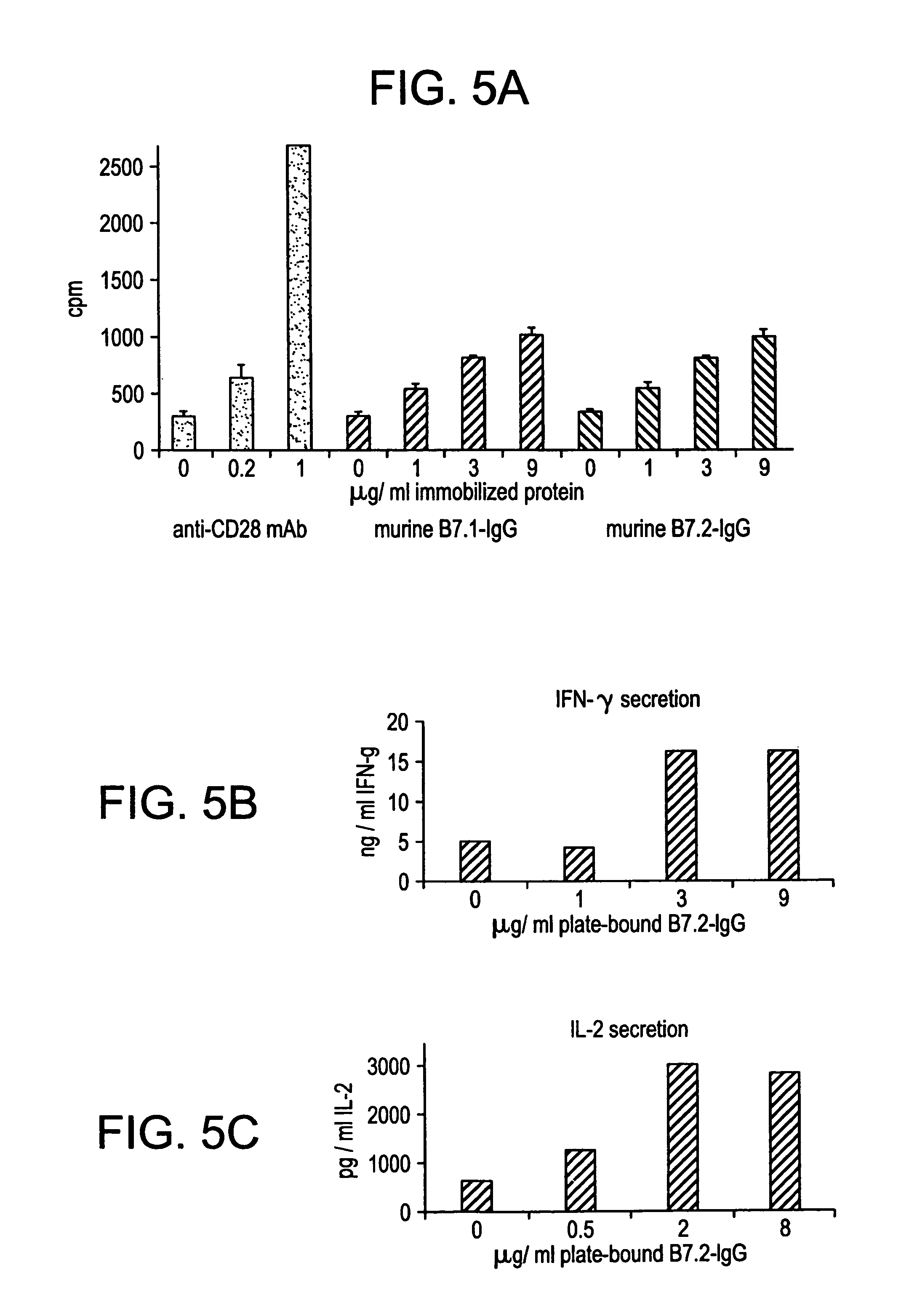
Enhancing immune responses with B7-1 or B7-2 in the absence of a crosslinking agent
InactiveUS7011833B1Enhance immune responseCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsAntigenInfectious agent
Owner:GENETICS INST INC
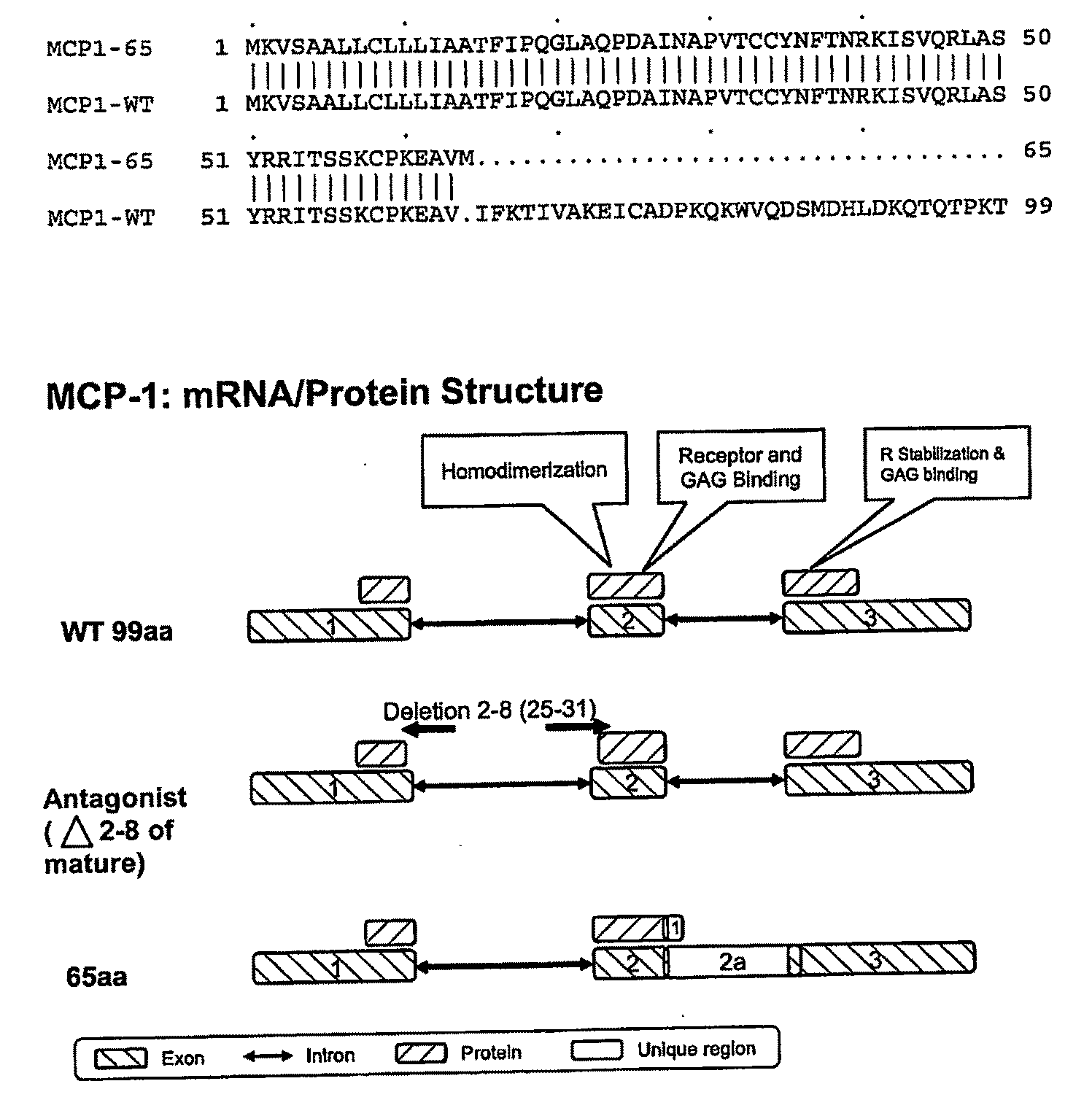
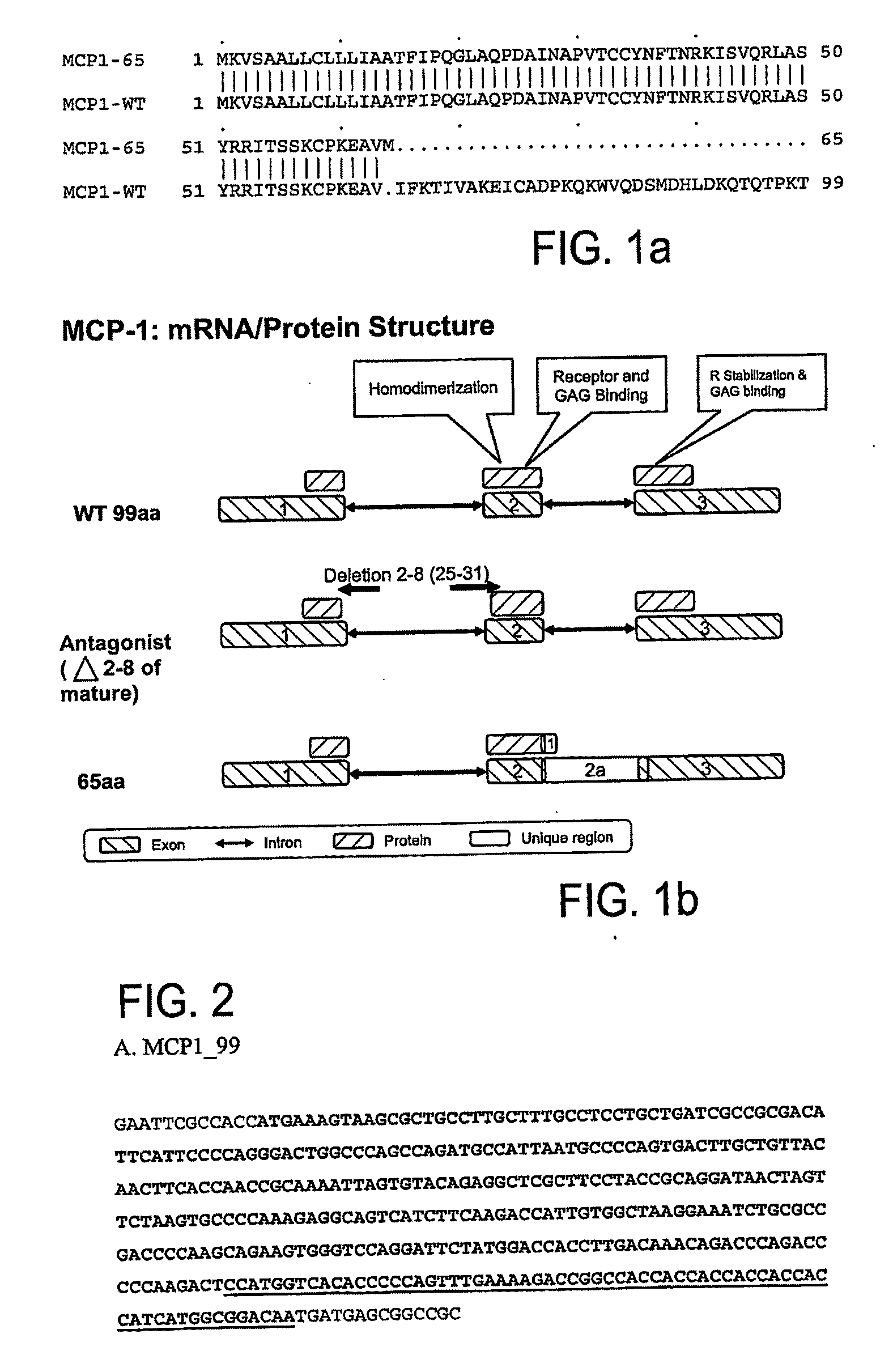
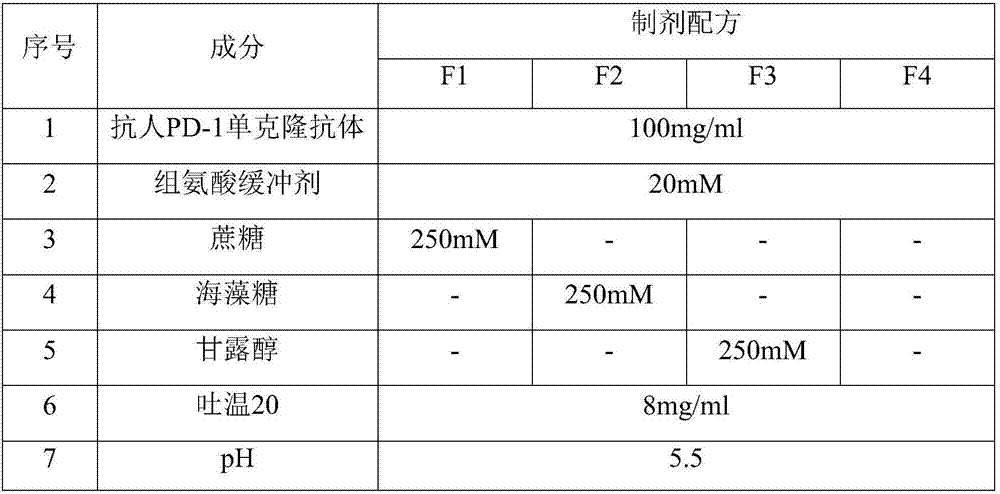
Mcp-1 splice variants and methods of using same
Owner:COMPUGEN
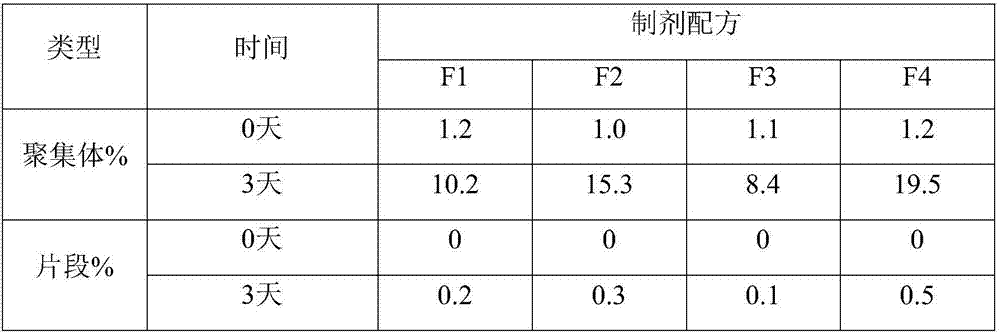
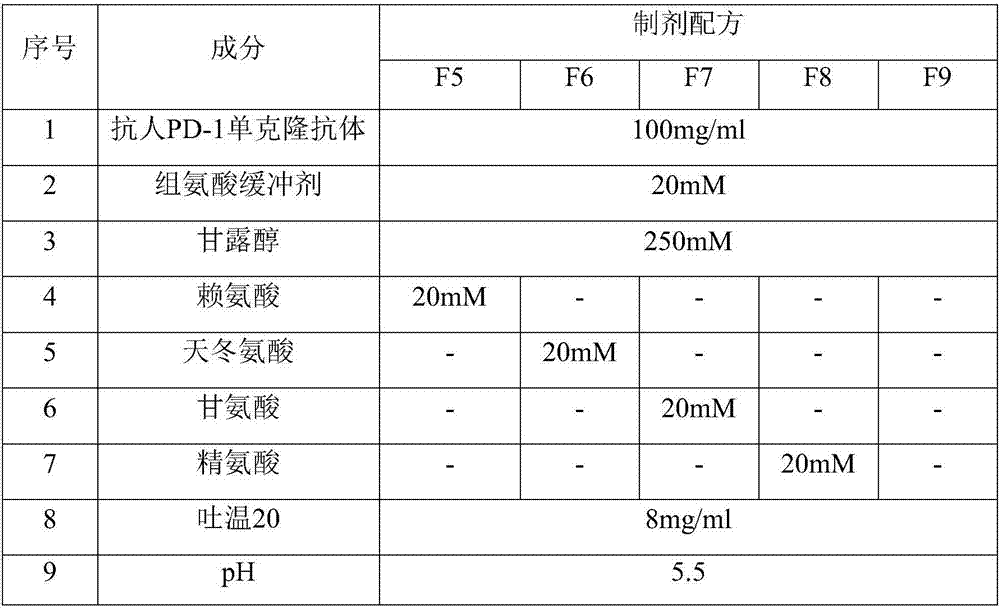
Anti-human PD-1 monoclonal antibody preparation suitable for subcutaneous injection
InactiveCN107325180AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAutoimmune diseaseHypodermoclysis
Owner:SHANGHAI NAT ENG RES CENT OF ANTIBODY MEDICINE
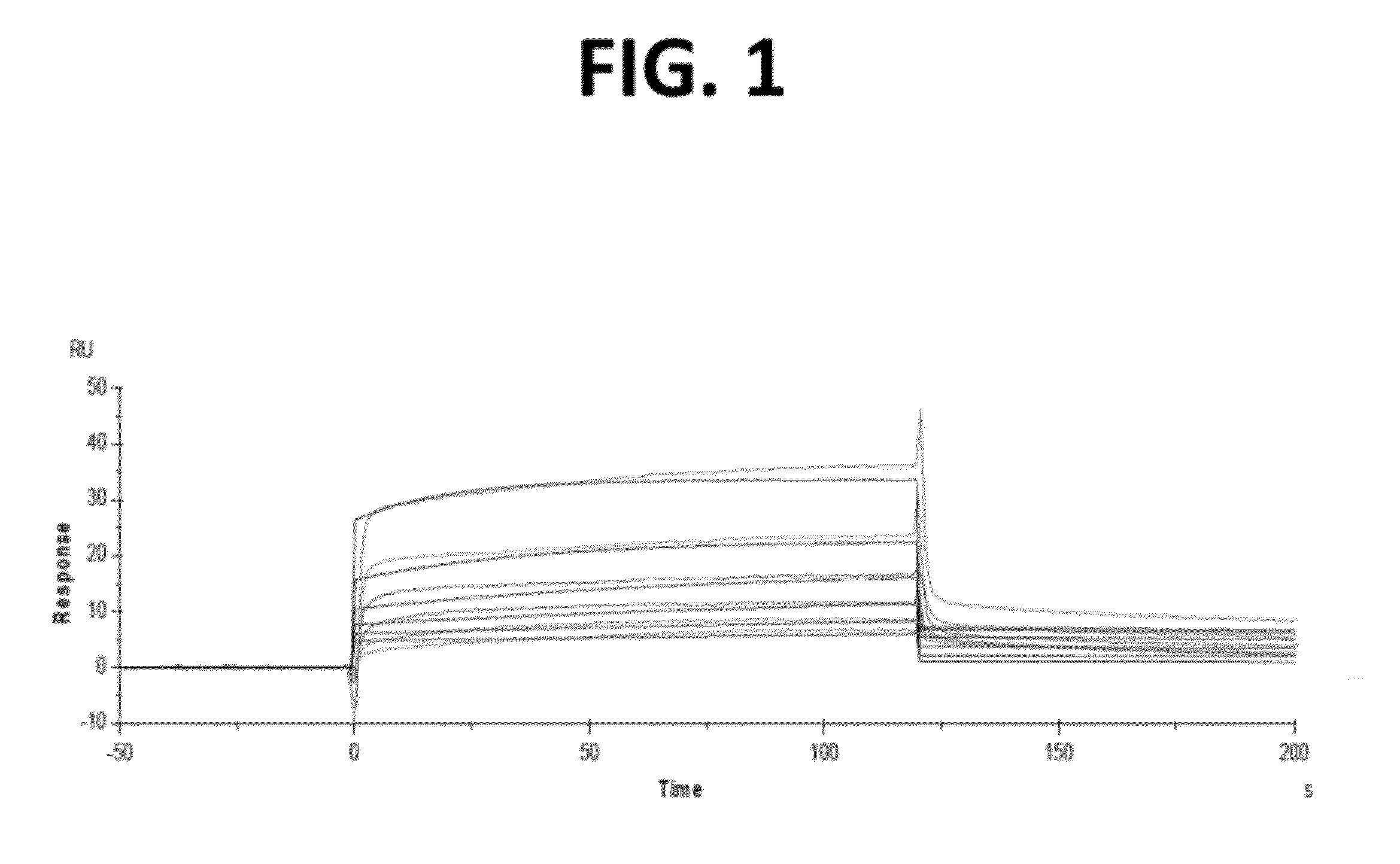
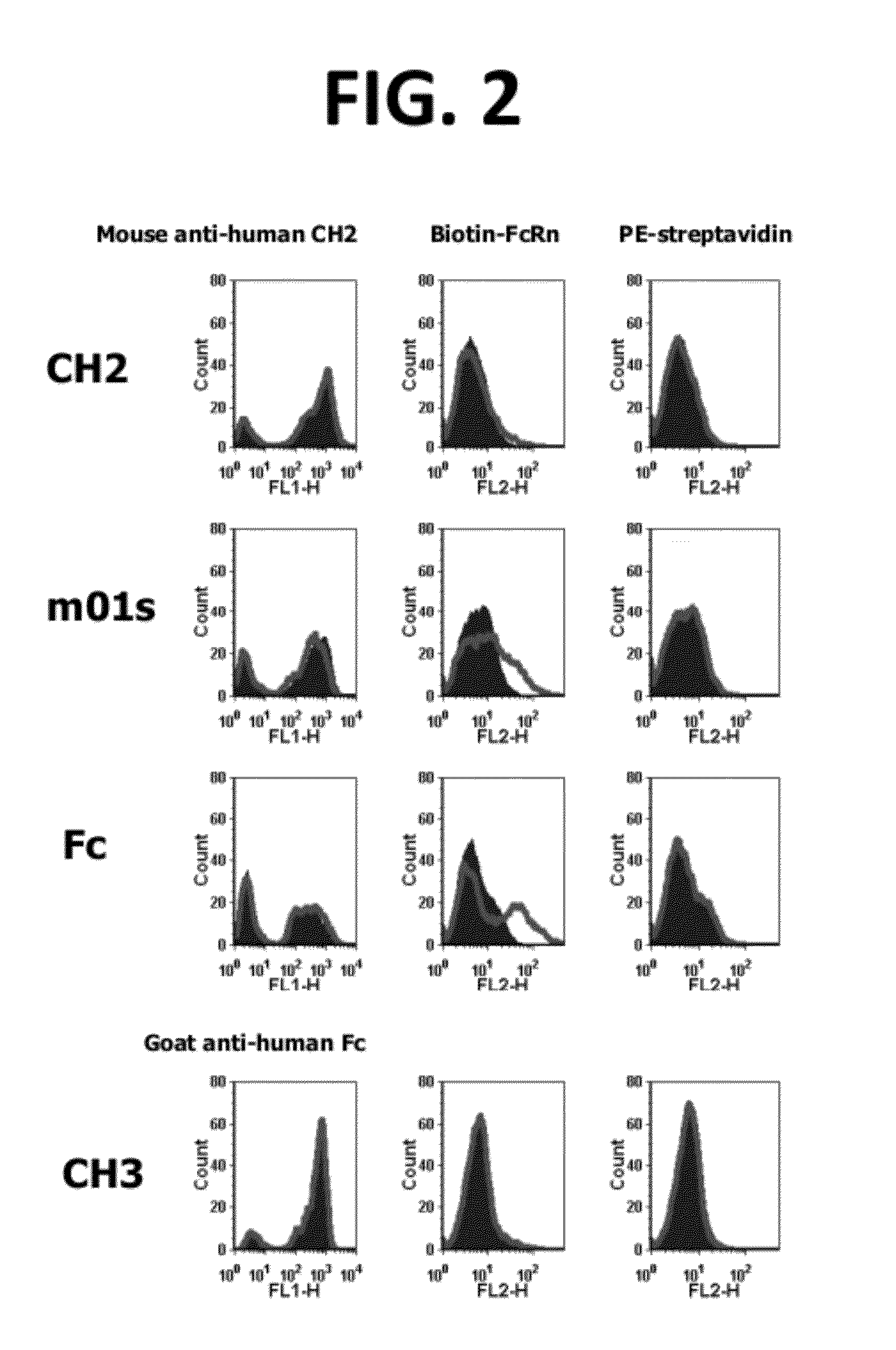
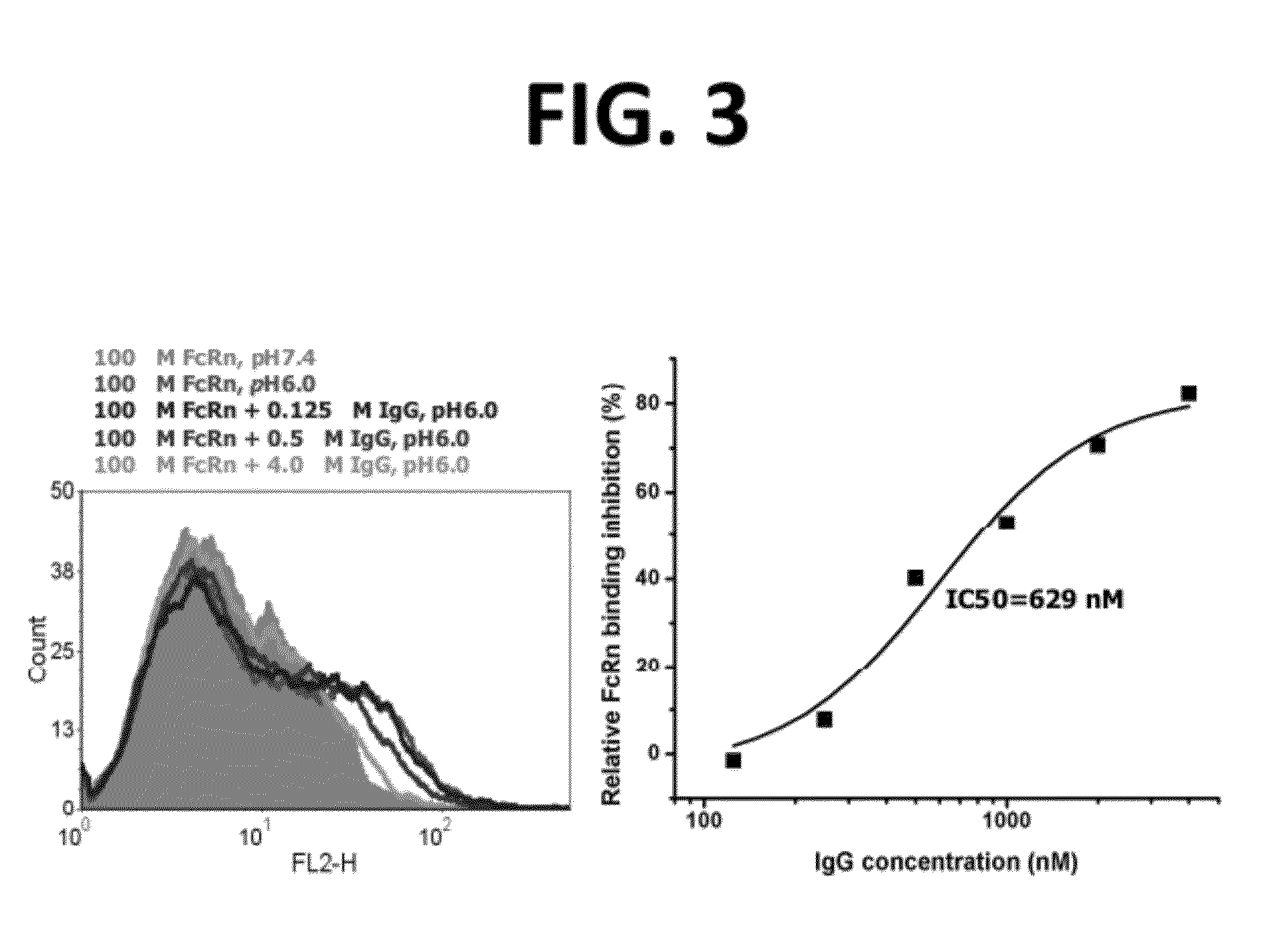
CH2 Domain Template Molecules Derived From Rational Grafting Of Donor Loops Onto CH2 Scaffolds
Owner:RES CORP TECH INC
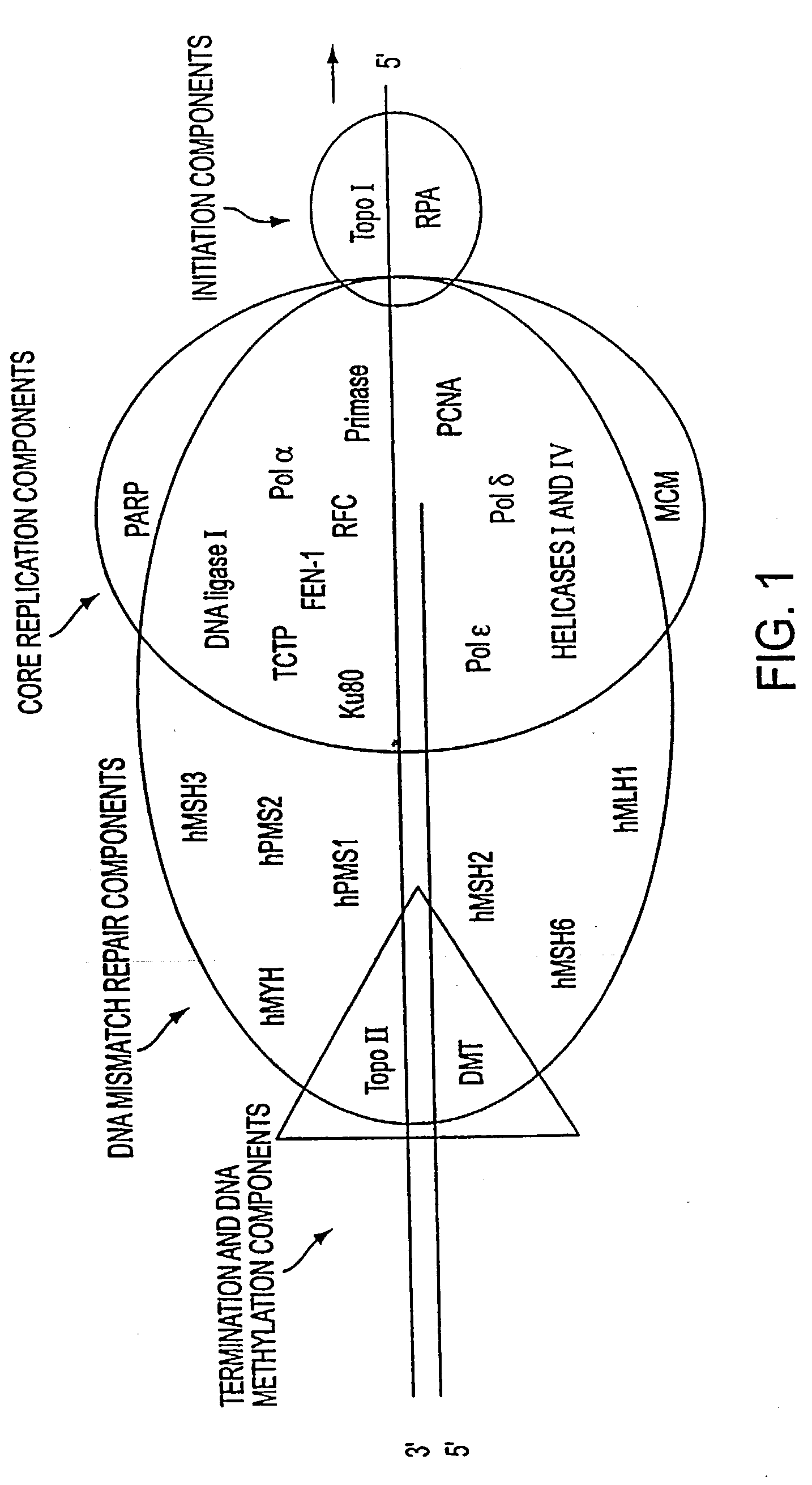
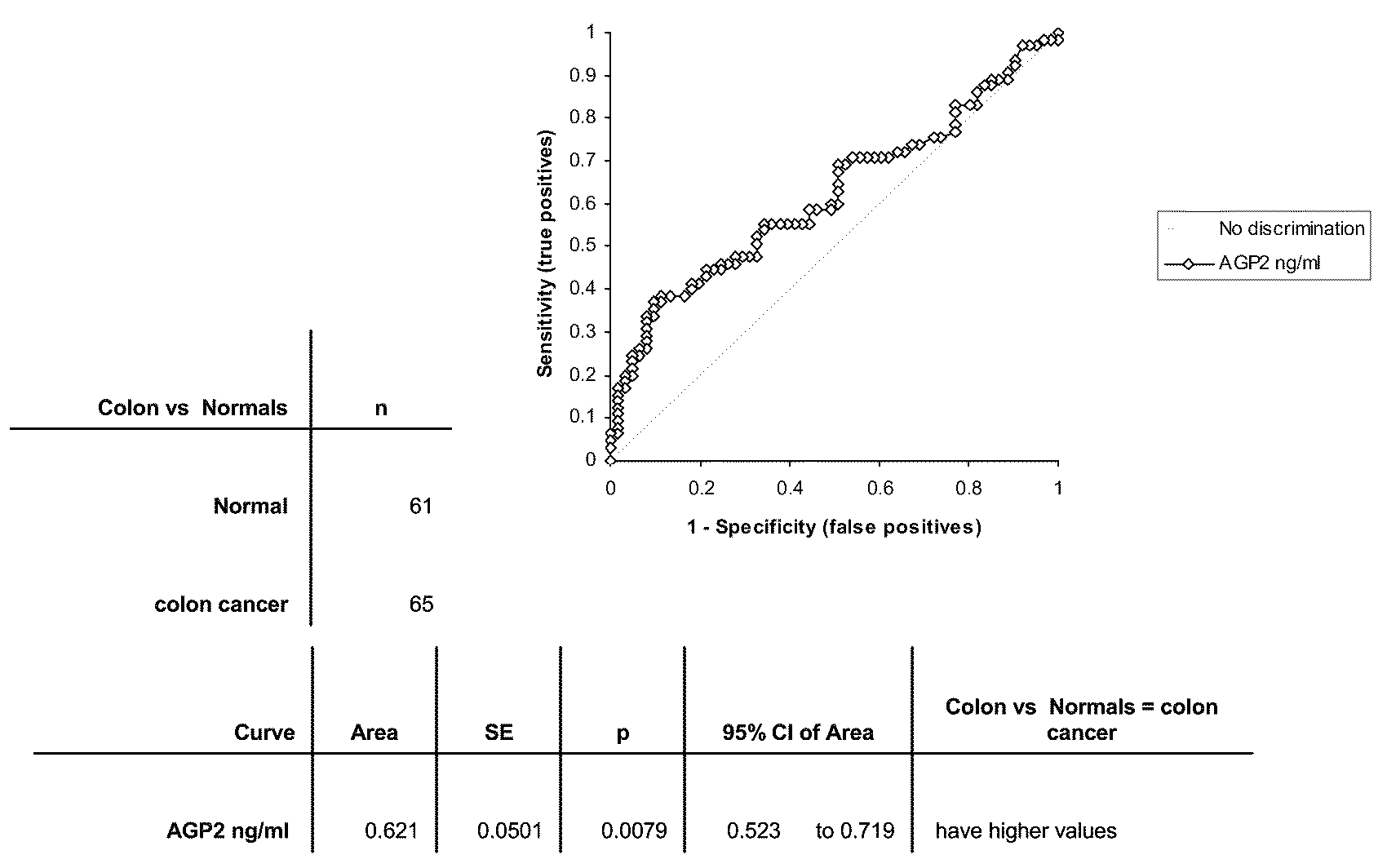
Altered DNA synthesome components as biomarkers for malignancy
InactiveUS20060073477A1Guaranteed functionChange activityPeptide/protein ingredientsMicrobiological testing/measurementMalignant phenotypeNeoplasm
Owner:SCHNAPER LAUREN
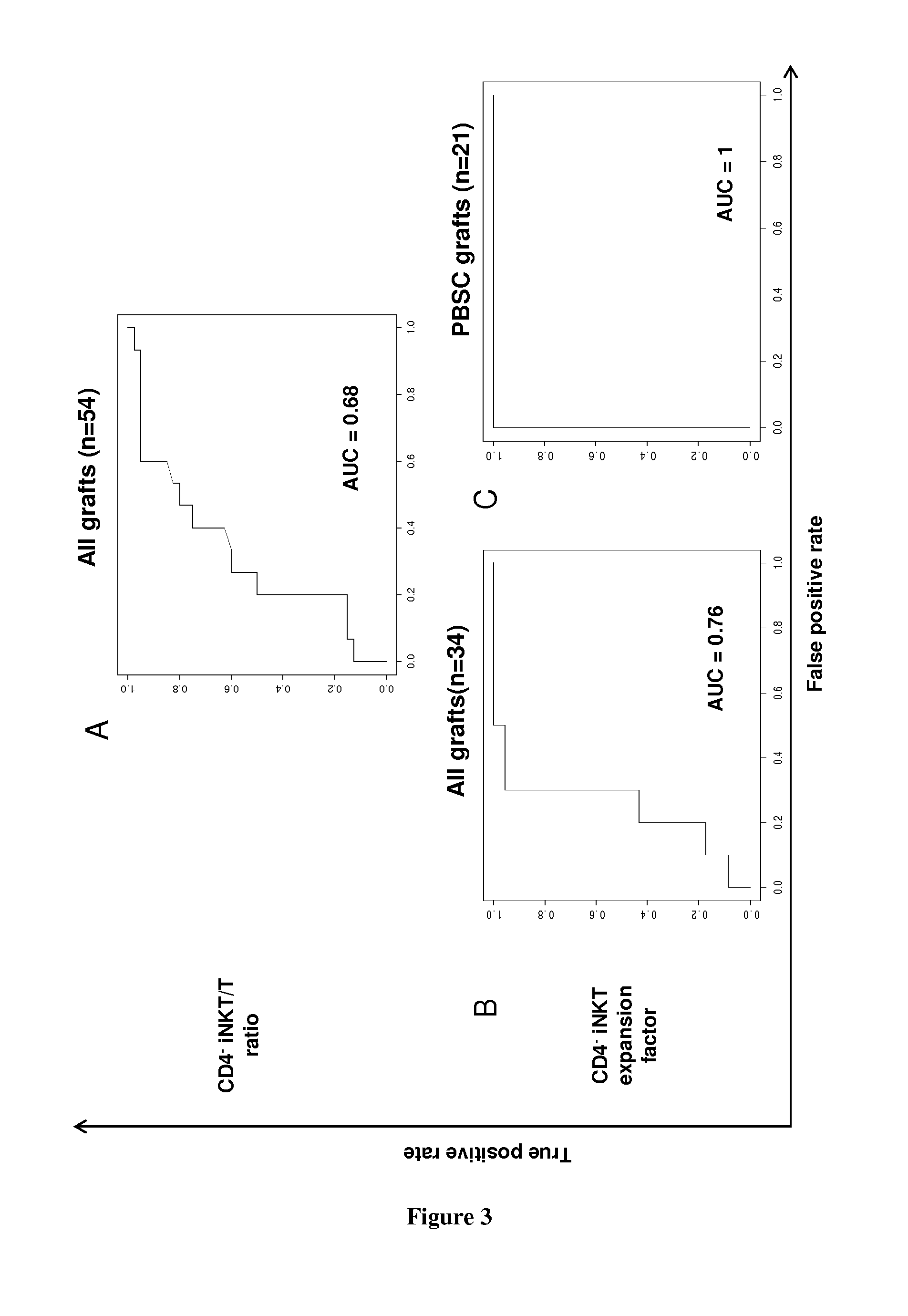
Methods for determining the risk of acute graft versus host disease
ActiveUS20150301022A1Increased riskBiocidePhosphorous compound active ingredientsCandidate donorSub populations
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Chemical inhibition of ferrochelatase as an antiangiogenic therapy
ActiveUS20160222388A1Organic active ingredientsPharmaceutical delivery mechanismDiseaseAntiangiogenic therapy
Methods for treating angiogenesis-mediated diseases are disclosed. More particularly, the present disclosure relates to methods of inhibiting ferrochelatase as an antiangiogenic therapy.
Owner:INDIANA UNIV RES & TECH CORP
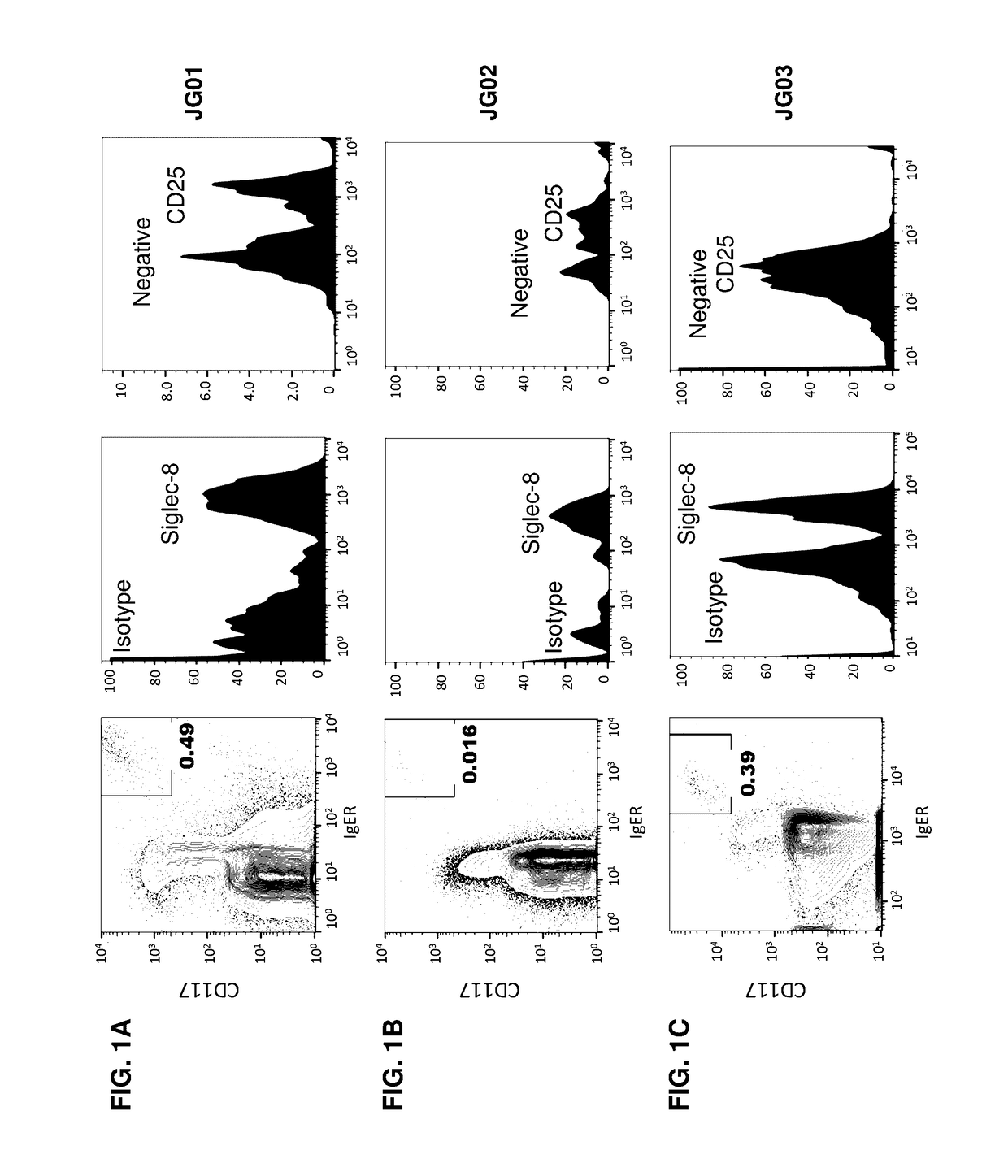
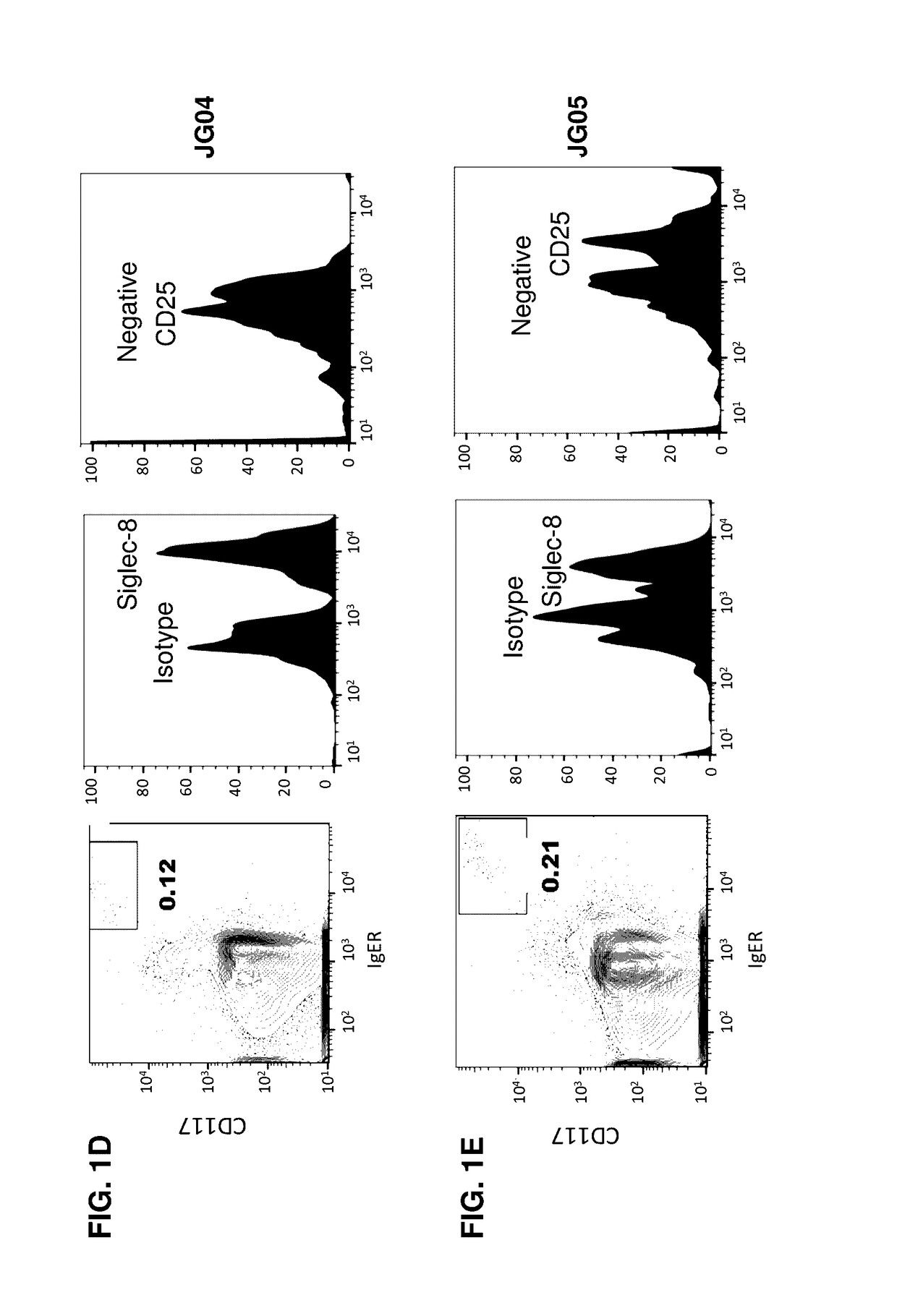
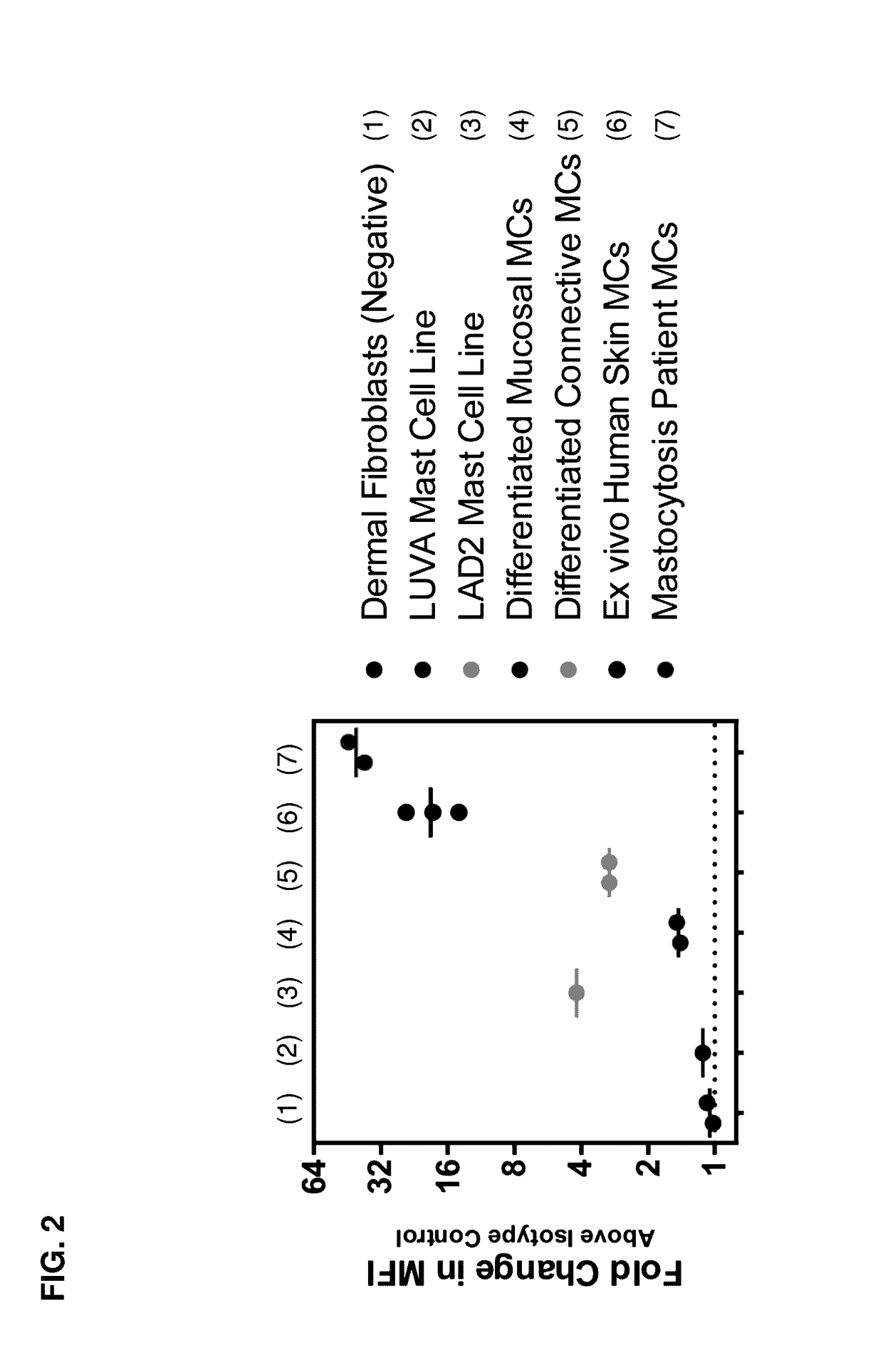
Methods and compositions for treating systemic mastocytosis
ActiveUS20170114138A1Improve antibody-dependent cell-mediated cytotoxicity (ADCC) activityEnhanced ADCC activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAgonist
Owner:ALLAKOS
Anti-il36r antibodies
ActiveUS20200017592A1Maintain good propertiesReduce skin thicknessAntipyreticAnalgesicsPalmoplantar pustulosisAntiendomysial antibodies
Owner:REGENERON PHARM INC
Multivalent molecules comprising DR5-binding domains
ActiveUS10501552B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsChemistryAntibody
Owner:MACROGENICS INC
Cerebral Vasospasm Inhibitor
InactiveUS20090175878A1Few side-effectsImmunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsSubarachnoid spaceSide effect
An objective of the present invention is to provide a cerebral vasospasm inhibitor which is effective on cerebral vasospasm occurring after subarachnoid hemorrhage and has few side-effects. The cerebral vasospasm inhibitor of the invention is characterized in containing an anti-HMGB1 monoclonal antibody as an active ingredient.
Owner:UNIV OKAYAMA
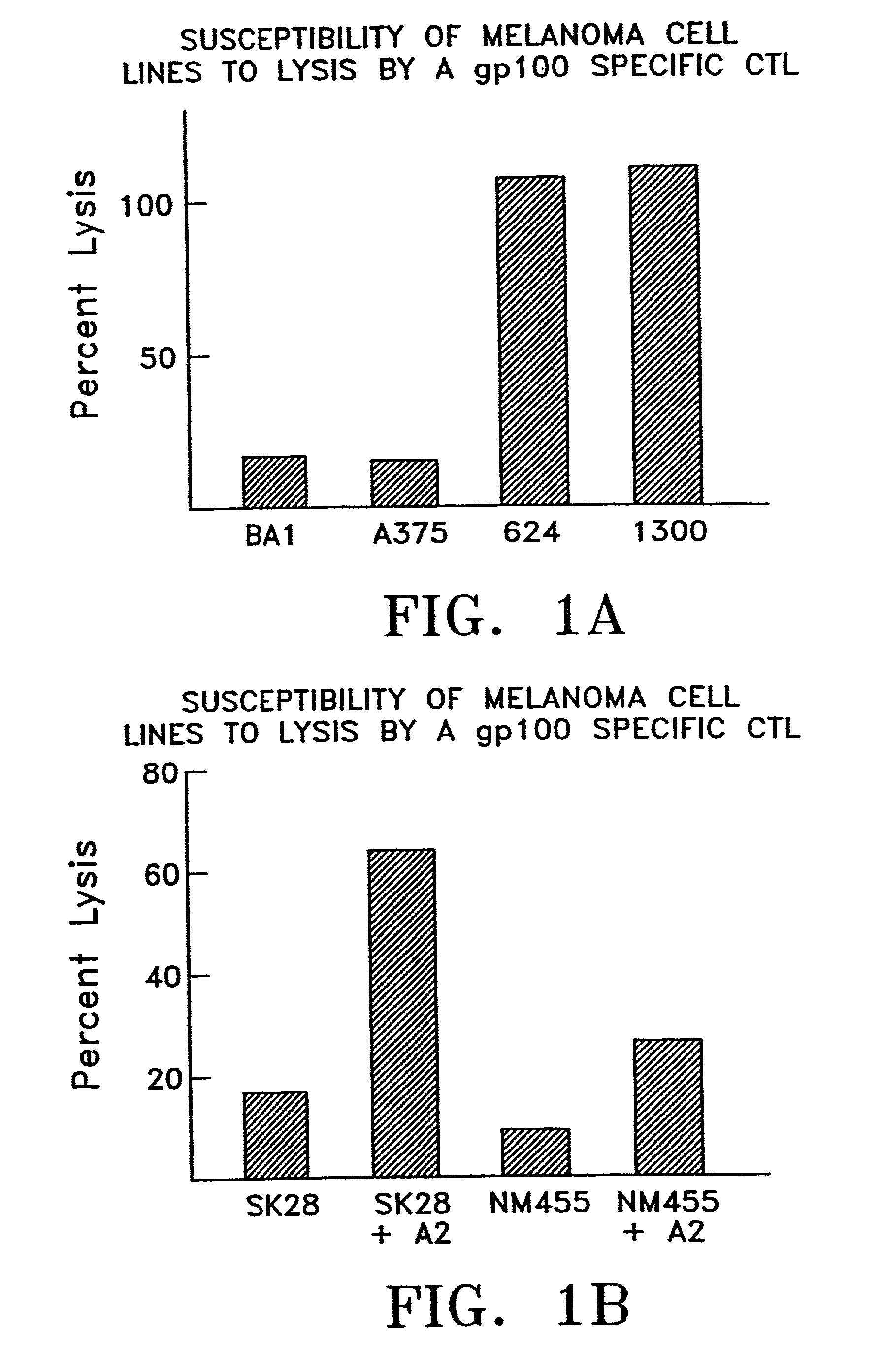
Antibodies specific for cancer associated antigen SM5-1 and uses thereof
The invention concerns antibodies which is specific for SM5-1 antigen expressed in melanoma, breast cancer and hepatocellular carcinoma, and polynucleotides encoding the antibodies. The invention further concerns use of such antibodies and / or polynucleotides in diagnosing and treating malignancies.
Owner:ONCOMAX ACQUISITION CORP
Methods and kits for predicting the risk of diabetes associated complications using genetic markers and arrays
InactiveUS20130209447A1Nucleotide librariesMicrobiological testing/measurementDiabetic kidneyGenetics predisposition
A method for diagnosing a genetic predisposition in a subject for diseases, disorders or conditions including a diabetic kidney complication such as kidney disease of type 2 diabetes or type 1 diabetes, end stage renal disease (ESRD) due to type 2 diabetes, ESRD due to hypertension in type 2 diabetes, ESRD due to type 1 diabetes; cardiovascular diseases due to type 2 diabetes or type 1 diabetes such as atherosclerotic peripheral vascular disease, hypertension, ischemic cardiomyopathy, and myocardial infarction due to type 2 diabetes or type 1 diabetes; and cerebrovascular accident due to type 2 diabetes. At least one polynucleotide is analyzed to detect a single nucleotide polymorphism (SNP), in which the presence of the single nucleotide polymorphism indicates that the subject is suffering from, at risk for, or suspected of suffering from the diseases, disorders or conditions. Also provided is an array or kit for diagnosing the genetic predisposition.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Anti-human GPC3 monoclonal antibody
ActiveCN110627904AGood water solubilityImprove patienceImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsImmunotherapyPhage display
The invention discloses an anti-human GPC3 monoclonal antibody which is a specific high-affinity nanometer antibody developed by using human GPC3 as a target on the basis of a phage display technology. Compared with the prior art, the anti-human GPC3 monoclonal antibody is successfully prepared; the antibody has high specificity and high affinity, and can be combined with the human GPC3 expressedon the cell surfaces; and the anti-human GPC3 monoclonal antibody belongs to potential medicine for tumor immunotherapy.
Owner:NANJING BLUE SHIELD BIOTECH CO LTD
Therapeutic adjuvant
InactiveUS20050271649A1Enhance immune responseEfficient productionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAdjuvantAntigen binding
Owner:ALTAREX MEDICAL
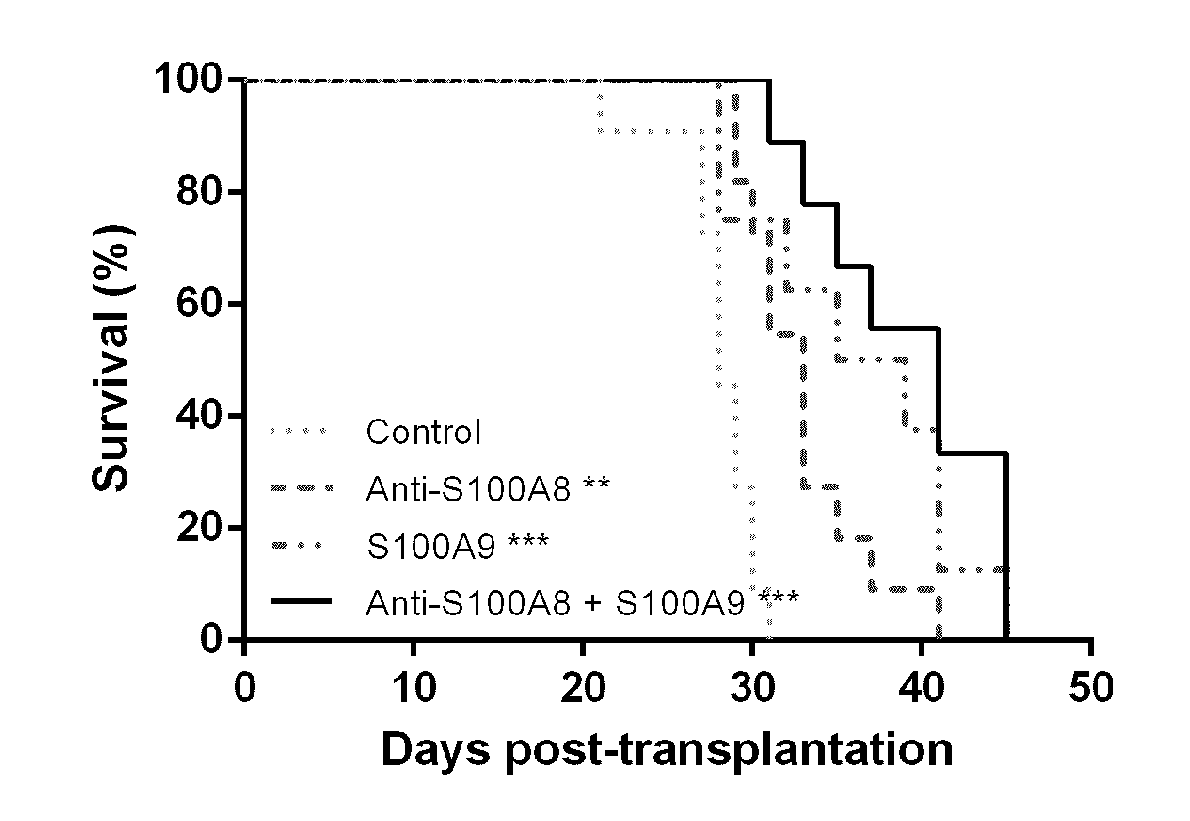
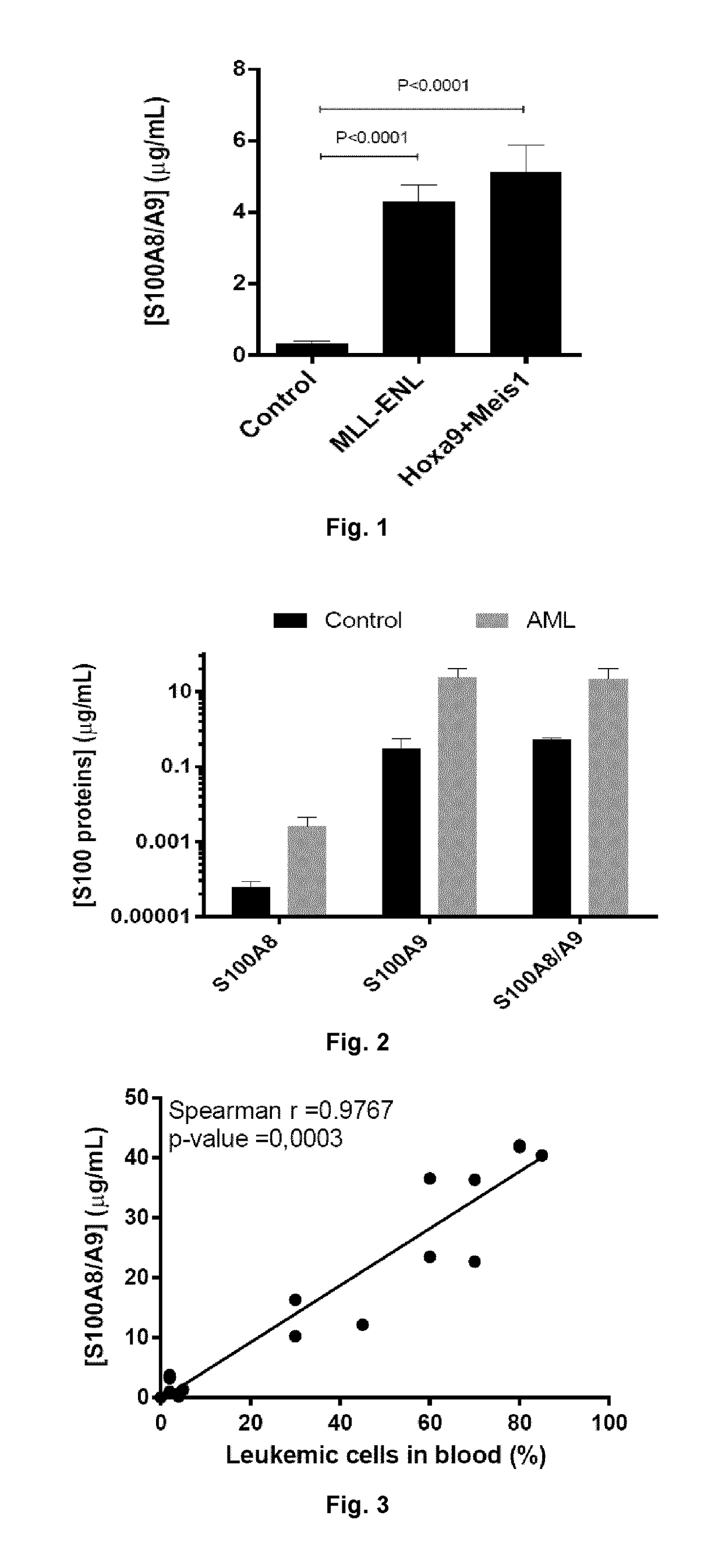
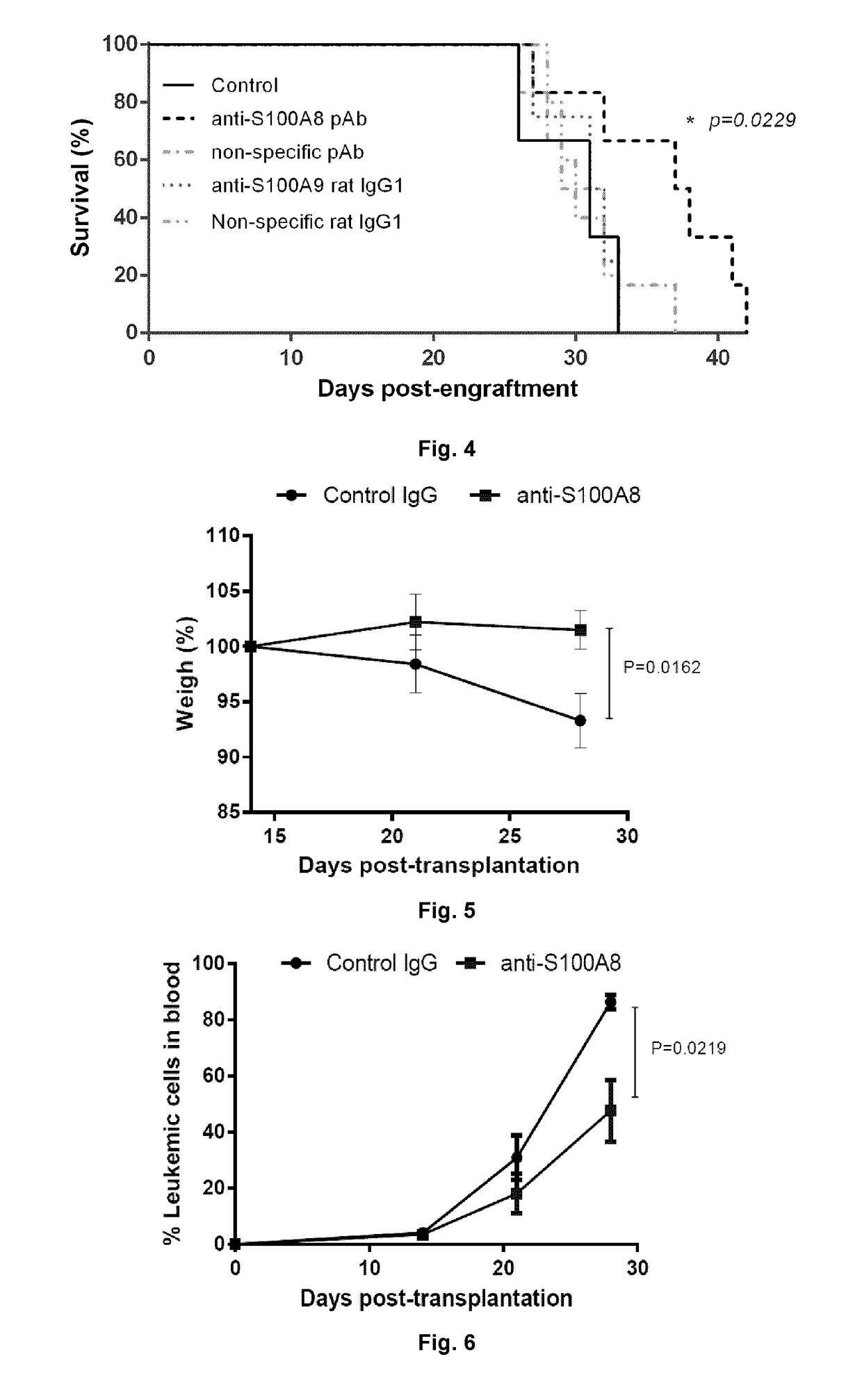
Anti-s100a8 for treating leukemia
ActiveUS20180256710A1Inhibit cell proliferationOrganic active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsLeukemiaAntibody
Owner:UNIV LAVAL
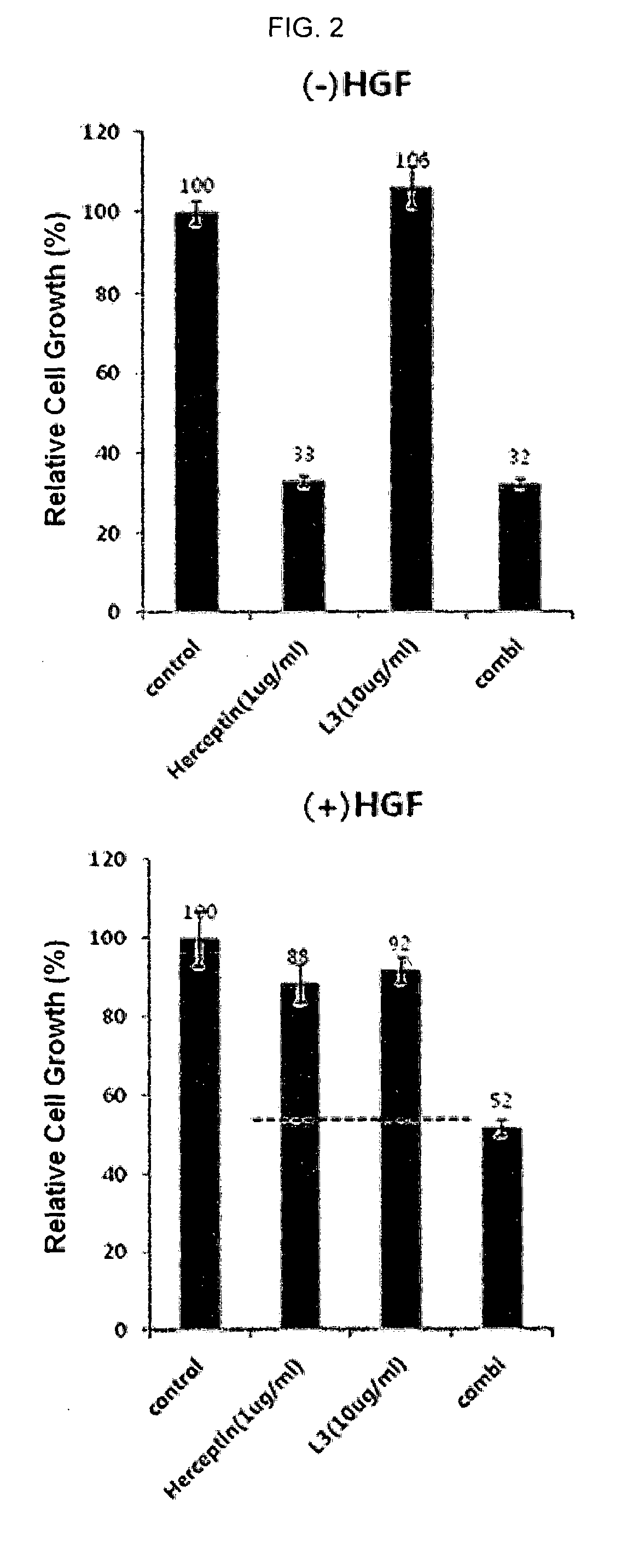
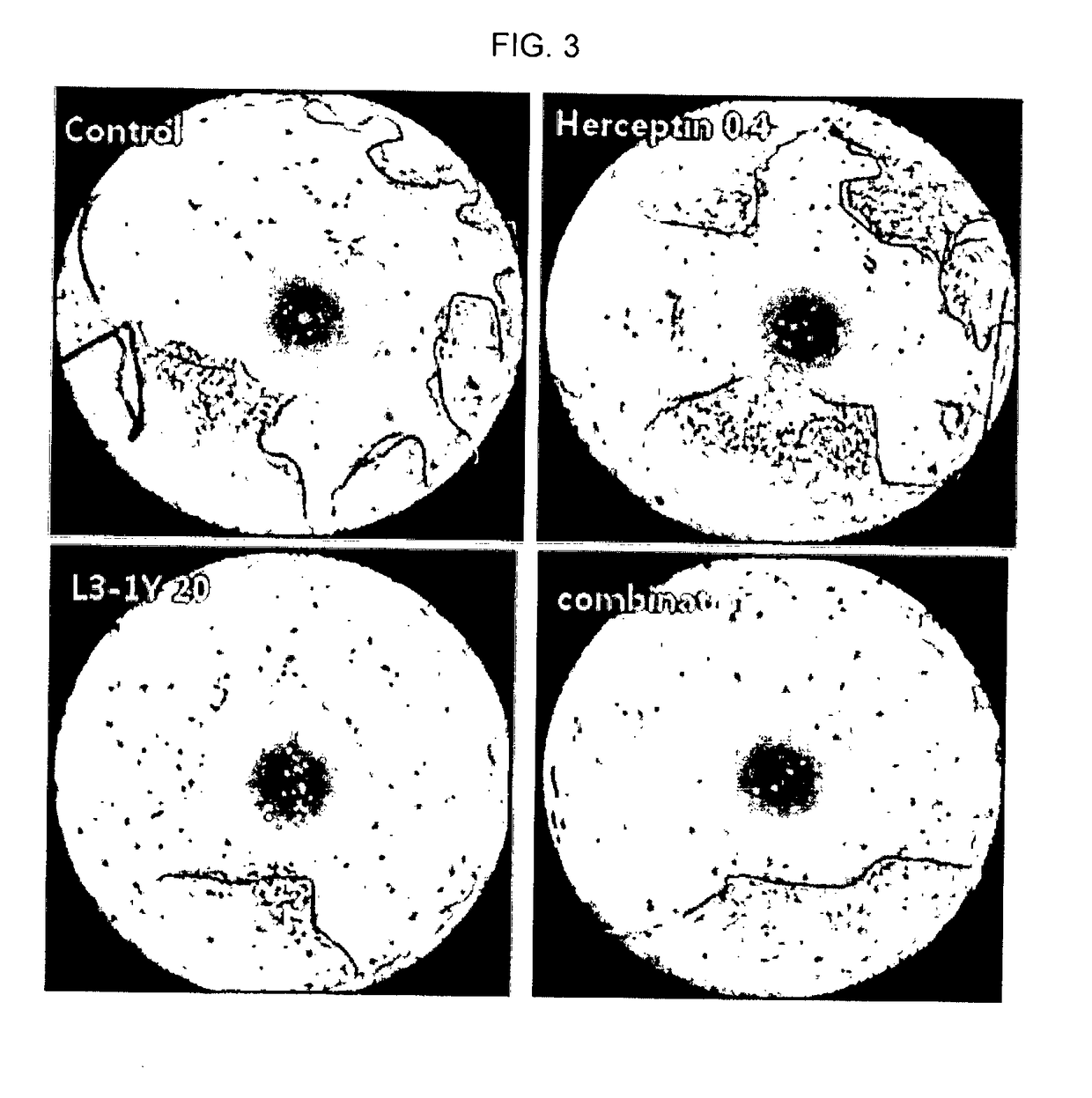
Composition for combination therapy comprising Anti-her2 antibody and Anti-c-met antibody
InactiveUS20170233489A1Inhibit angiogenesisImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsC-MetAngiogenesis growth factor
Owner:SAMSUNG ELECTRONICS CO LTD
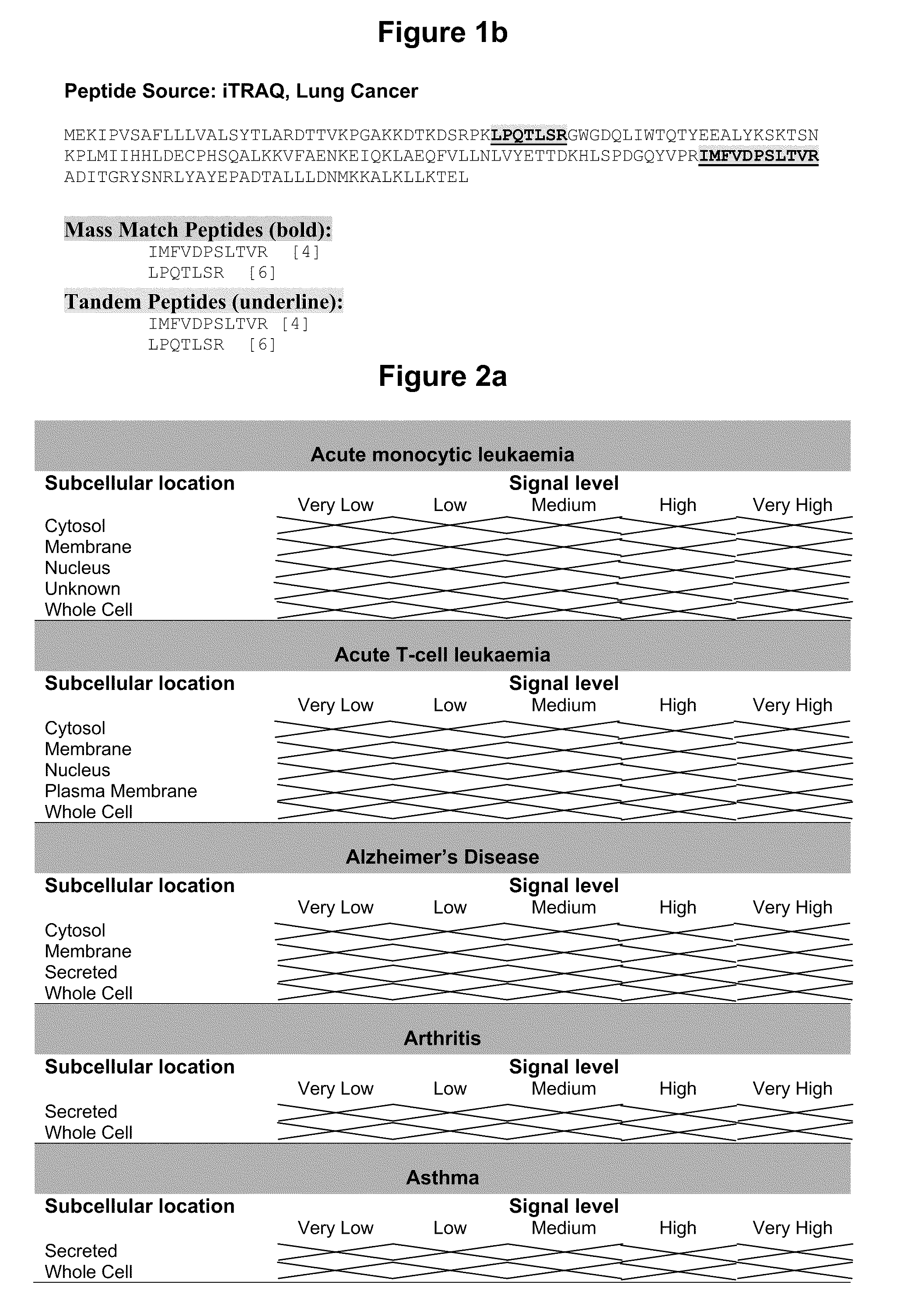
Protein
InactiveUS20090238833A1Compound screeningApoptosis detectionDrug developmentLung cancer
Owner:OXFORD BIOTHERAPEUTICS
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap