Fusion protein VT-GL-B3, coding gene thereof and applications thereof
A gene and encoding technology, applied in the fusion protein VT-GL-B3 and its encoding gene and application field, can solve problems such as abnormal cardiac conduction, achieve the effects of prolonging half-life, promoting tumor angiogenesis, and inhibiting tumor rescue response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the preparation of VT-GL-B3 fusion protein
[0038] 1. Construction of recombinant plasmids
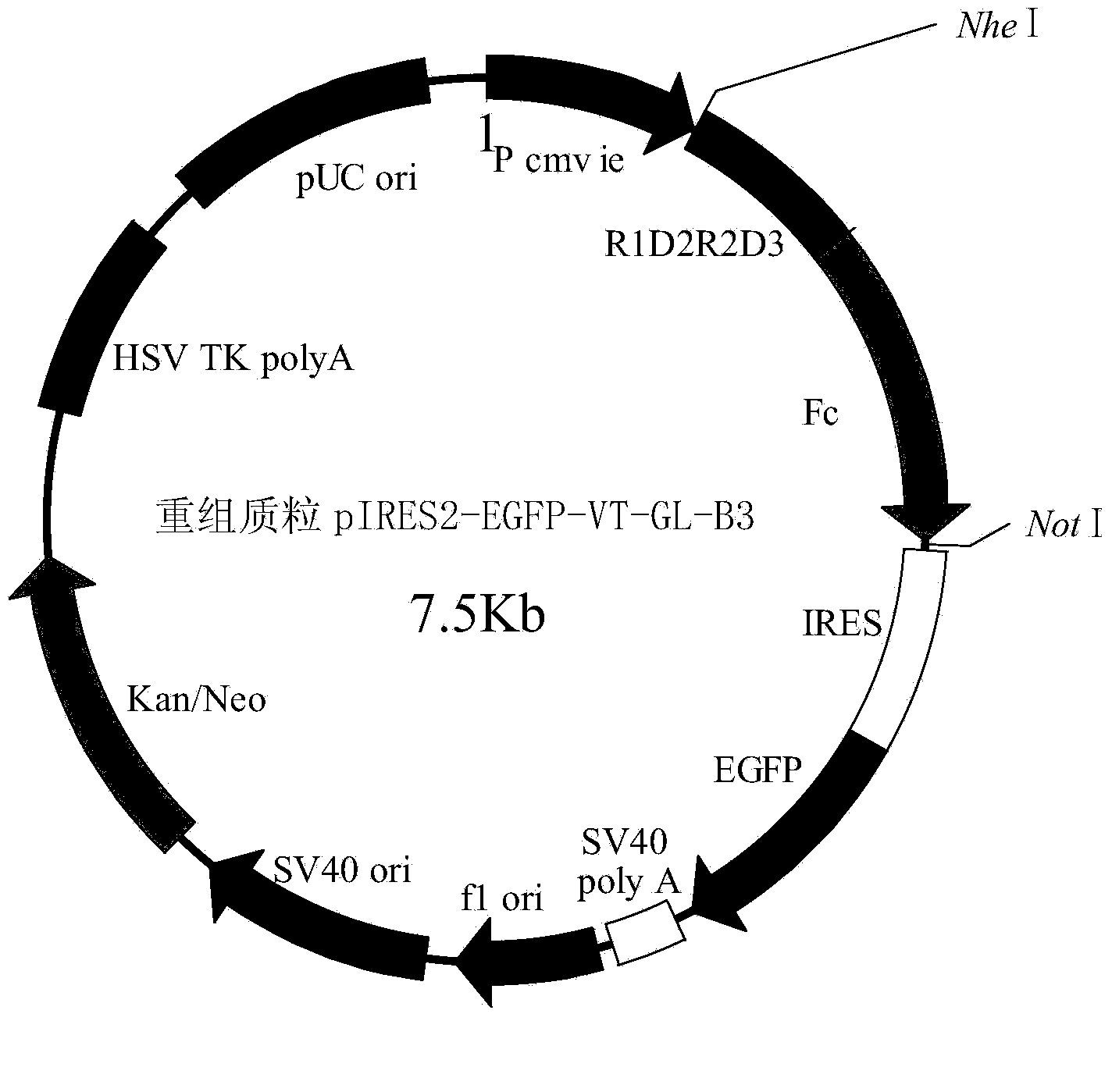
[0039] Insert the double-stranded DNA molecule shown in Sequence 2 of the sequence table between the NheI and NotI restriction sites of the pIRES2-EGFP vector to obtain the recombinant plasmid pIRES2-EGFP-VT-GL-B3. The schematic diagram of the structure of the recombinant plasmid pIRES2-EGFP-VT-GL-B3 is shown in figure 1 .
[0040] The double-stranded DNA molecule shown in Sequence 2 of the Sequence Listing was named VT-GL-B3 gene. The double-stranded DNA molecule shown in sequence 2 of the sequence listing encodes the protein shown in sequence 1 of the sequence listing. The protein shown in Sequence 1 of the sequence listing is named VT-GL-B3 fusion protein.
[0041] In sequence 1 of the sequence listing, amino acid residues 1 to 20 from the N-terminal are signal peptides, and amino acid residues 21-236 are VEGFR1D2R2D3 fragments (VEGFR1D2R2D3 fragments consist
Embodiment 2
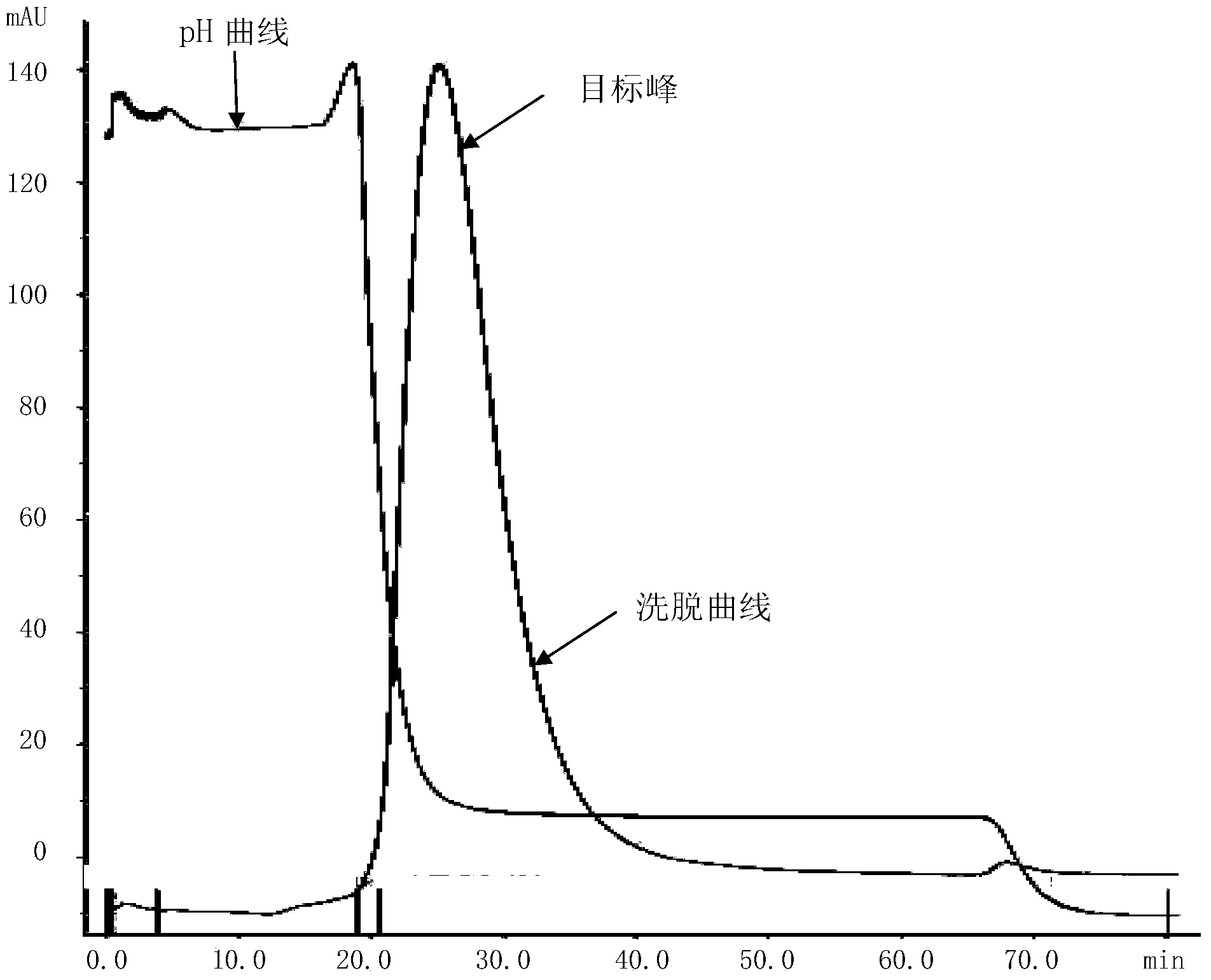
[0056] Example 2, the biological activity of VT-GL-B3 mature peptide
[0057] 1. Affinity between VT-GL-B3 mature peptide and rhVEGF165
[0058] Biacore T200, CM5 sensor chip (Lot No.10148134), Amine Coupling Kit (Lot No.2057704), Human Antibody Capture Kit (Lot No.10137214), HBS-EP+buffer (10×) (Lot No.BCBJ0266V) Both were purchased from GE Healthcare.
[0059] Anti-Human IgG(Fc) Antibody is a component of Human Antibody Capture Kit (Lot No.10137214). HBS-EP + Buffer (10×) is diluted to 10 times volume with deionized water, which is HBS-EP + buffer solution.
[0060] 1. Anti-Human IgG (Fc) Antibody combined with the surface of CM5 sensor chip
[0061] (1) Embed the CM5 sensor chip into Biacore T200.
[0062] (2) Prepare Anti-Human IgG(Fc) Antibody solution with pH 5.0, 10mM sodium acetate buffer.
[0063] (3) According to the sample placement method in the Immobilization program setting, place the solution in the Amine Coupling Kit in the appropriate position of the Biaco
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap