Composition for combination therapy comprising Anti-her2 antibody and Anti-c-met antibody
a combination therapy and anti-her2 antibody technology, applied in the direction of immunoglobulins, peptides, drug compositions, etc., can solve the problems of ineffective anti-her2 antibody, inability to achieve significant anticancer effects of combination therapy of two antibodies, and strict restrictions on approval
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
i-c-Met Antibody
1.1. Production of “AbF46”, a Mouse Antibody to c-Met
1.1.1. Immunization of Mouse
[0117]To obtain immunized mice necessary for the development of a hybridoma cell line, each of five BALB / c mice (Japan SLC, Inc.), 4 to 6 weeks old, was intraperitoneally injected with a mixture of 100 μg of human c-Met / Fc fusion protein (R&D Systems) and one volume of complete Freund's adjuvant. Two weeks after the injection, a second intraperitoneal injection was conducted on the same mice with a mixture of 50 μg of human c-Met / Fc protein and one volume of incomplete Freund's adjuvant. One week after the second immunization, the immune response was finally boosted. Three days later, blood was taken from the tails of the mice and the sera were 1 / 1000 diluted in PBS and used to examine a titer of antibody to c-Met by ELISA. Mice found to have a sufficient antibody titer were selected for use in the cell fusion process.
1.1.2. Cell Fusion and Production of Hybridoma
[0118]Three days be
example 1
Screening of Receptor Expression
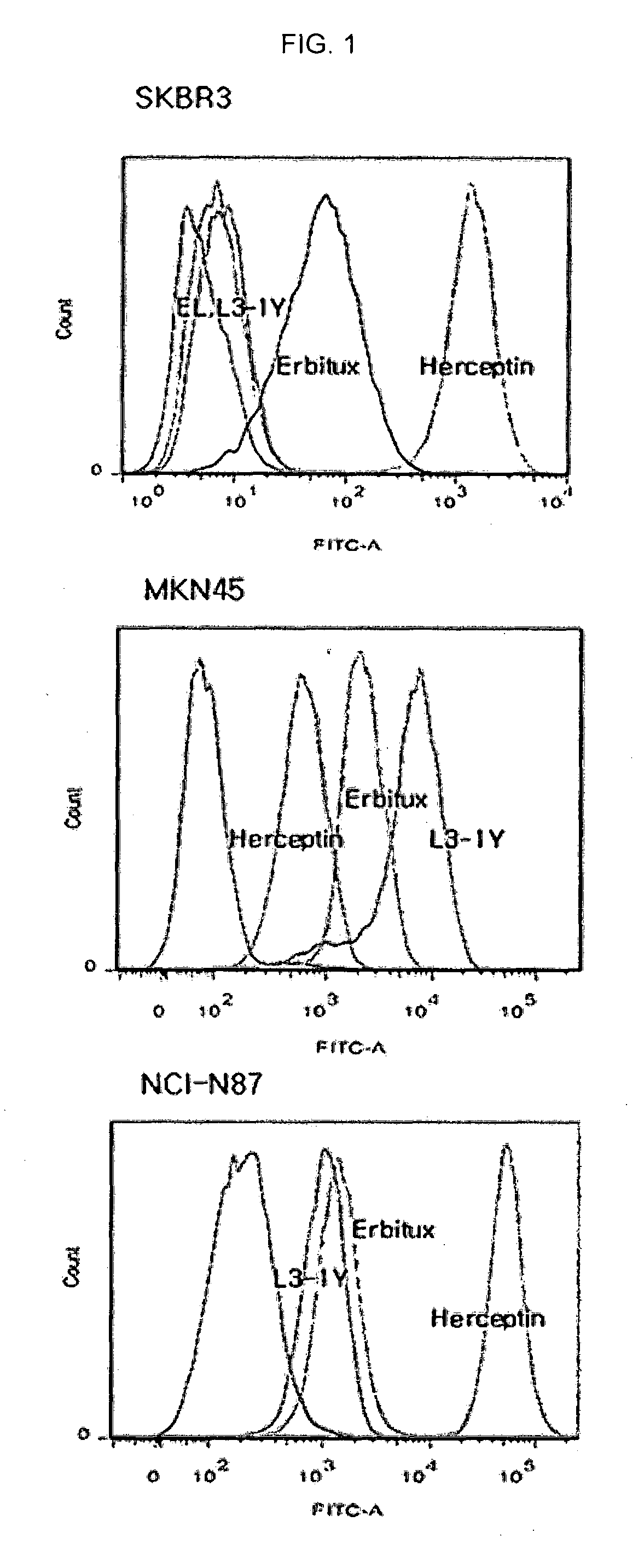
[0155]In various human cancer cell lines purchased from ATCC (SKBR3 (breast cancer cell line), MKN45 (gastric cancer cell line), NCI-N87 (gastric cancer cell line)), expressions of cMET and HER2 were examined. Specifically, 5×105 cells were incubated with 1 μg / mL of a primary antibody (c-Met(L3-1Y), EGFR (Erbitux), HER2 (Herceptin)) at 4° C. for 1 hour in a FACS buffer, and then, incubated with a secondary antibody of anti-human-FITC (Jackson ImmunoResearch) at 4° C. for 30 minutes, and analyzed by FACS (FACS Canto, BD), and the results are shown in FIG. 1.
[0156]As shown in FIG. 1, particularly, high HER2 expression was observed in NCI-N87 (gastric cancer cell line), which was used in the following Examples.
example 2
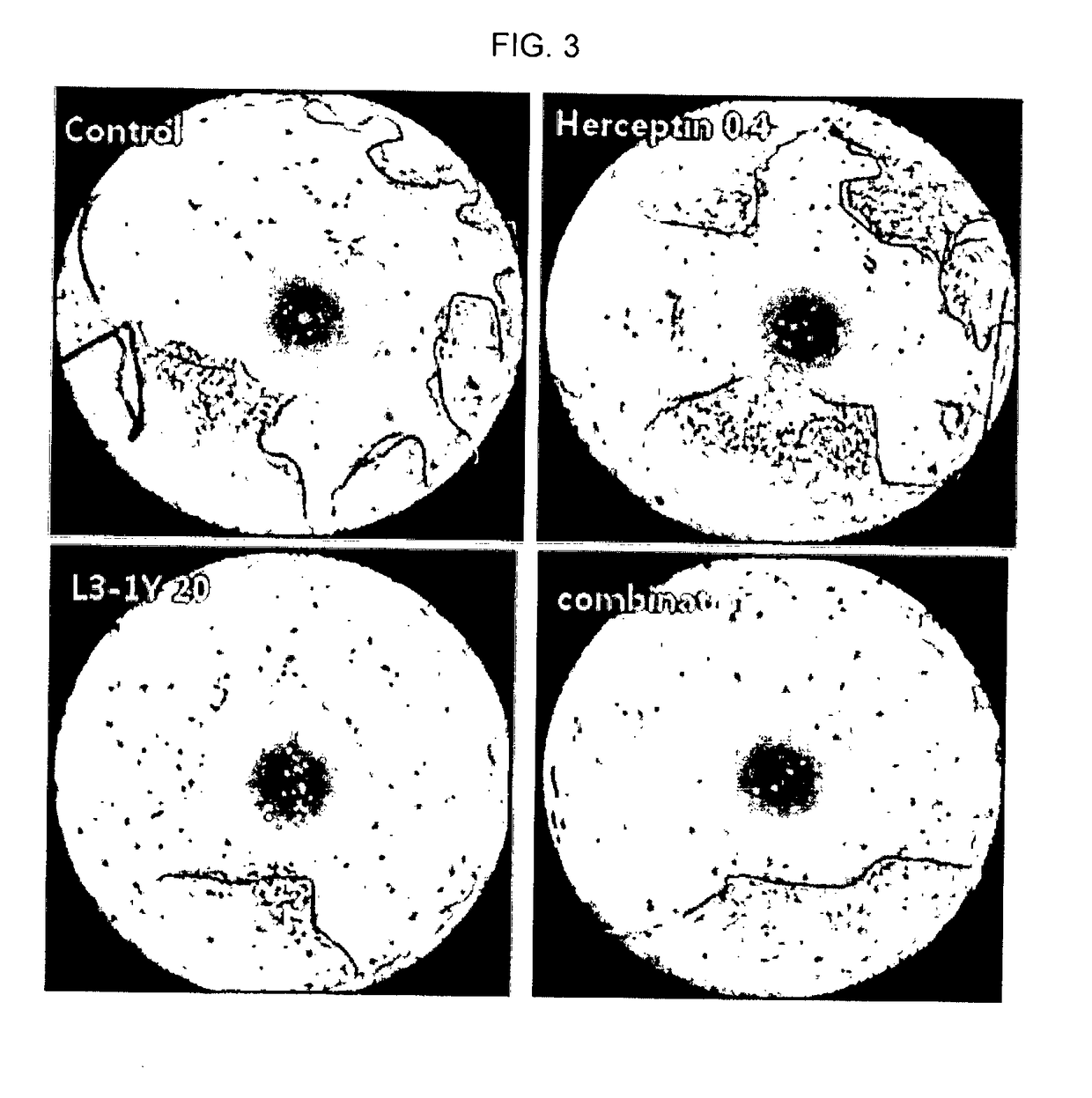
Confirmation of the Effect of Combination Treatment of Anti-cMet Antibody L3-1Y and Anti-HER2 Antibody Herceptin
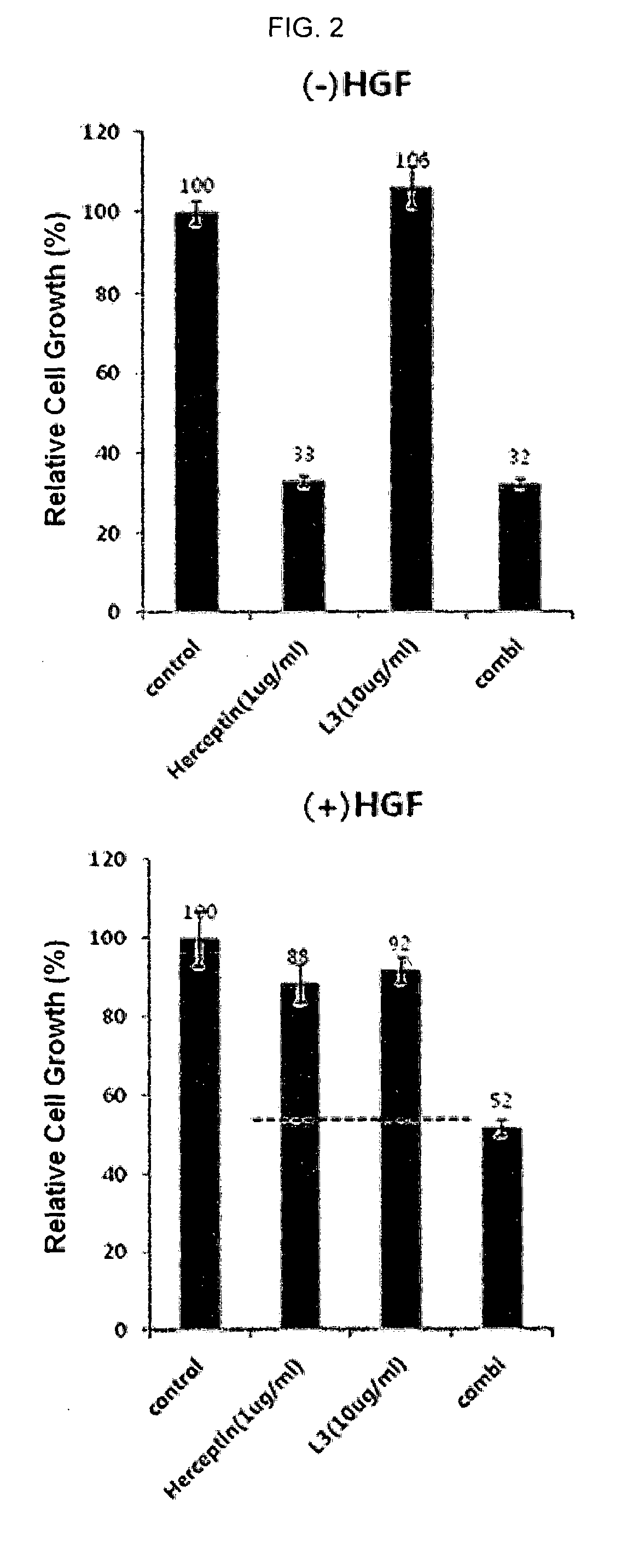
[0157]NCI-N87 cells were seeded at 5×103 cells / well in a 96 well plate, and then, the 96 wells were treated with Herceptin and L3-1Y each alone or in combination under 5% (v / v) FBS, without HGF or with HGF(100 ng / mL) conditions (37° C., 96 hours). At 96 hours, NCI-N87 cells were measured with Cell-Titer Glo (Promega), and the results are shown in FIG. 2. The HGF used in this experiment was purchased from PANGEN.
[0158]As shown in FIG. 2, when an anti-HER2 antibody Herceptin was treated alone under conditions without HGF, an excellent inhibition effect of cell line growth was exhibited. Under conditions with HGF, acquired resistance was exhibited, and when Herceptin was treated alone, cell line growth reached 88%, thus confirming that inhibition effect of cancer cell growth was significantly decreased. However, when an anti-c-Met antibody L3-1Y and an anti-HER2 antibody H
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap