Therapeutic adjuvant
a technology of adjuvants and adjuvants, applied in the field of therapeutic adjuvants, can solve the problems of/or diagnostics, affecting the effect of subsequent therapies, and unable to demonstrate meaningful therapeutic benefits, etc., to achieve enhancement or production of effective immune responses, enhance effective immune responses, and enhance effective immune responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0111] The following examples are intended to further illustrate certain particularly preferred embodiments of the invention and are not intended to limit the scope of the invention. The contents of all cited references (including literature references, issued patents, published patent applications as cited throughout this application) are hereby expressly incorporated by reference.
[0112] The practice of the present invention will employ, unless otherwise indicated, conventional techniques of cell biology, cell culture, molecular biology, microbiology and recombinant DNA, which are within the skill of the art. Such techniques are explained fully in the literature. See, for example, Molecular Cloning: A Laboratory Manual, 2nd Ed., ed. by Sambrook, Fritsch and Maniatis (Cold Spring Harbor Laboratory Press: 1989); DNA Cloning, Volumes I and II (D. N. Glover ed., 1985); Oligonucleotide Synthesis (M. J. Gait ed., 1984); Mullis et al. U.S. Pat. No. 4,683,195; Nucleic Acid Hybridization (B.
example i
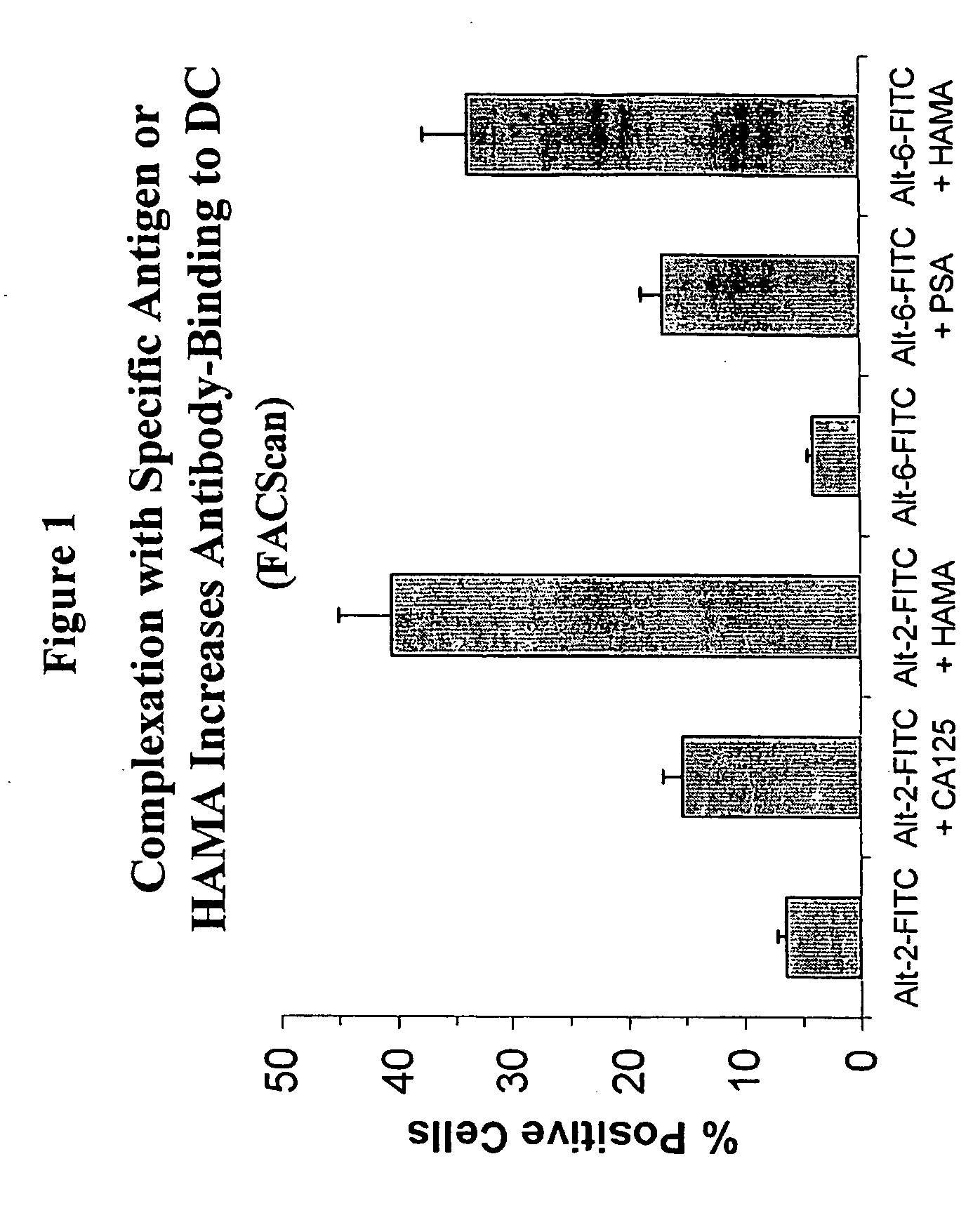
Effect Of HAMA On Antigen Uptake By Dendritic Cells
[0113] Complexation of antibody with specific antigen or with HAMA and binding to dendritic cells was measured. To do this, human anti-mouse antibody (HAMA) was purified from patient serum samples with high HAMA concentrations after MAb Alt-2 injection via affinity chromatography on Protein G, and a MAb-Alt-1 column (Alt-1 specifically binds to the MUC-1 antigen) to eliminate Ab2 (i.e., human antibody that binds to the idiotype of the MAb Alt-2 antibody).
[0114] Five micrograms (5 μg) of fluorescein (FITC)-labeled Alt-2 (anti-CA125) or Alt-6 (anti-PSA) murine monoclonal antibody was incubated together with the corresponding antigen (1μg) and / or HAMA (2.5 μg) and 5×105 immature dendritic cells at 37° C. for 60 minutes. The mixture was then washed once and resuspended in 0.5% formalin+PBS and subjected to flow cytometry (FACScan) on a FACSCalibur machine (Becton-Dickinson).
[0115] As shown in FIG. 1, antibody plus antigen bound to den
example ii
Effect Of HAMA On Antigen Uptake By Monocytes
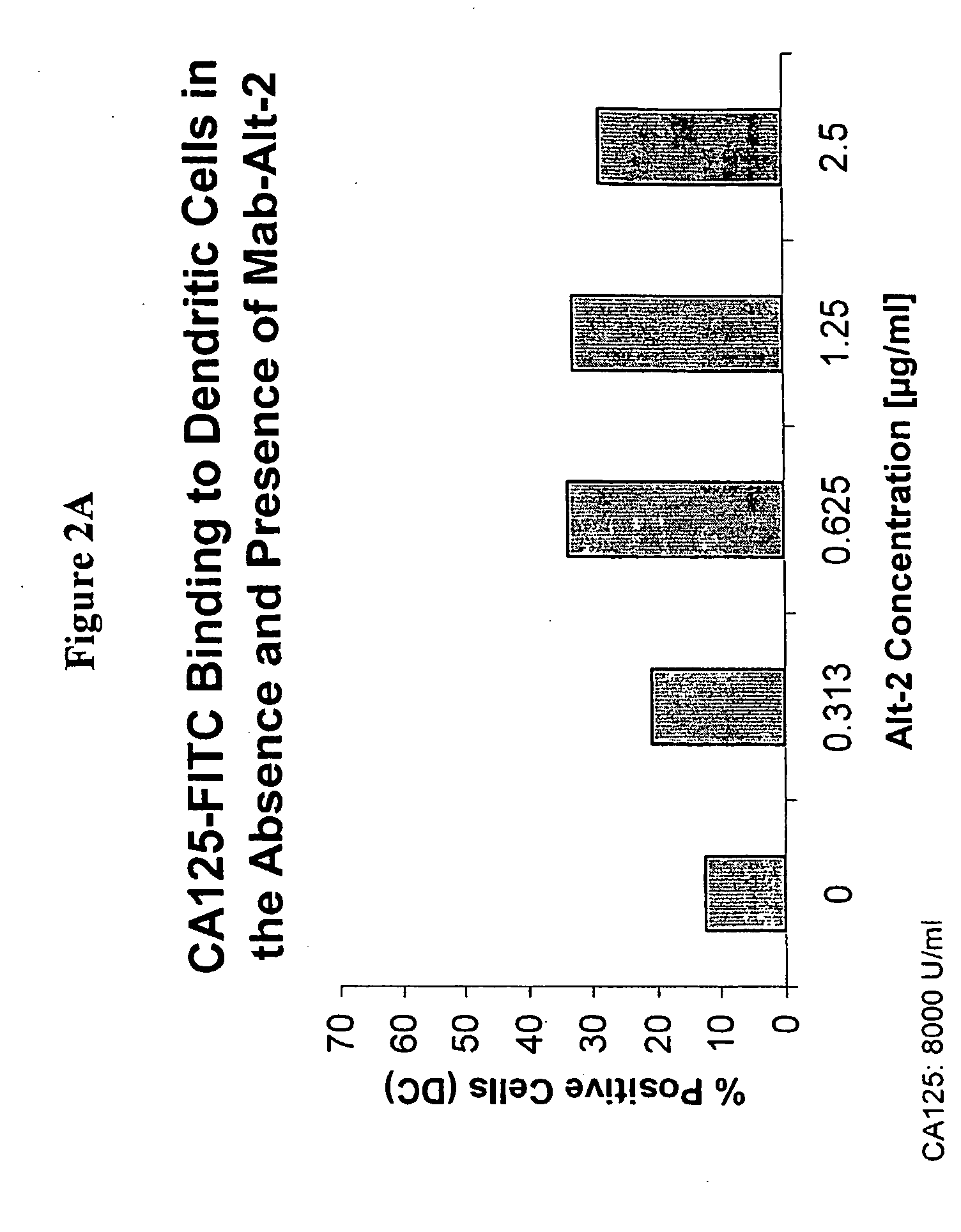
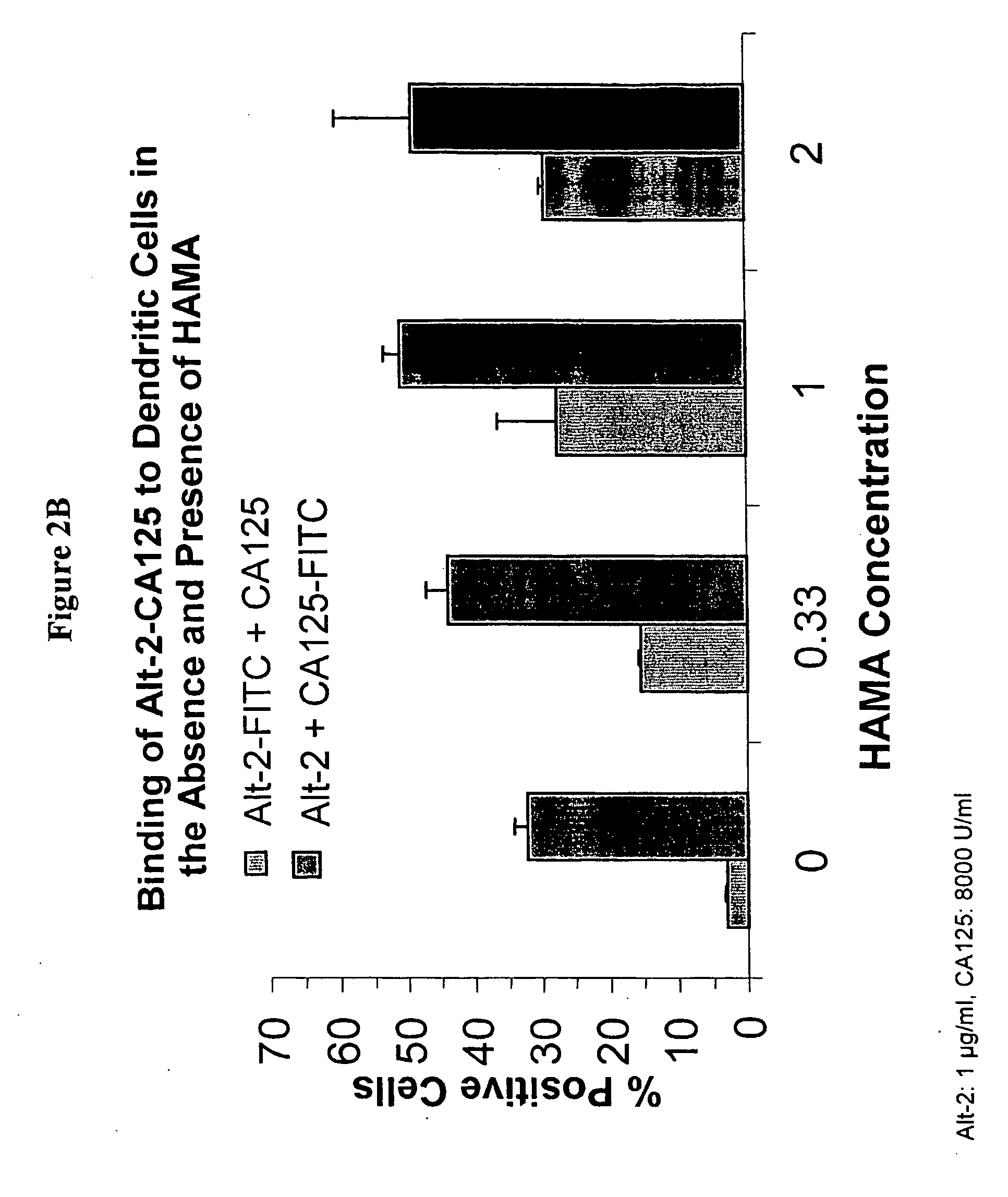
[0118] In another experiment, CA125 or MAb-Alt-2 was labeled with FITC and incubated at 1000 U / ml of CA125 and 1 μg / ml of MAb-Alt-2 with monocytes for 1 hour at 37° C. The binding studies were conducted in the absence and presence of HAMA at three concentrations. The binding to cells was assessed by flow cytometry.
[0119] As shown in FIGS. 3A and 3B, both the CA125 antigen as well as the Alt-2 murine monoclonal antibody are taken up more efficiently in the presence of HAMA. FIG. 3A shows the increase in the number of monocytes bound by either FITC-labeled CA125 or FITC labeled Alt-2 in the presence of 0, 0.33, 1, or 2 μg / ml HAMA; FIG. 3B shows the increase in overall fluorescence per cell.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap