Protein
a technology of protein and lipid, applied in the field of proteins, can solve the problems of damage to nearby organs, bleeding from surgery, and thrombosis of deep veins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Membrane Proteins Expressed in Colorectal Cancer Blood and Tissue Samples Using 1D Gel Electrophoresis
[0227]Using the following Reference Protocol, membrane proteins extracted from colorectal cancer tissue samples were separated by 1D gel and analysed.
1.1 Materials and Methods
1.1.1—Plasma Membrane Fractionation
[0228]The cells recovered from the epithelium of a colorectal adenocarcinoma were lysed and submitted to centrifugation at 1000 G. The supernatant was taken, and it was subsequently centrifuged at 3000 G. Once again, the supernatant was taken, and it was then centrifuged at 100 000 G.
[0229]The resulting pellet was recovered and put on 15-60% sucrose gradient.
[0230]A Western blot was used to identify sub cellular markers, and the Plasma Membrane fractions were pooled.
[0231]The pooled solution was either run directly on 1D gels (see section 1.1.4 below), or further fractionated into heparin binding and nucleotide binding fractions as described below.
1.1.2—Plasma M
example 2
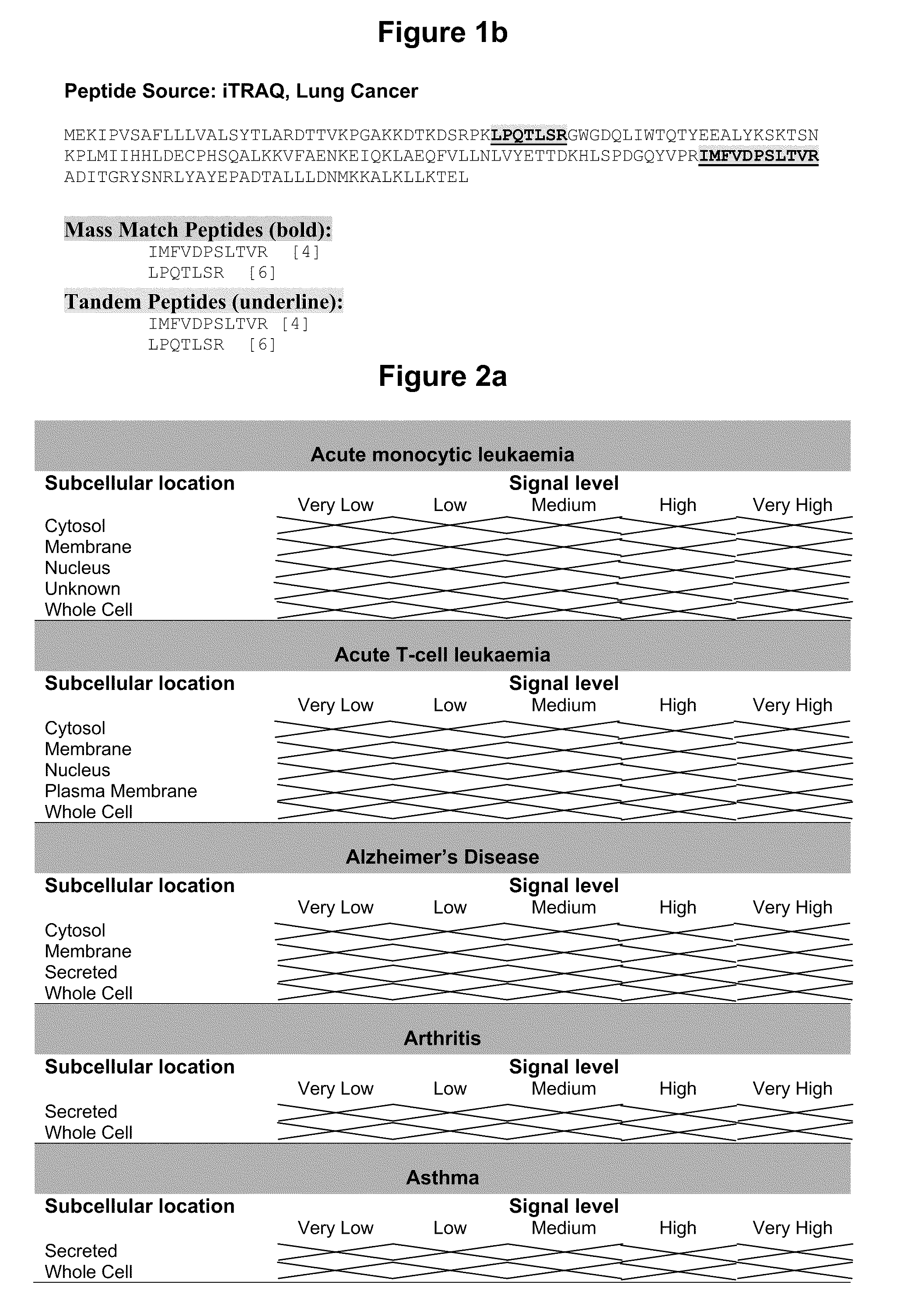
Identification of Membrane Proteins Expressed in Colorectal Cancer or Lung Cancer Blood and Tissue Samples Using Isotope tagging for absolute and relative quantitation (iTRAQ)
[0263]Using the following Reference Protocol, membrane proteins extracted from colorectal cancer and lung cancer tissue and corresponding normal adjacent colorectal and lung tissue samples were digested, labelled with Isotope Tagging for Absolute & Relative Quantitation reagents (iTRAQ; Applied Biosystems, Foster City, Calif., USA) and resulting peptides sequenced by tandem mass spectrometry.
2.1 Materials and Methods
2.1.1—Plasma Membrane Fractionation
[0264]The cells recovered from a colorectal cancer or lung cancer or corresponding normal adjacent tissue were lysed and submitted to centrifugation at 1000 G. The supernatant was taken, and it was subsequently centrifuged at 3000 G. Once again, the supernatant was taken, and it was then centrifuged at 100 000 G.
[0265]The resulting pellet was recovered and put on 15-6
example 3
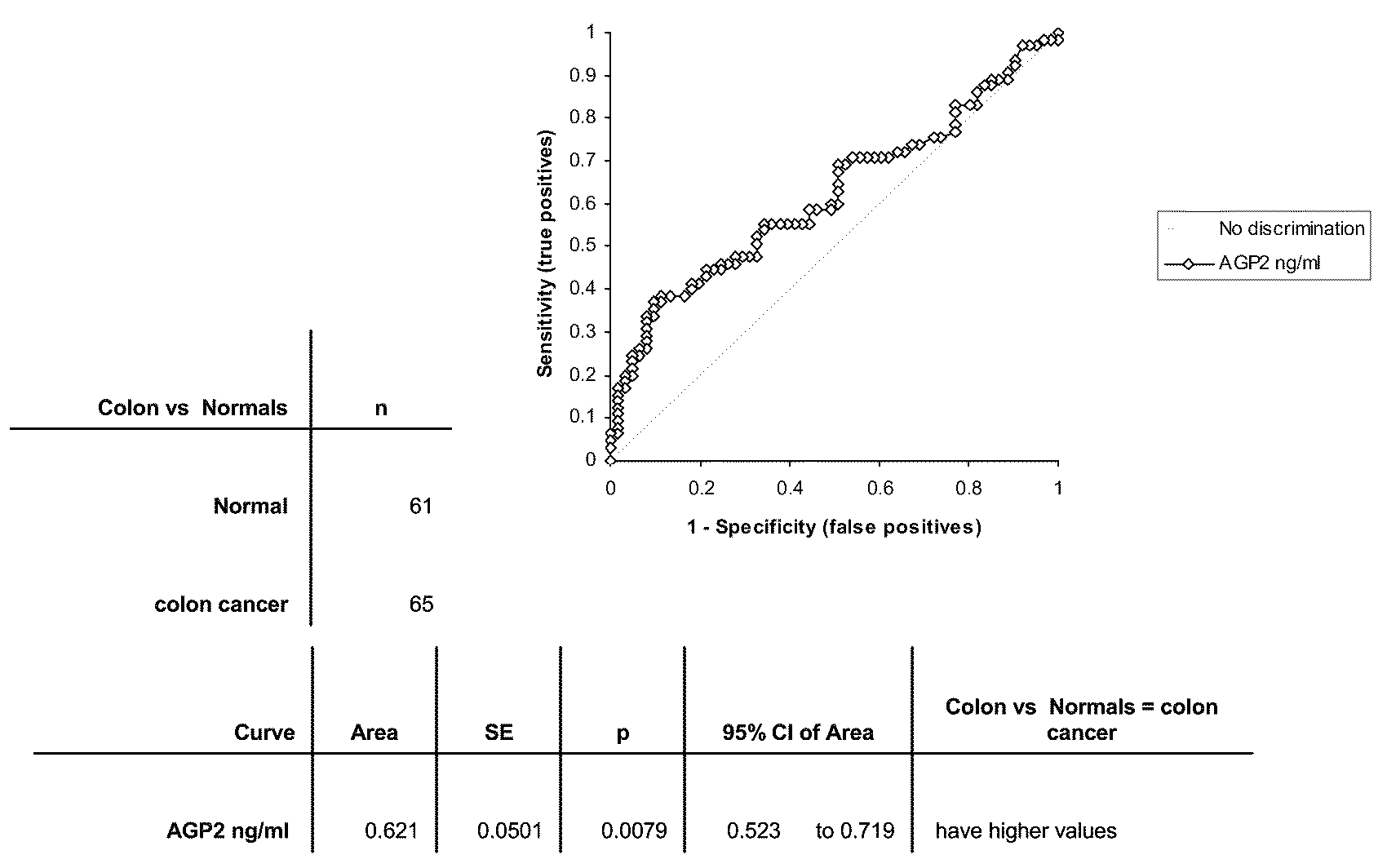
Assay to Detect Soluble OGTA019 in Patient Serum Using Sandwich ELISA
[0278]Using the following Reference Protocol, sandwich ELISAs were performed using antibodies to OGTA019.
3.1 Materials and Methods
[0279]Antibodies to OGTA019 (as defined by SEQ ID No: 1) for the sandwich ELISAs were developed at Biosite. Biotinylated antibody (primary antibody) was diluted into assay buffer (10 mM Tris, 150 mM NaCl, 1% BSA) to 2 ug / ml and added to 384 well neutravidin coated plate (Pierce Chemical Company, Rockford Ill.) and allowed to incubate at room temperature for 1 hour. Wells were then washed with wash buffer (20 mM Borate, 150 mM NaCl, 0.2% Tween 20). Samples and standards were added and allowed to incubate at room temperature for 1 hour. Wells again were washed. An antibody conjugated to fluorescein (secondary antibody) was diluted into assay buffer to 2 ug / ml and was then added to the plate and allowed to incubate at room temperature for 1 hour. Wells again were washed. Anti-fluorescein a
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap