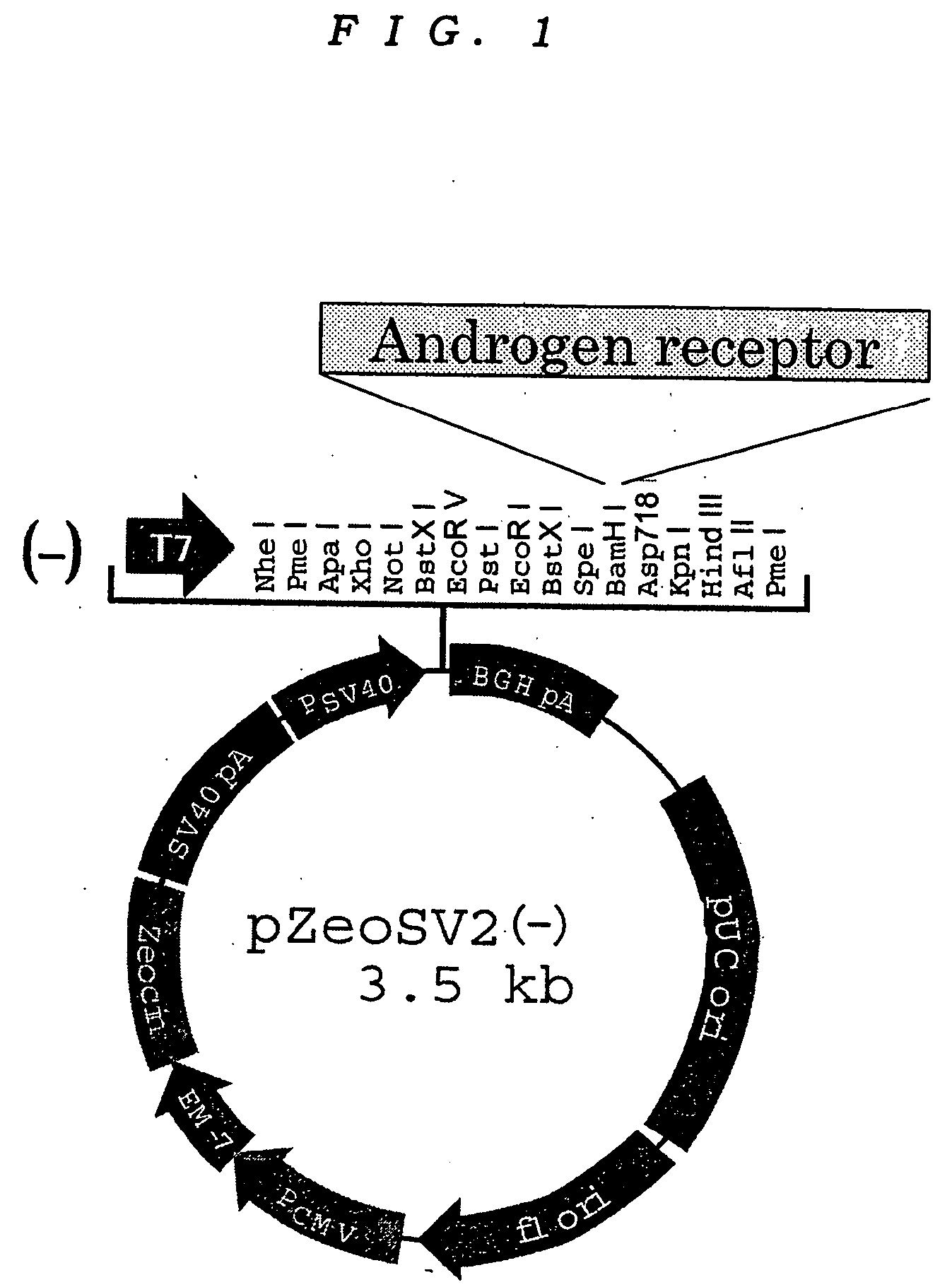
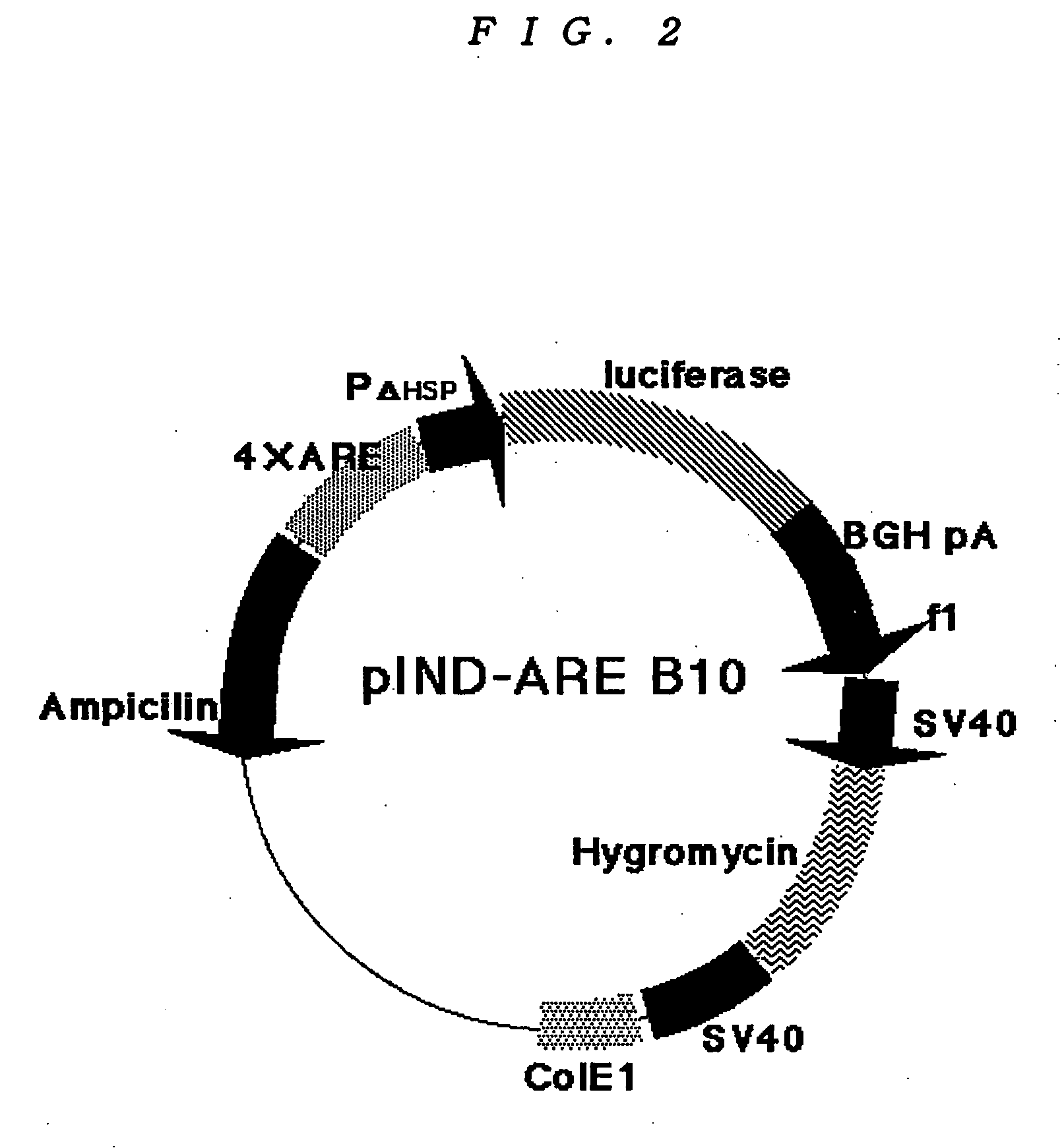
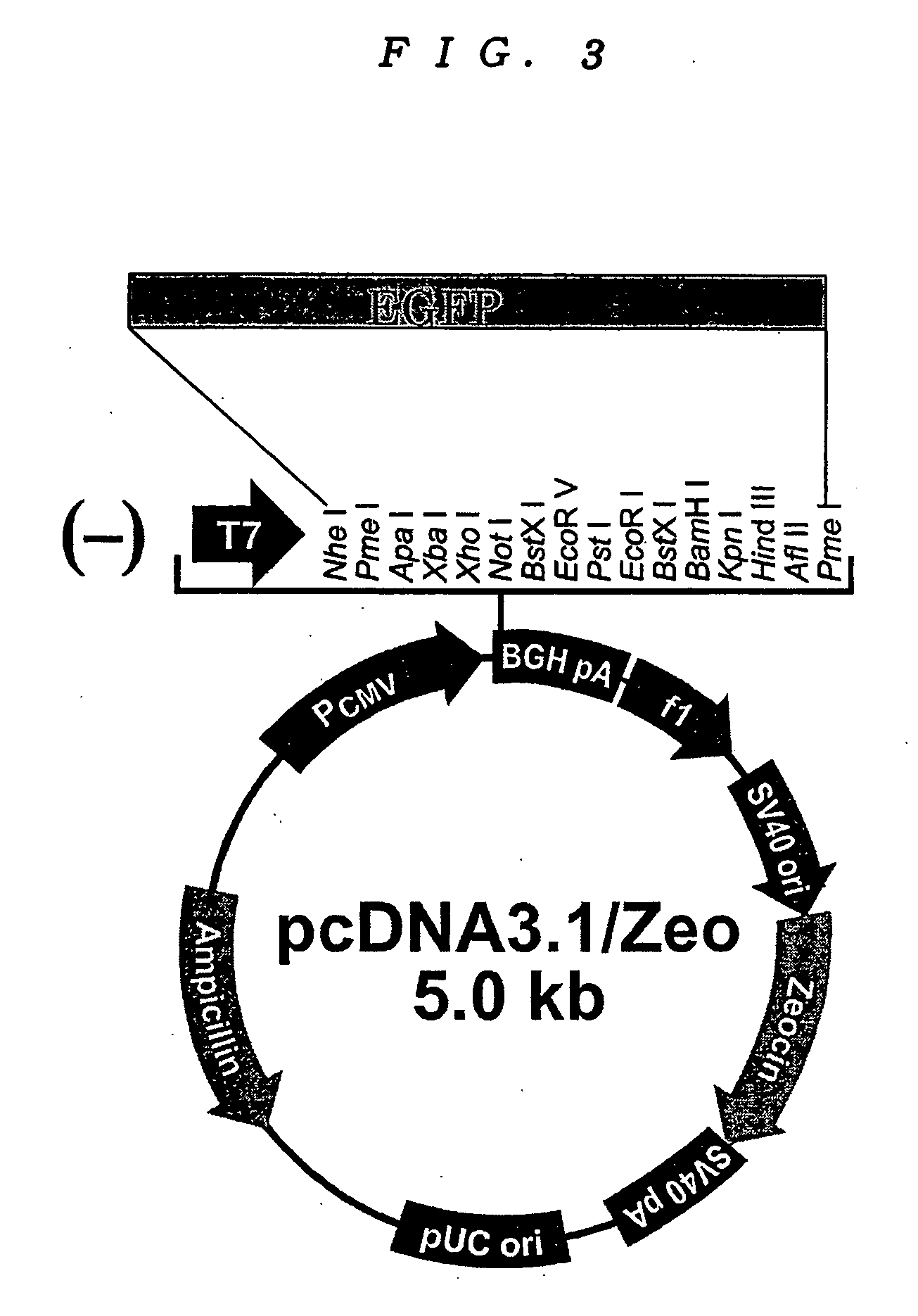
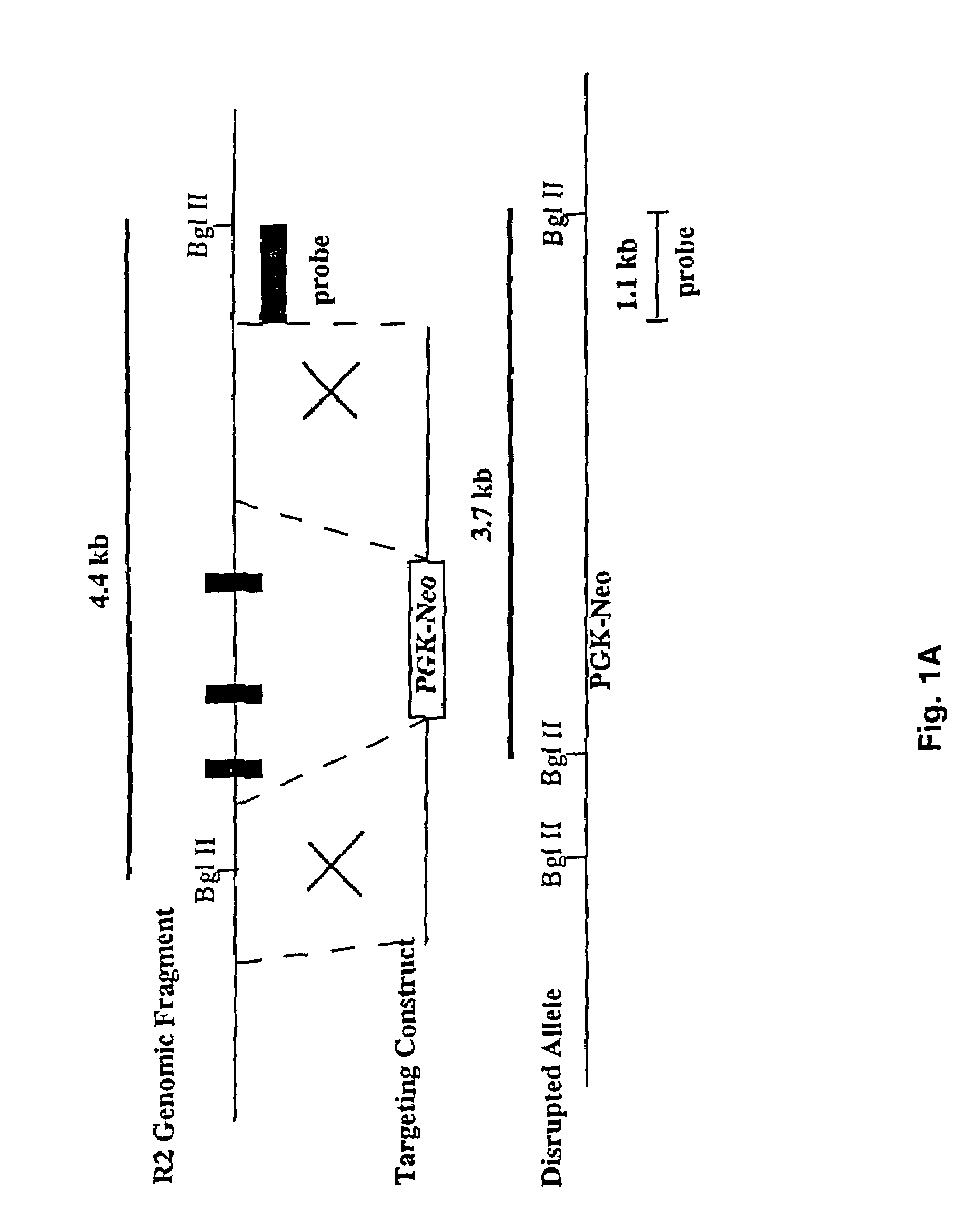
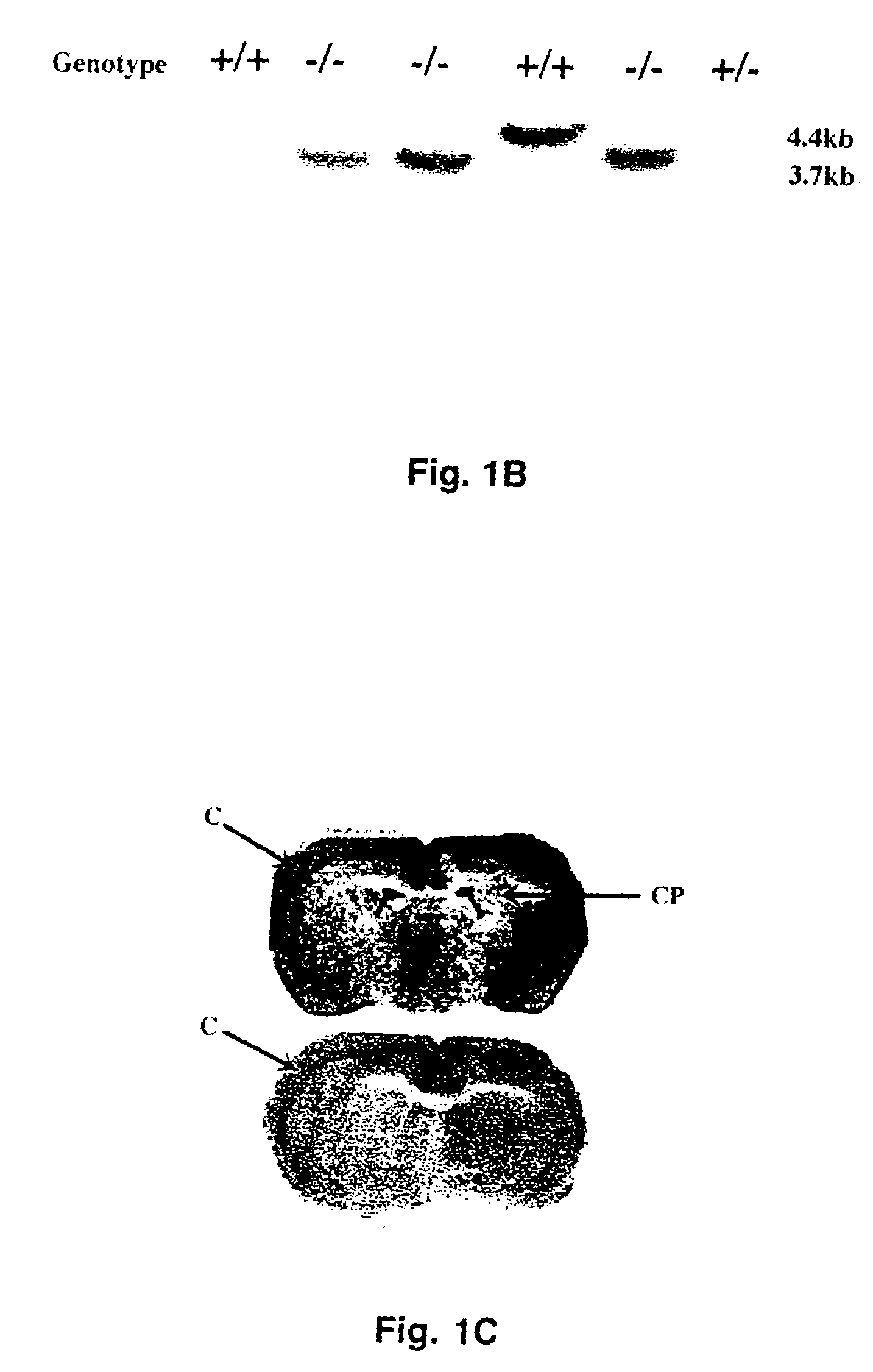
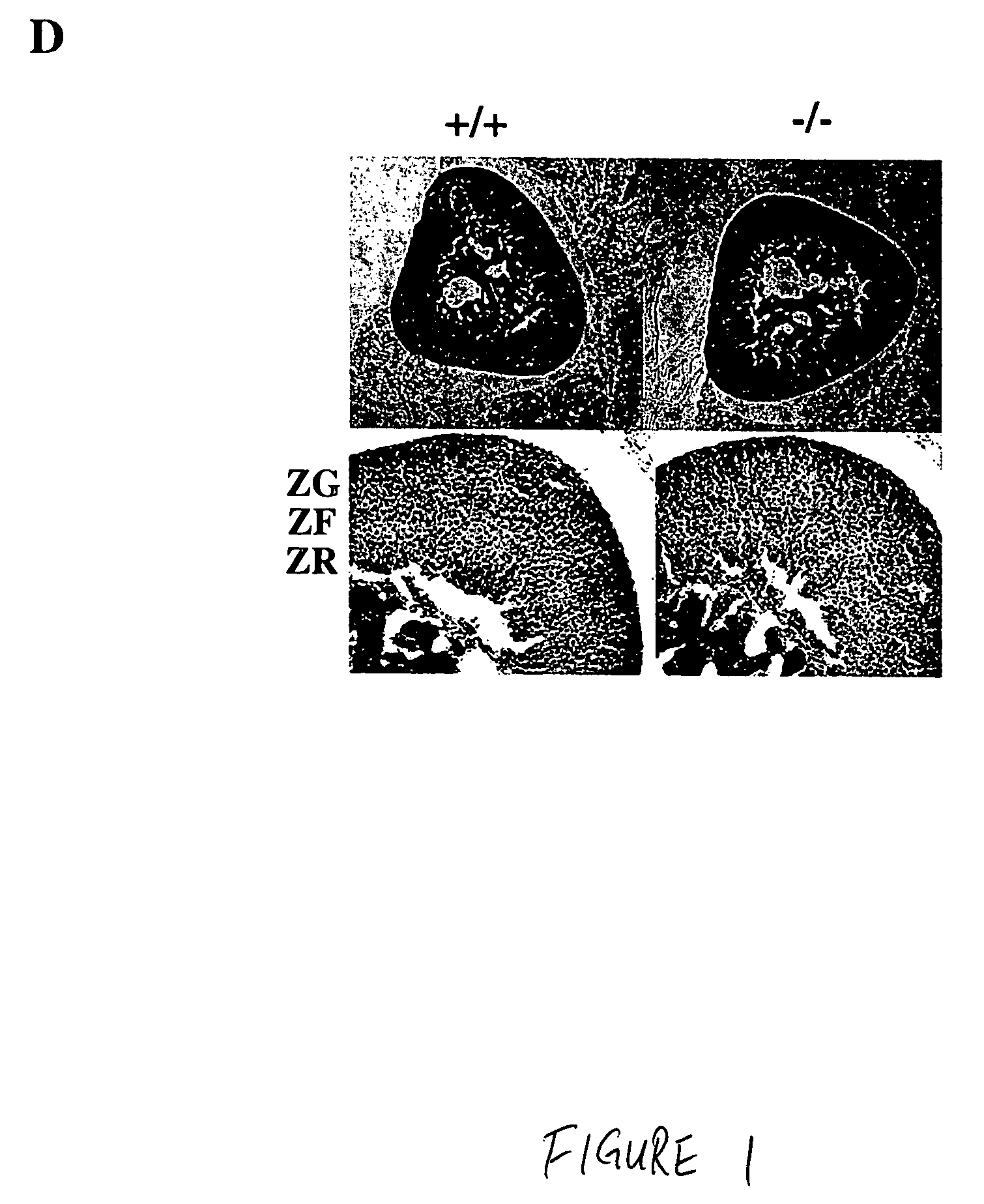
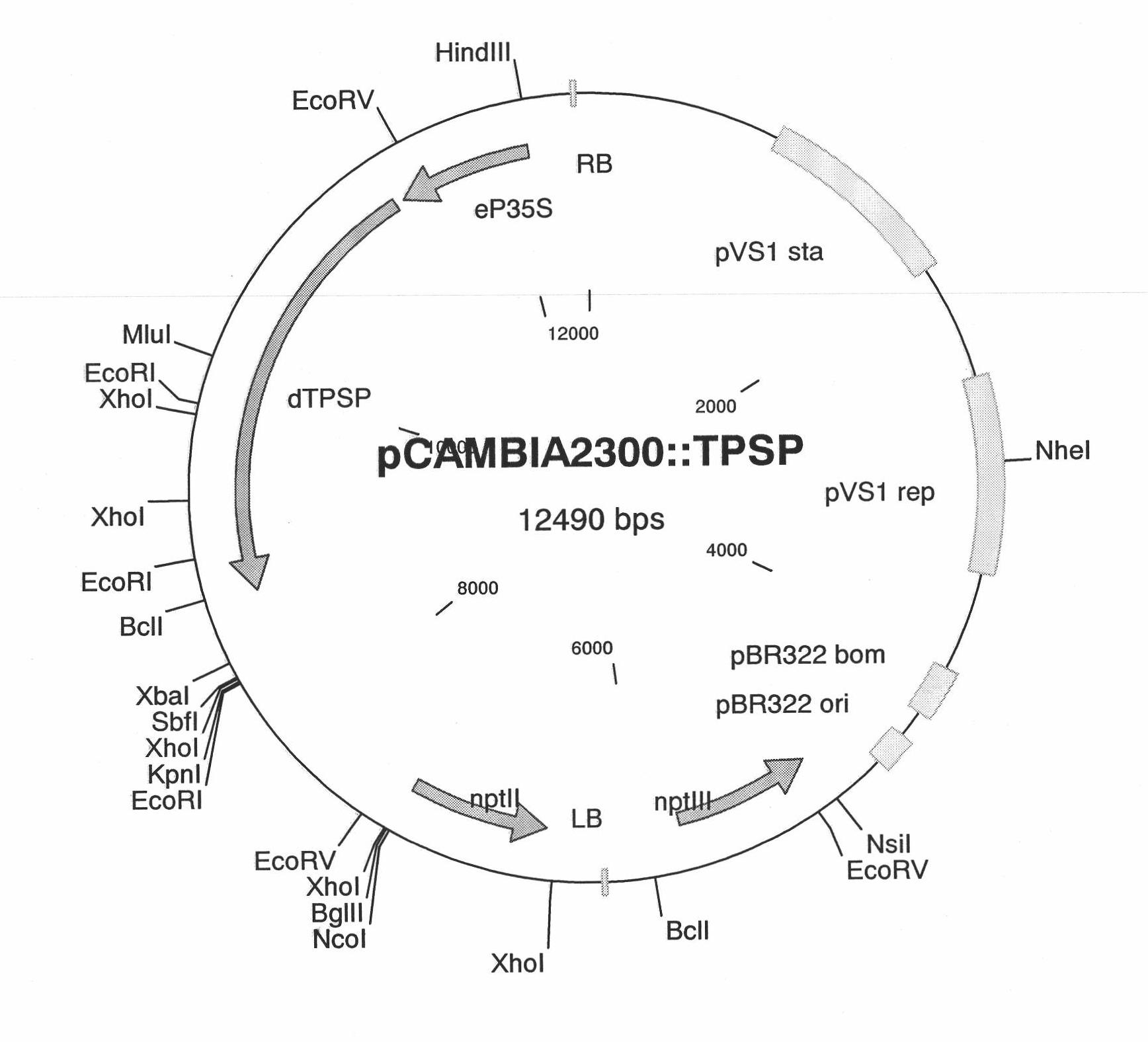
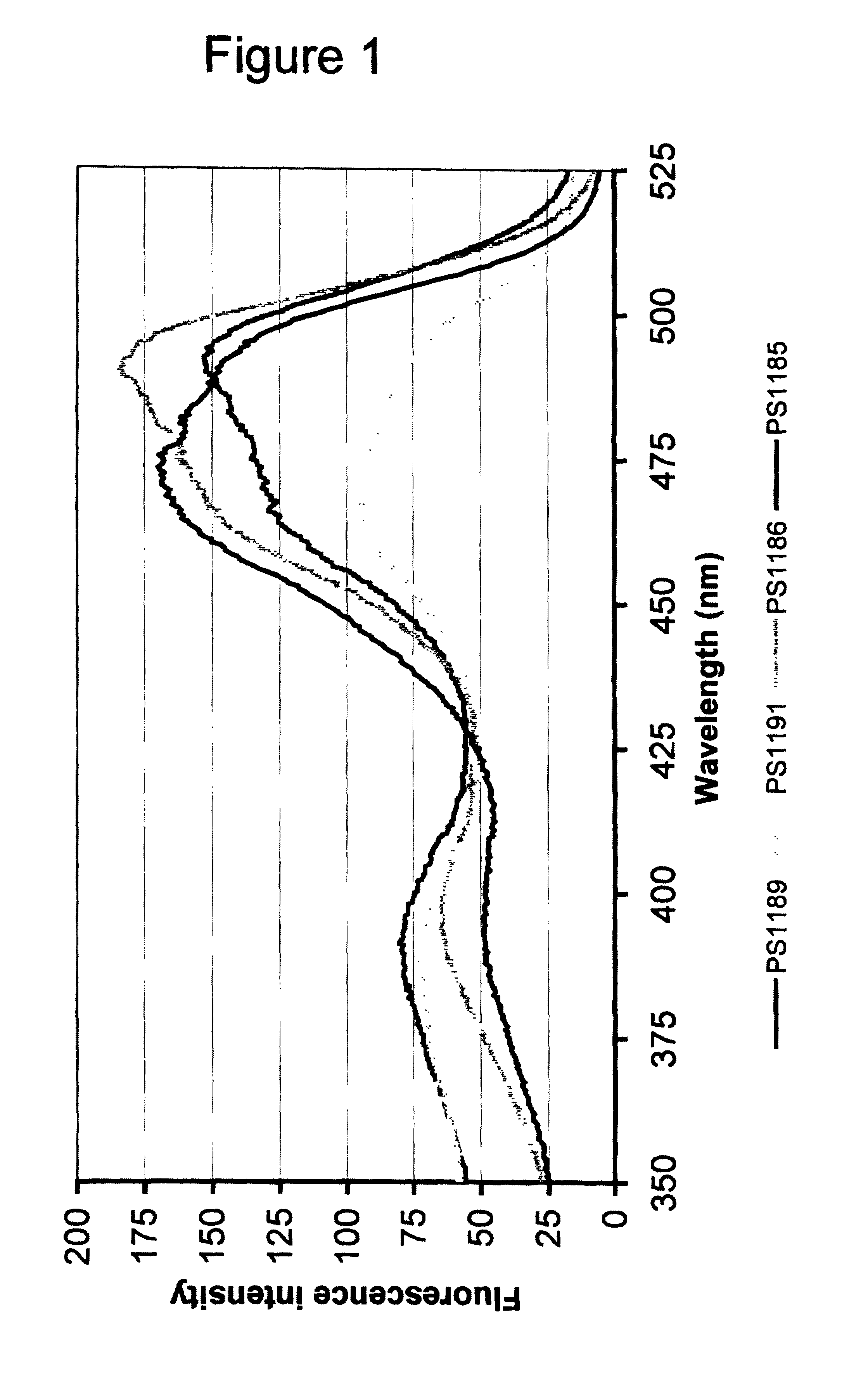
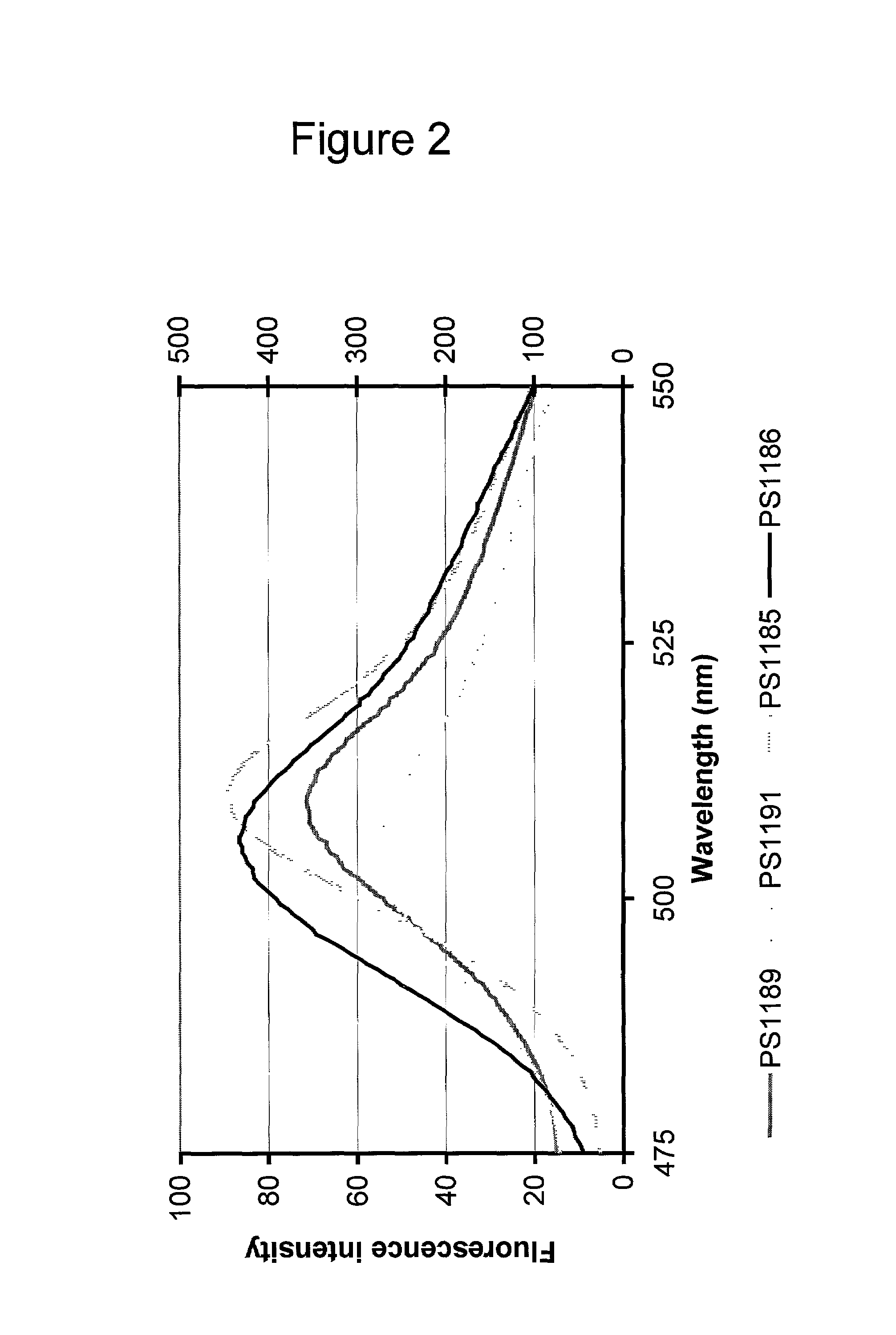
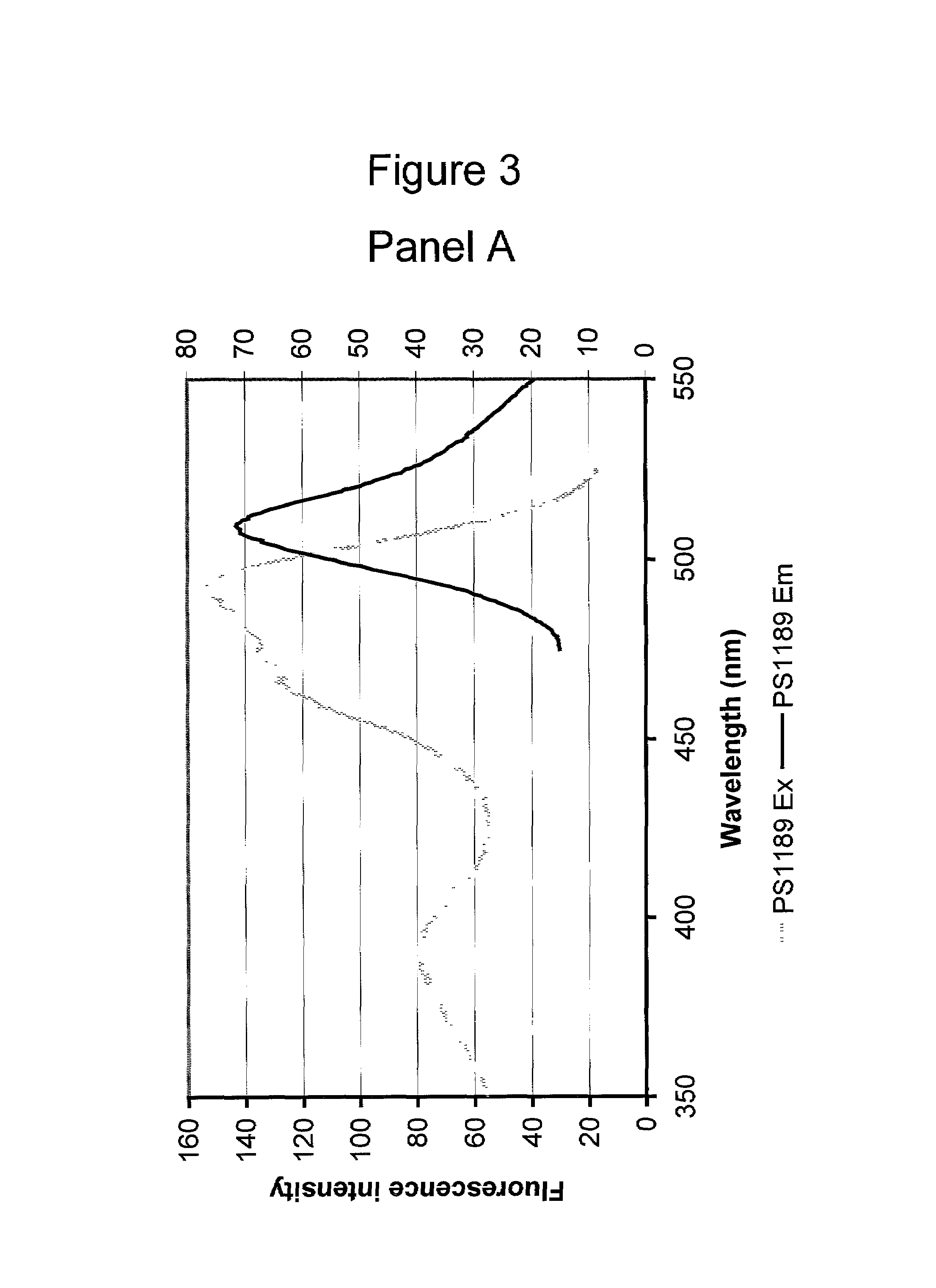
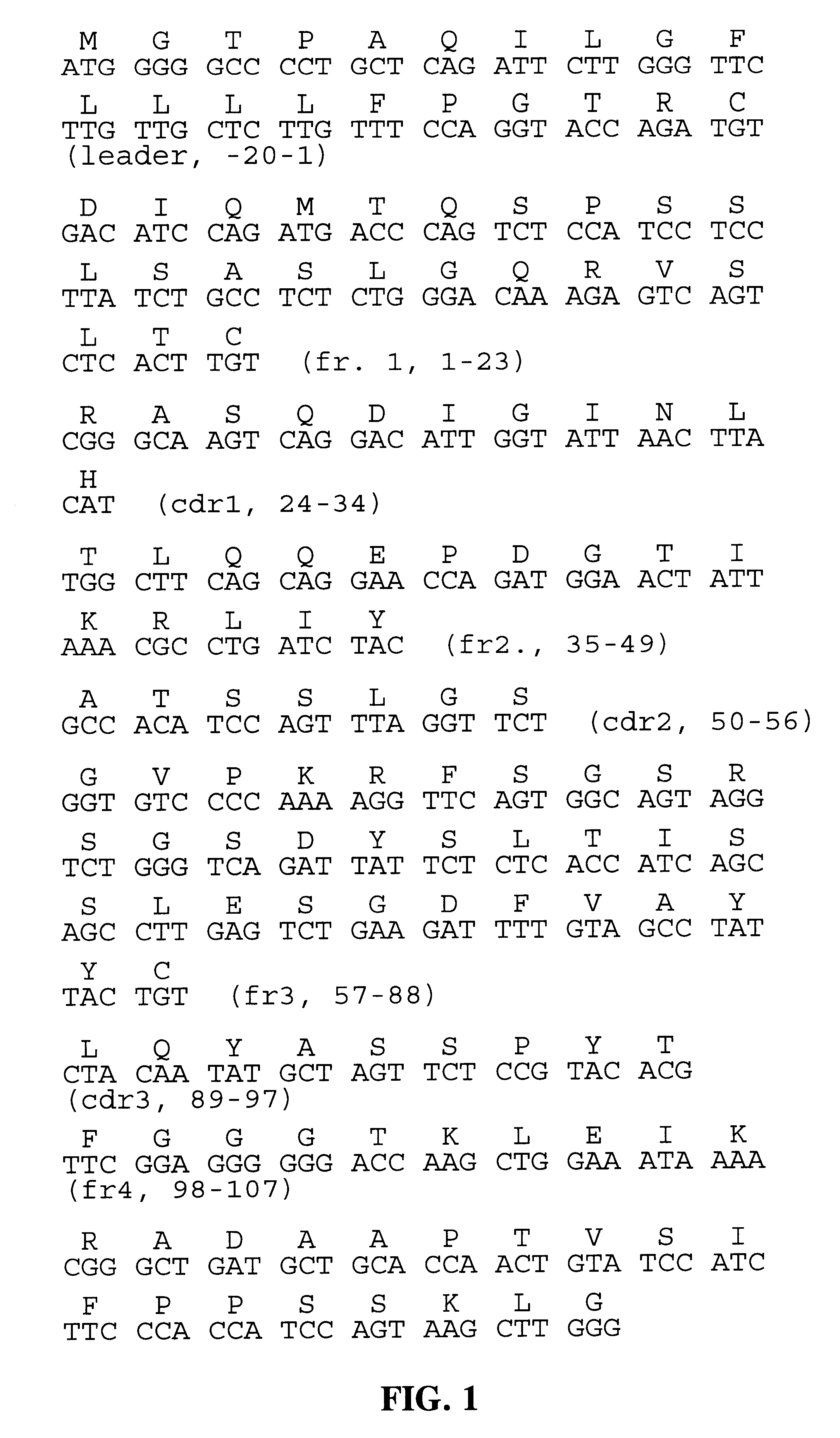Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33results about "Foreign genetic material cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Murine monoclonal anti-idiotype antibody 11D10 and methods of use thereof
Owner:UNIVERSITY OF KENTUCKY
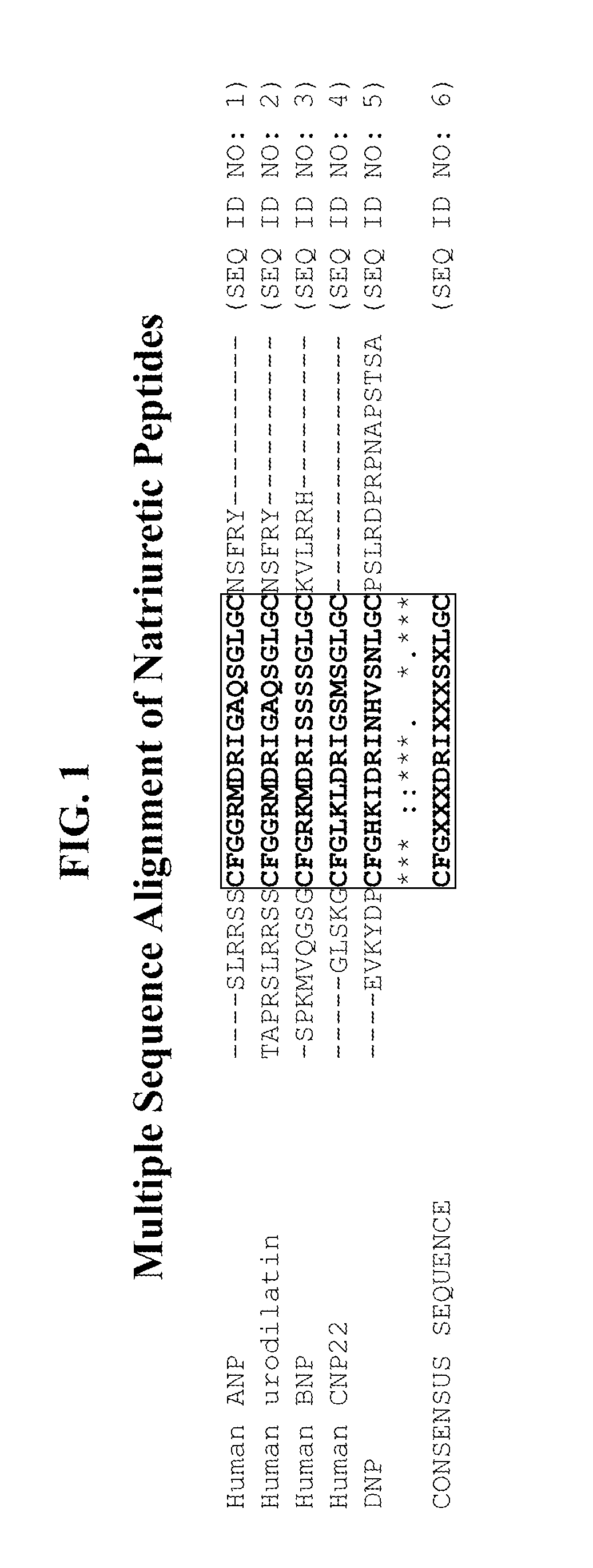
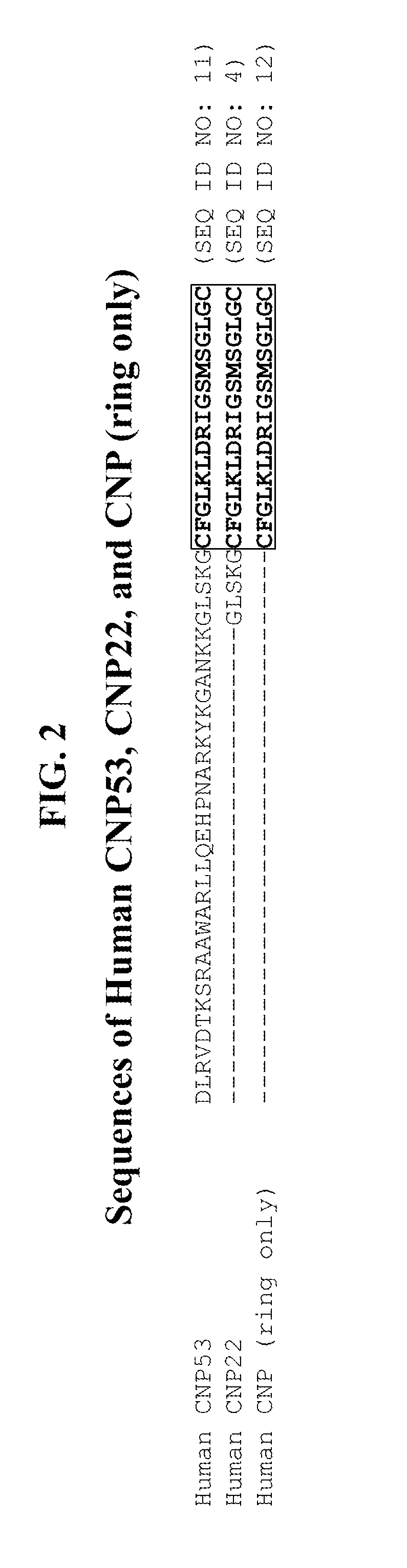
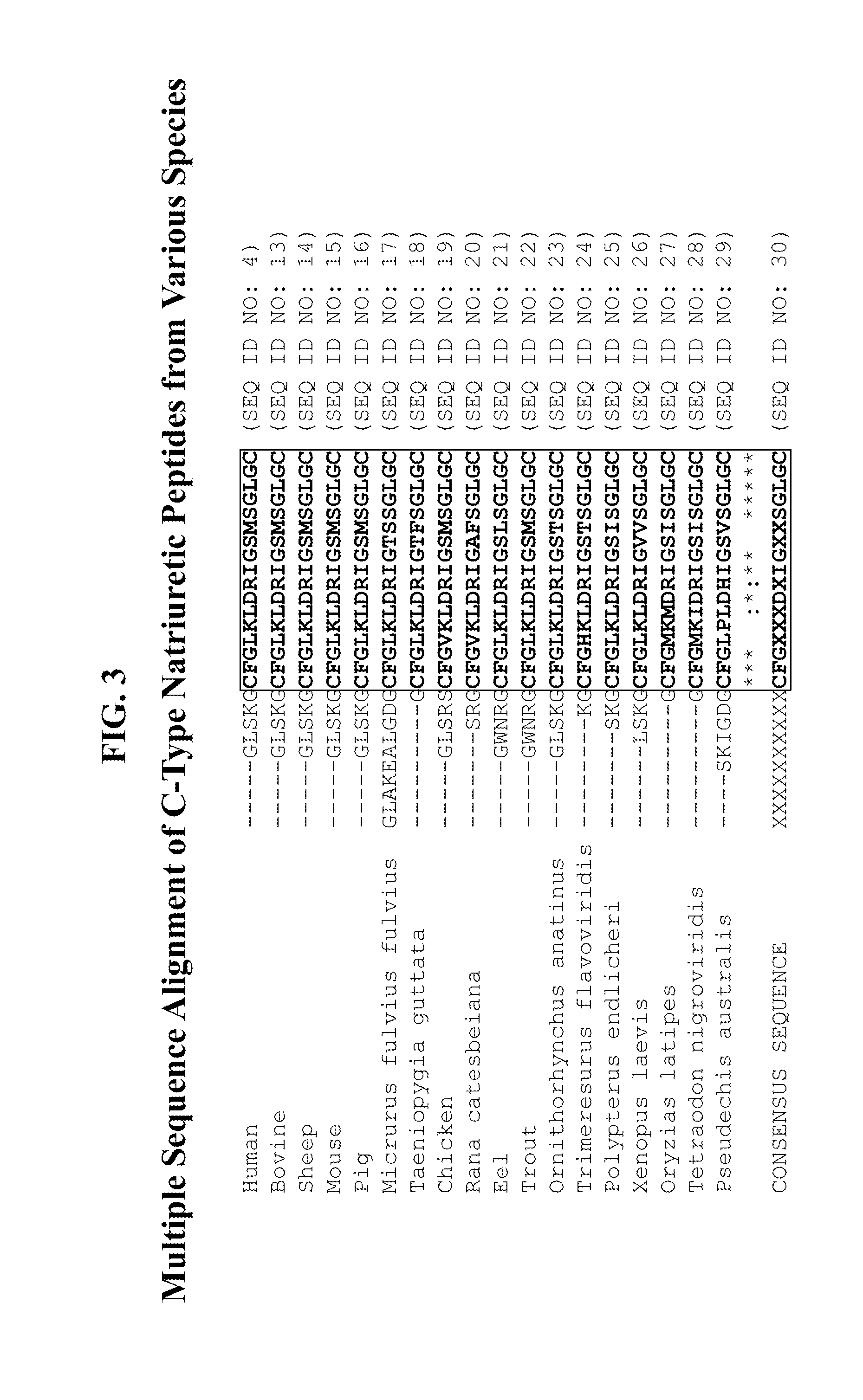
Compositions comprising natriuretic peptides and methods of use thereof
Owner:ALEXION PHARMA INC
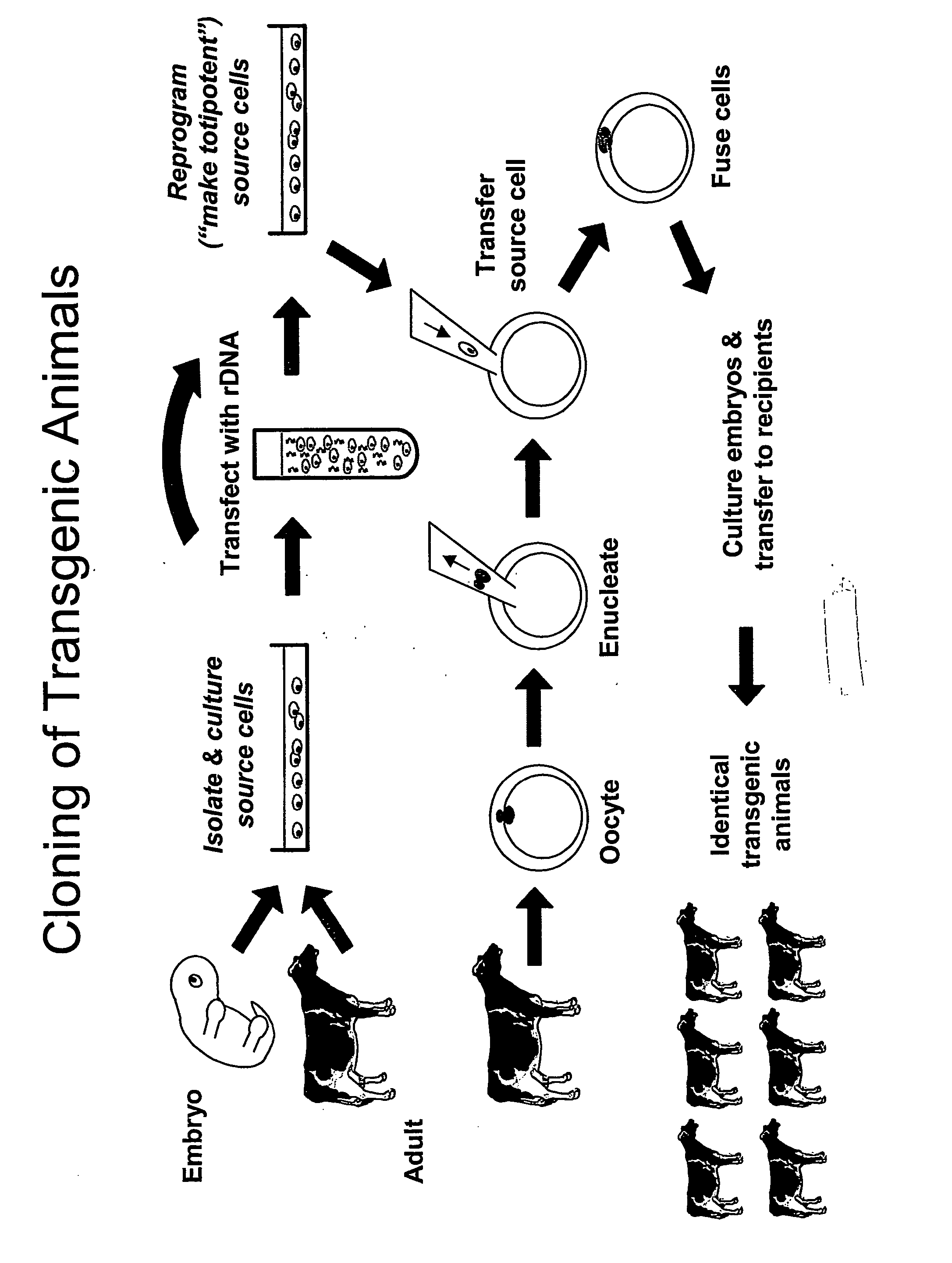
Method and system for fusion and activation following nuclear transfer in reconstructed embryos
InactiveUS20050177882A1Improve usabilityPrevent rejectionNew breed animal cellsRecombinant DNA-technologyActivation methodPrimate
Owner:GTC BIOTHERAPEUTICS INC
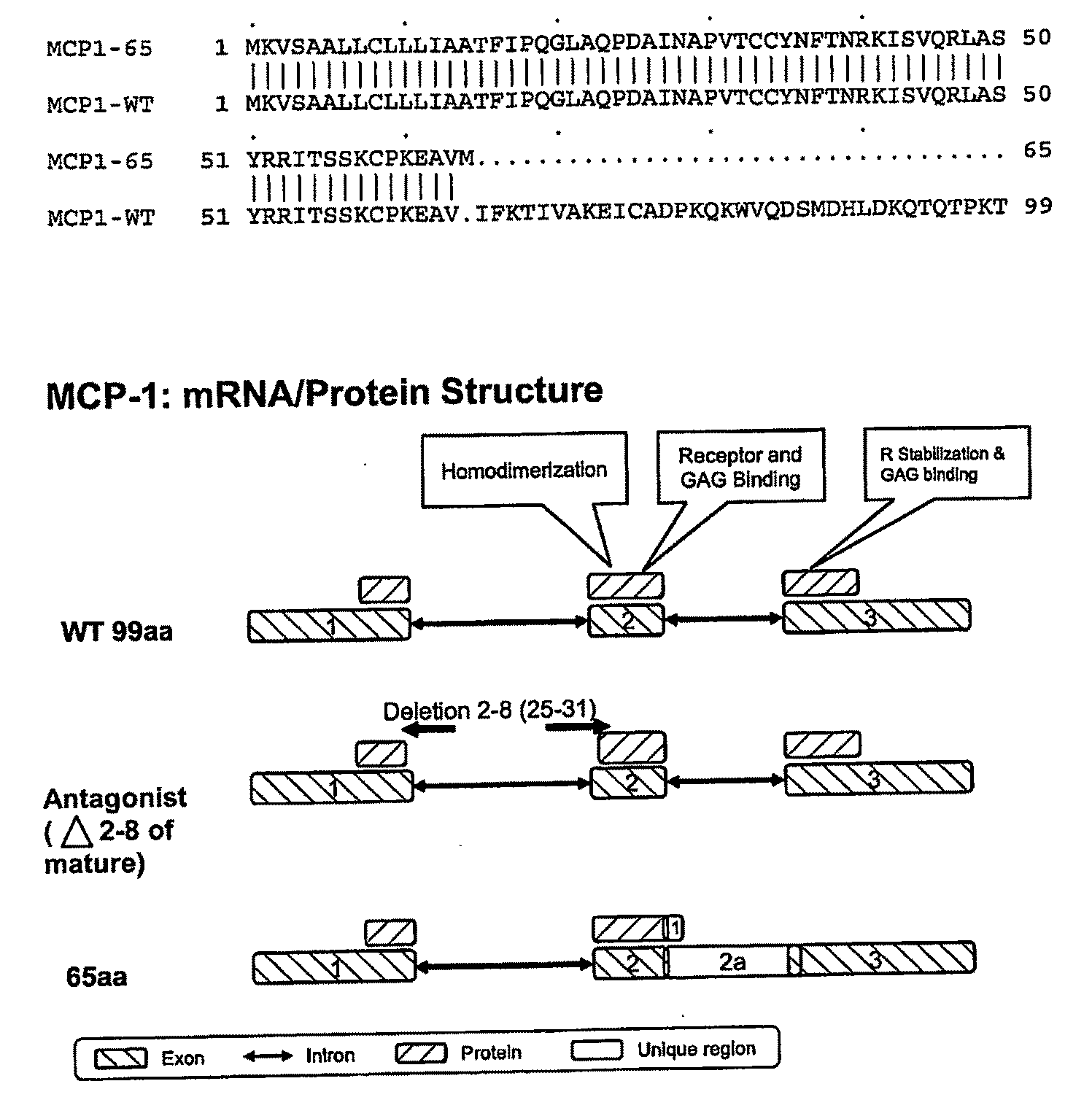
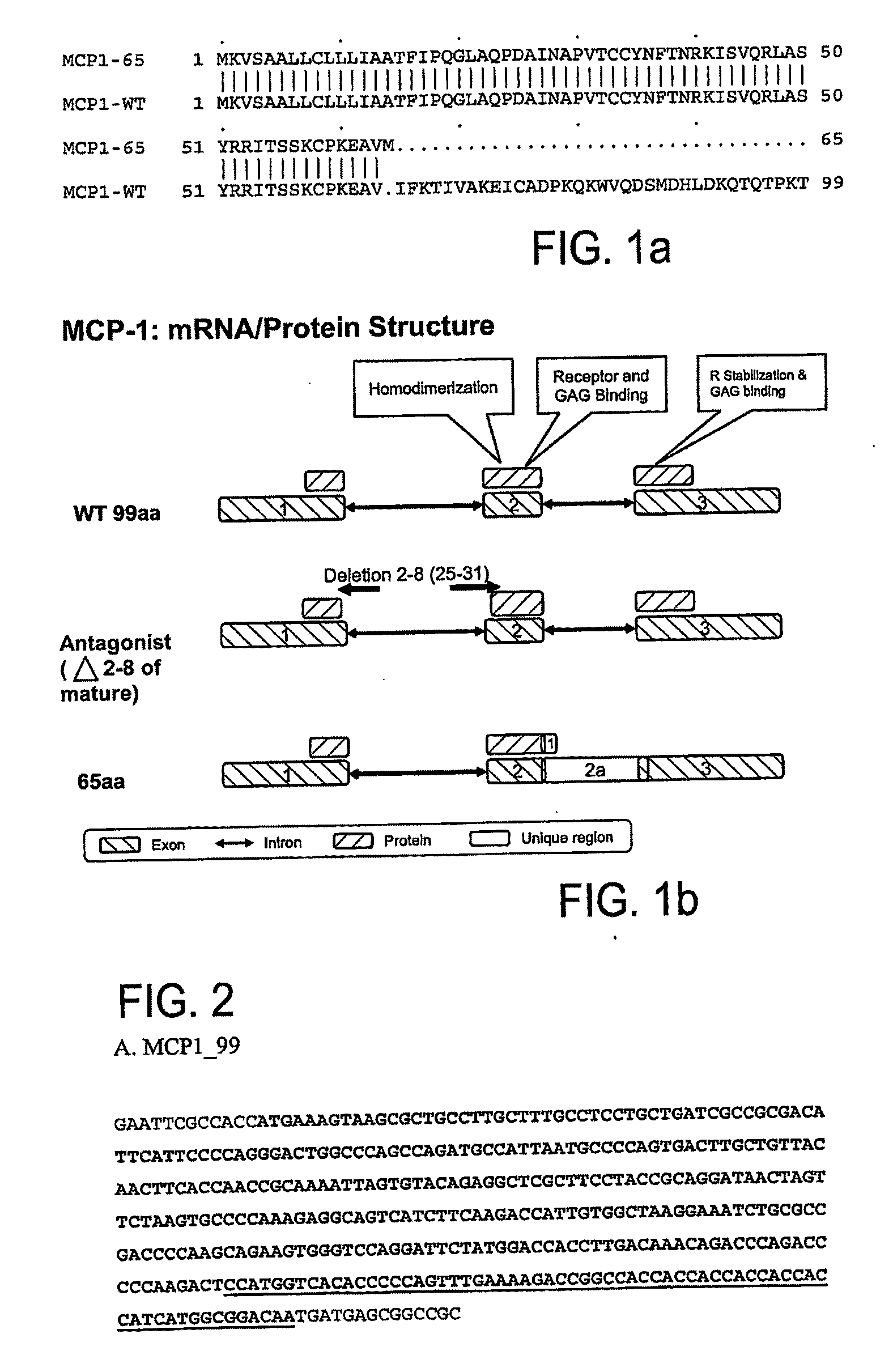
Mcp-1 splice variants and methods of using same
Owner:COMPUGEN
Prepn and application of epiderm or hair follicle stem cell from souce of human early embryo
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Long-chain non-coding RNA PRALR, and expression plasmid and use thereof
ActiveCN110129318AUseful for researchSuppress drug resistanceOrganic active ingredientsGenetically modified cellsChemotherapeutic drugsNon-coding RNA
The invention relates to a long-chain non-coding RNA PRALR, and an expression plasmid and a use thereof. Starting from TCONS-00013523 sequence, a complete gene sequence is discovered by 5'-RACE and 3'-RACE technologies and is named as PRALR (paclitaxel resistance-associated lncRNA, paclitaxel resistance-associated long-chain non-coding RNA). Then, the PRALR expression plasmid and siRNA are constructed, and the PRALR is further confirmed to be a novel ovarian cancer drug resistance-associated lncRNA; the constructed PRALR expression plasmid can significantly increase the expression quantity ofthe PRALR, and the designed siRNA can significantly reverse drug resistance of paclitaxel-resistant ovarian carcinoma cells. A powerful tool is provided for study of the mechanism of paclitaxel resistance and other chemotherapeutic drug resistance, and a reference is provided for clinical development of drugs to reverse the drug resistance of tumor to paclitaxel.
Owner:JINSHAN HOSPITAL FUDAN UNIV
Callery pear ascorbate peroxidase gene and use thereof in resisting heavy metal stress
The invention relates to a callery pear ascorbate peroxidase gene and a use thereof in resisting heavy metal stress. The gene has a nucleotide sequence shown in the sequence SEQ ID No. 1. The total RNAs of the leaves of the callery pear are extracted after cadmium treatment and the ascorbate peroxidase gene Pc. APX is cloned by combination of bioinformatics and PCR so that the complete coding genesequence of 753 bp is obtained. The escherichia coli expression vector pET-22b(+)-Pc. APX is constructed and through the escherichia coli heterologous expression system, the functions of the cloned ascorbate peroxidase gene Pc. APX are identified and the recombinant escherichia coli with the cloned ascorbate peroxidase genes Pc. APX have strong tolerance to cadmium. At the same time, a binary plant expression vector pRI201-AN-GUS-Pc. APX is contructed, the vector is transferred into agrobacterium tumefaciens GV3101 cells by a freezing-thawing method, and the cells are transformed into Arabidopsis thaliana, and the obtained transgenic Arabidopsis thaliana has higher cadmium tolerance than wild-type Arabidopsis thaliana.
Owner:JIANGSU ACAD OF AGRI SCI
Reporter gene assay method
InactiveUS20060166196A1Accurate identificationAnimal cellsMaterial analysis by observing effect on chemical indicatorCell activityHormones regulation
Owner:OTSUKA PHARM CO LTD
Lettuce variety 41-137 rz, "delibes rz"
InactiveUS20140020129A1Microbiological testing/measurementFood preservationBremia lactucaePemphigus bursarius
Owner:RIJK ZWAAN ZAADTEELT & ZAADHANDEL BV
Anti-nematode peptides and methods of use thereof
The invention provides novel polypeptides, and variants and fragments thereof, having pesticidal activity against nematodes. Particular embodiments of the invention provide isolated nucleic acids encoding pesticidal proteins, biopesticide compositions, expression cassettes, and transformed microorganisms and plants comprising a nucleic acid of the invention. These compositions find use in methods for controlling pests, especially plant parasitic nematodes.
Owner:PIONEER HI BRED INT INC
Method of inhibiting angiogenesis by administration of a corticotropin releasing factor receptor 2 agonist
InactiveUS7674463B1Alters anxiety stateReduce appetiteCompounds screening/testingOrganic active ingredientsCorticotropinsTransgene
Owner:RES DEVMENT FOUND
Fusion protein capable of synthetizing trehalose and application thereof in culture of dwarfed lawn grass
Owner:BEIJING NORTH ELITE BIOTECH
General CAR-T cell and preparation method and application thereof
PendingCN109777782ASimple structureNon-pathogenicStable introduction of DNAMammal material medical ingredientsAbnormal tissue growthT cell
The invention discloses a physiological general CAR-T cell which enables expression of CAR to be controlled by an endogenous promoter and a preparation method and application thereof. A TCR constant region gene is knocked out by utilizing a CRISPR / Cas9 gene editing technique, and the CAR gene is inserted into a TCR constant region precisely by using an AAV-mediated homologous recombination technique. Therefore, the general CAR-T enabling the CAR expression controlled by the endogenous promoter and enabling the expression level of CAR be controlled within a normal physiological range can be obtained. The general CAR-T cell has the characteristics of being allogeneically infused, the expression of CAR is at the physiological level and uniform and consistent, no significant cytokine storm exists, and the cell is safer. The invention also relates to application of the CAR-T cell on anti-tumor aspect.
Owner:BEIJING MENLO BIOLOGICAL TECH CO LTD
Pair of transcriptional activation subsample effect factor nucleases and coding gene and application thereof
The invention discloses a pair of transcriptional activation subsample effect factor nucleases and a coding gene and application thereof. The pair of transcriptional activation subsample effect factor nucleases (TALEN) is obtained by merging a pair of DNA identifying proteins with two different source subunits of a Fok1 DNA incision enzyme respectively, and two adjacent locuses on a prion protein gene (PRNP) exon2 of a goat or a sheep can be identified in specificity mode. When the pair of transcriptional activation subsample effect factor nucleases is simultaneously transferred into a host cell, the nucleases can shoot targets of the exon2 locuses of a host cell PRNP gene and enable the target shooting locuses to have genic mutation so as to perform targeted modification on the PRNP gene of the goat or sheep. The nucleases have the advantages of being strong in specificity, high in target shooting efficiency and accuracy and the like.
Owner:ZHEJIANG UNIV
Fluorescent proteins
Owner:FISHER BIOIMAGE
Cryptosporidium parvum Cp15 recombinant invasive lactic acid bacteria live vector vaccine
InactiveCN113304254AImproving immunogenicityImprove responseProtozoa antigen ingredientsAntibody mimetics/scaffoldsCryptosporidium infectionCryptosporidium parvum
The invention discloses a cryptosporidium parvum Cp15 recombinant invasive lactic acid bacteria live vector vaccine. The used lactic acid bacteria are lactic acid bacteria expressing staphylococcus aureus invasive cell key protein FnBPA protein through transformation, and have the capability of delivering DNA. The Cp15 recombinant invasive lactic acid bacteria live vector vaccine for preventing cryptosporidiosis parvum is low in production cost, simple in preparation method and easy to standardize; and the use is convenient. The prepared Cp15 recombinant invasive lactic acid bacteria live vector vaccine for preventing cryptosporidiosis can stimulate cellular immunity and humoral immunity of a host and reduce oocyst discharge by 51.7%, which shows that the vaccine has good immune protection capability on cryptosporidium infection.
Owner:JILIN UNIV
Inhibitors of mTORC2
PendingCN114702552AInhibition of activationInhibit phosphorylationNervous disorderPeptide/protein ingredientsPhosphorylationKinase
The invention discloses an mTORC2 (mammalian TORC2) inhibitor. According to the application, an mTORC2 inhibitor is designed and developed on the basis of a protein region Sin1-N structure of an mTORC2 specific subunit Sin1, and the mTORC2 inhibitor specifically inhibits activation of mTORC2 and specifically inhibits phosphorylation of a downstream kinase Akt activation site Ser473; compared with the existing mTOR inhibition active center-oriented small-molecule inhibitor, the variable configuration inhibitor has the advantages of strong inhibition effect and small side effect.
Owner:SUZHOU SITRI INST OF IMMUNOLOGY CO LTD
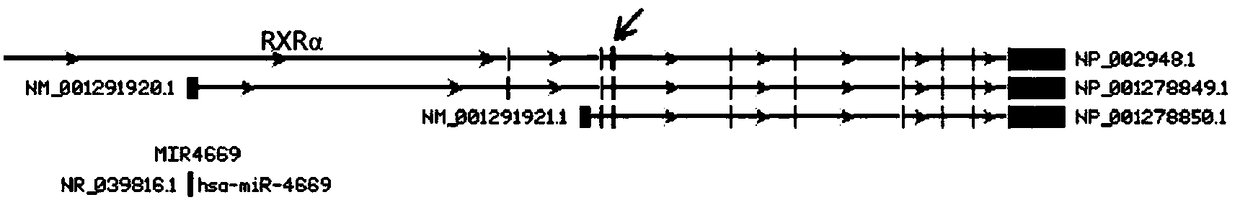
RXRalpha (Homo Sapiens Retinoid X Receptor alpha) gene knockout cell system with stable and low expression of RXRalpha protein and preparation method of RXRalpha gene knockout cell system
ActiveCN108753732AReduce the difficulty of proliferation and cultureStable genotypeCell receptors/surface-antigens/surface-determinantsGenetically modified cellsGenotypeCell system
Owner:SHENZHEN CENT FOR DISEASE CONTROL & PREVENTION
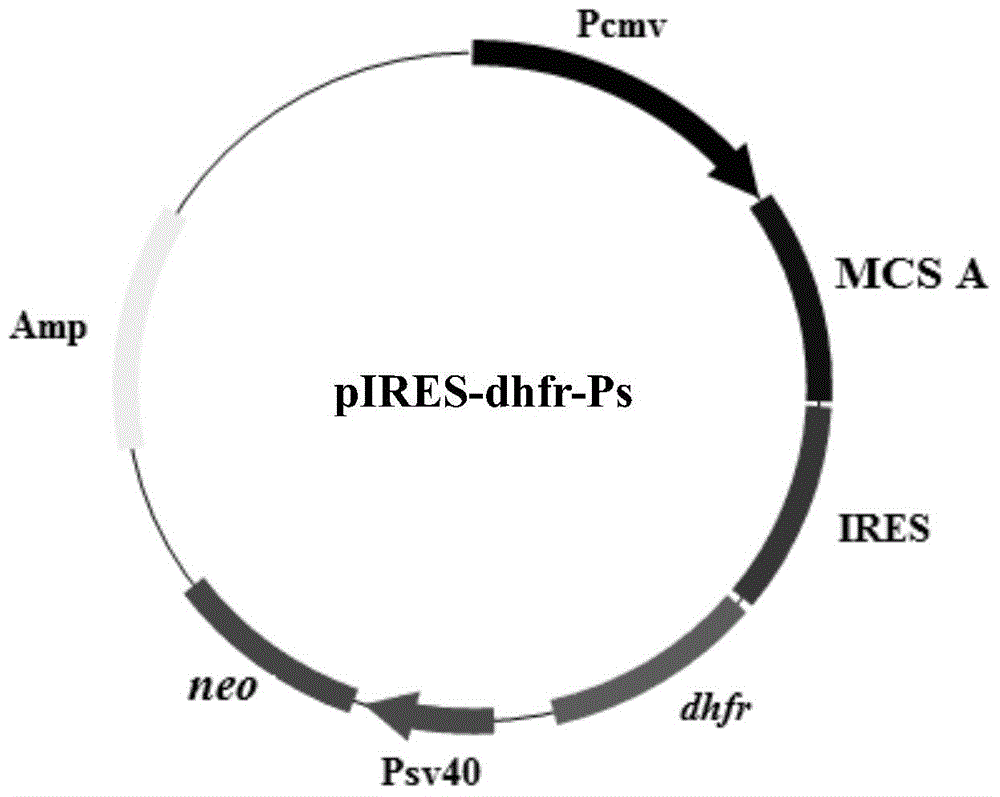
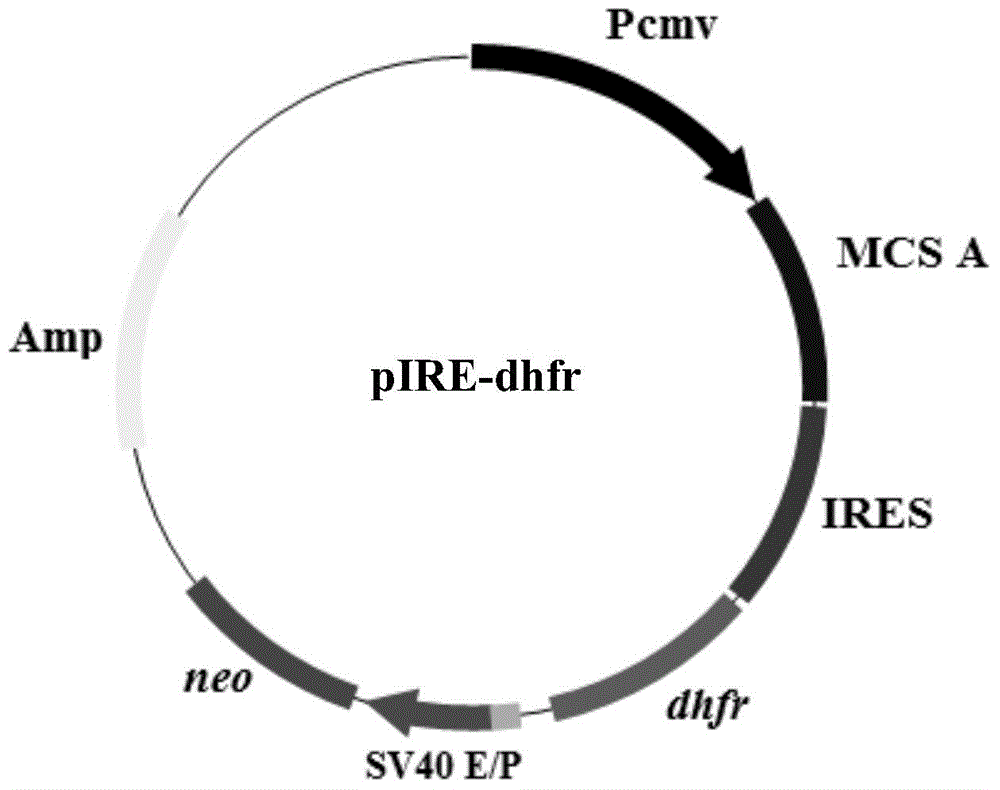
Expression vector for animal cell as well as preparation method and application of expression vector
ActiveCN104694568AReduce translation efficiencyIncrease productionVector-based foreign material introductionForeign genetic material cellsTranslational efficiencySusceptibility/Resistance Gene
Owner:SHENZHEN INST OF ADVANCED TECH
Methods for producing denetically modified plants, plant materials and plant products produced thereby
Owner:GENESIS RES & DEV +1
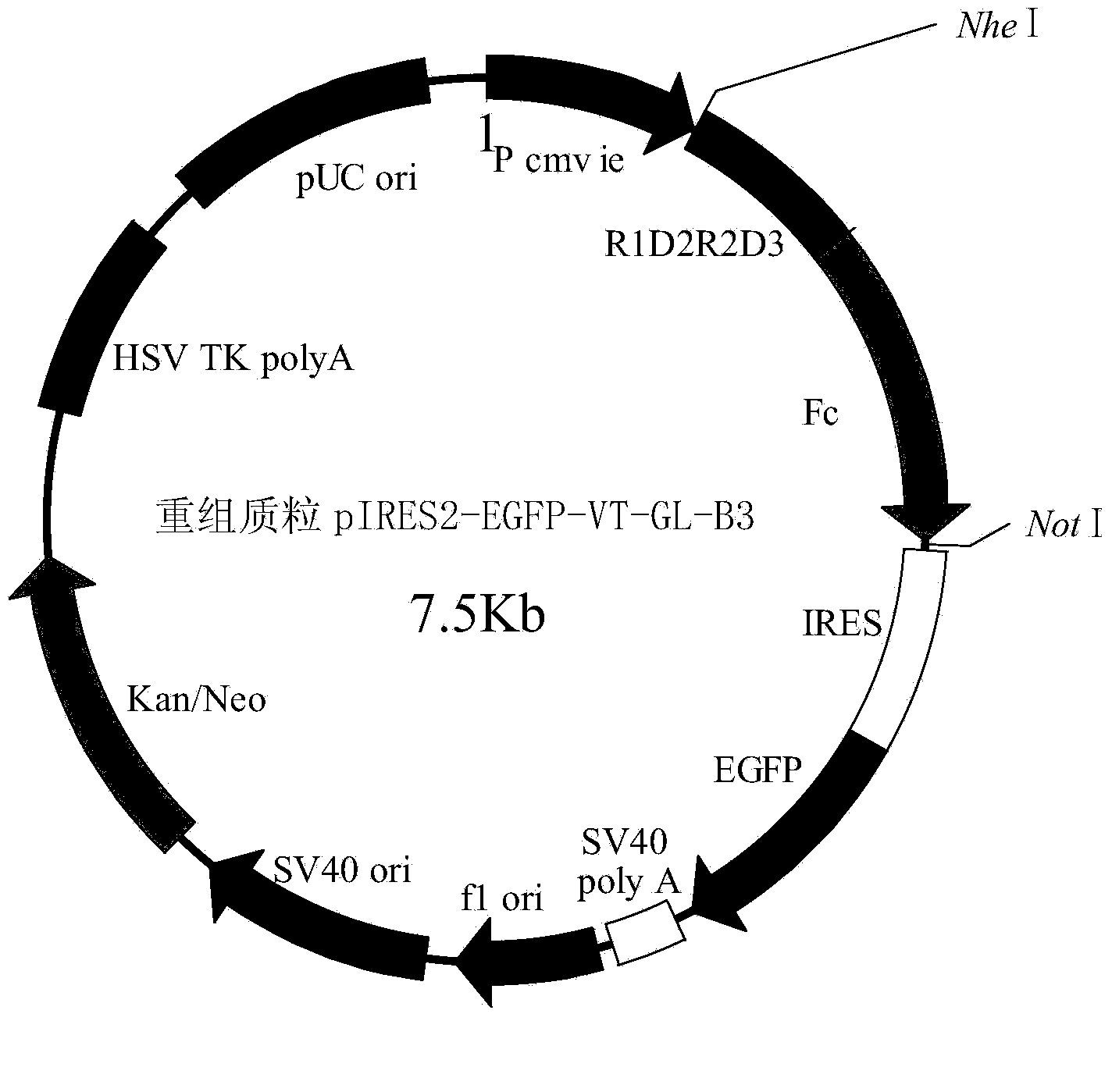
Fusion protein VT-GL-B3, coding gene thereof and applications thereof
InactiveCN103819566APromote generationTumor rescue response inhibitionPeptide/protein ingredientsPharmaceutical non-active ingredientsDiseaseAngiogenesis growth factor
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Novel transposon-like element
Owner:TOMITA MOTONORI
Immune cell CAR-T culture equipment with high cell survival rate and culture method
PendingCN113913295ARealize automatic deliveryNurturing status blockBioreactor/fermenter combinationsBiological substance pretreatmentsHigh cellCulture cell
Owner:上海芙普瑞生物科技有限公司
Recombinant cell strain HSCT6-rADHI as well as construction method and application thereof
PendingCN114107216AEasy to solveBeneficial for targeted therapyGenetically modified cellsDiagnosticsEthanol dehydrogenaseReceptor
Owner:ZHENGZHOU UNIV
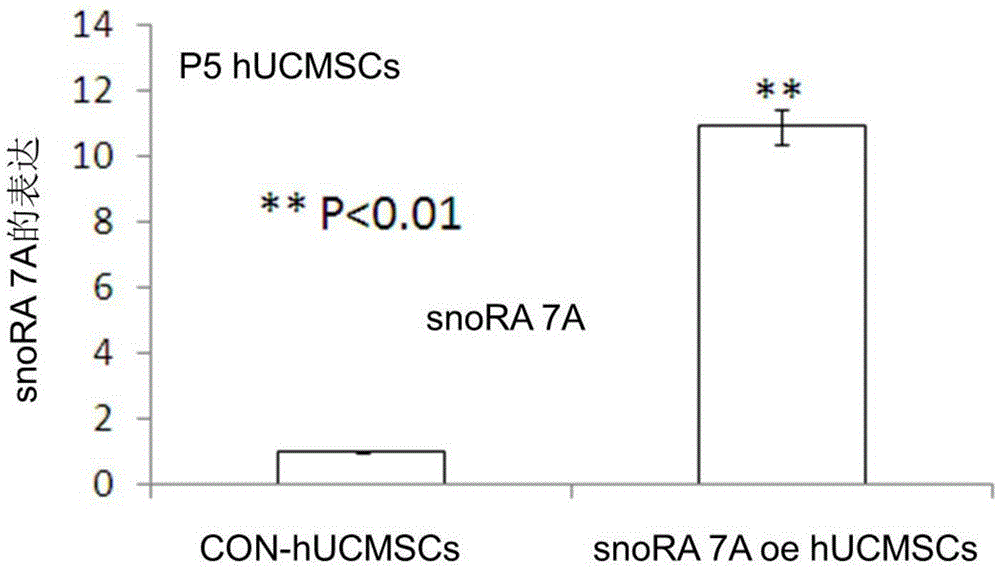
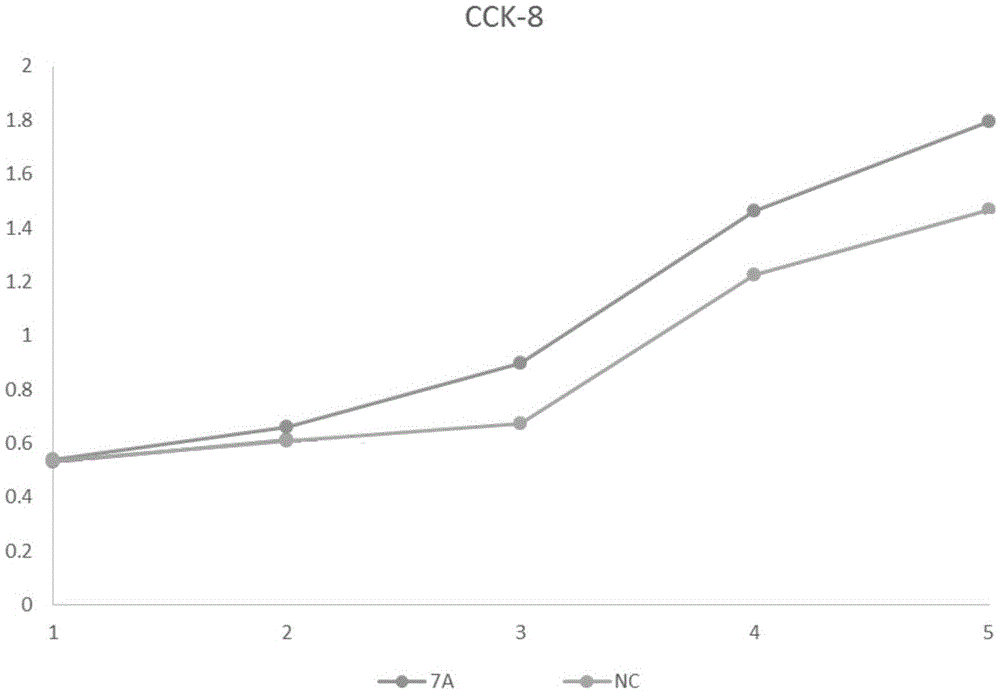
Method for enhancing proliferative capacity of hUCMSCs and application of method
ActiveCN105463019AVector-based foreign material introductionForeign genetic material cellsBiologyPlasmid
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
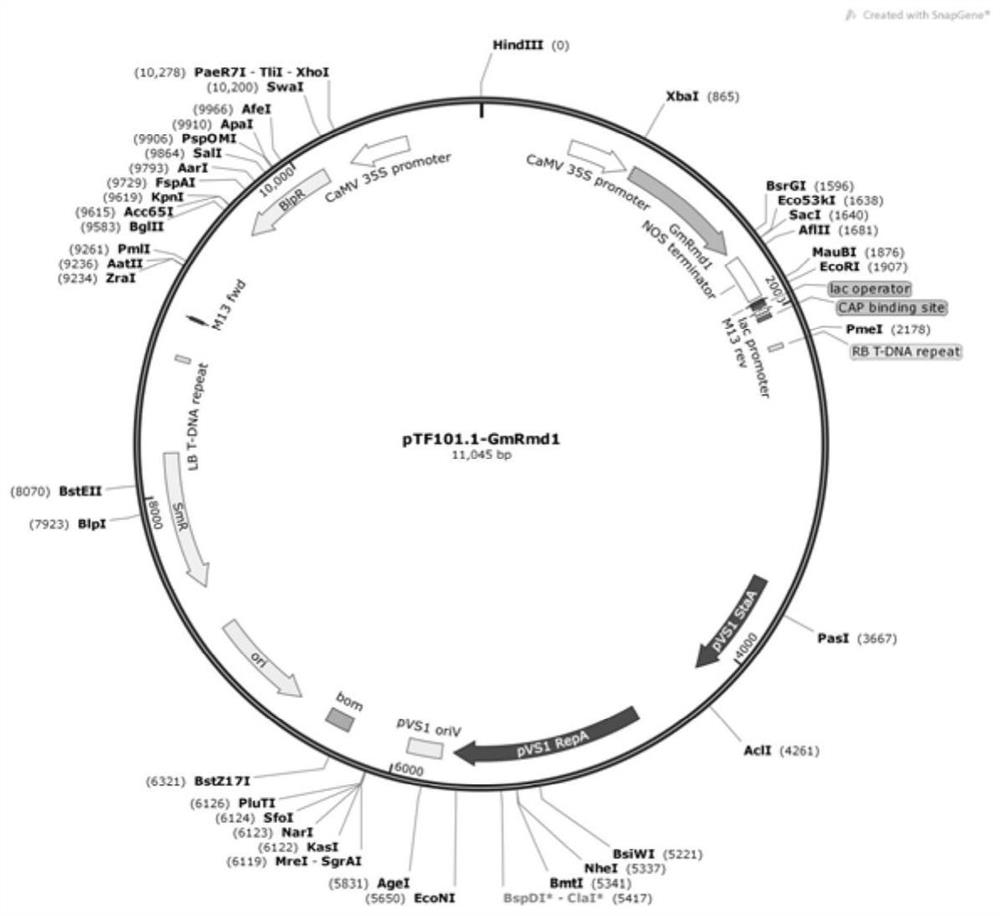
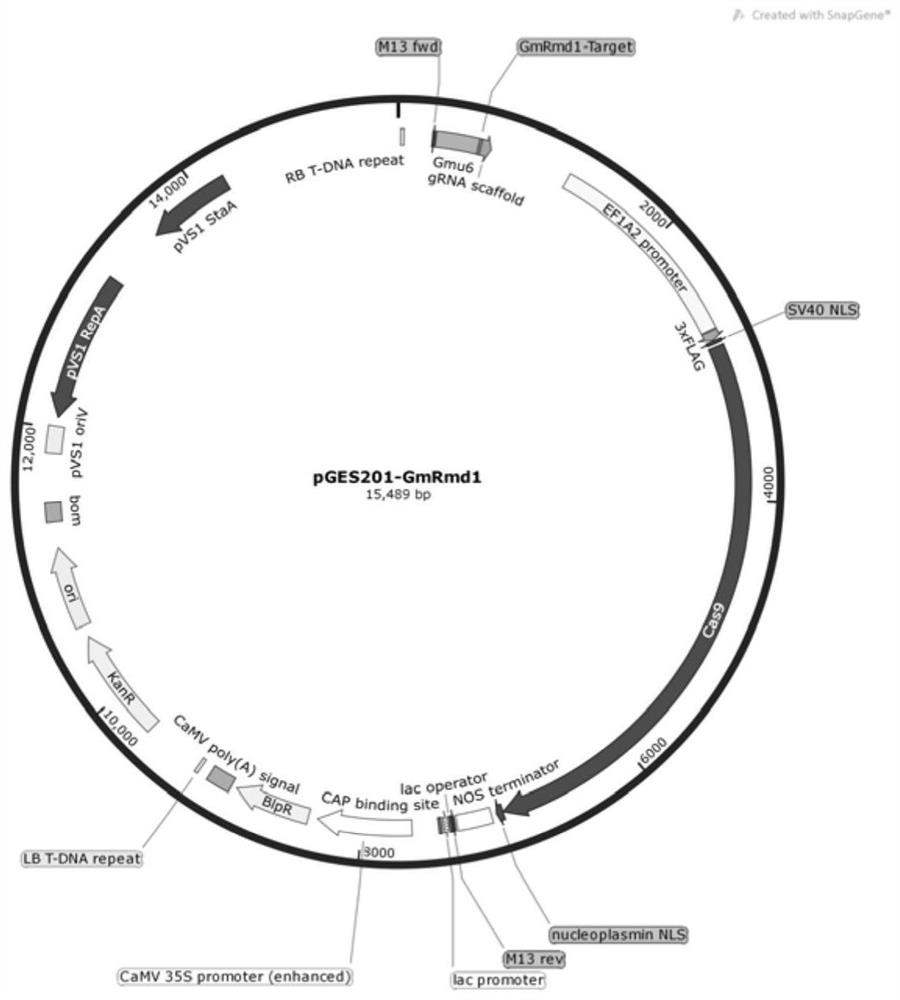
Gene GmRmd1 for resisting powdery mildew of soybean as well as encoded protein and application of gene GmRmd1
Owner:SOUTH CHINA AGRI UNIV
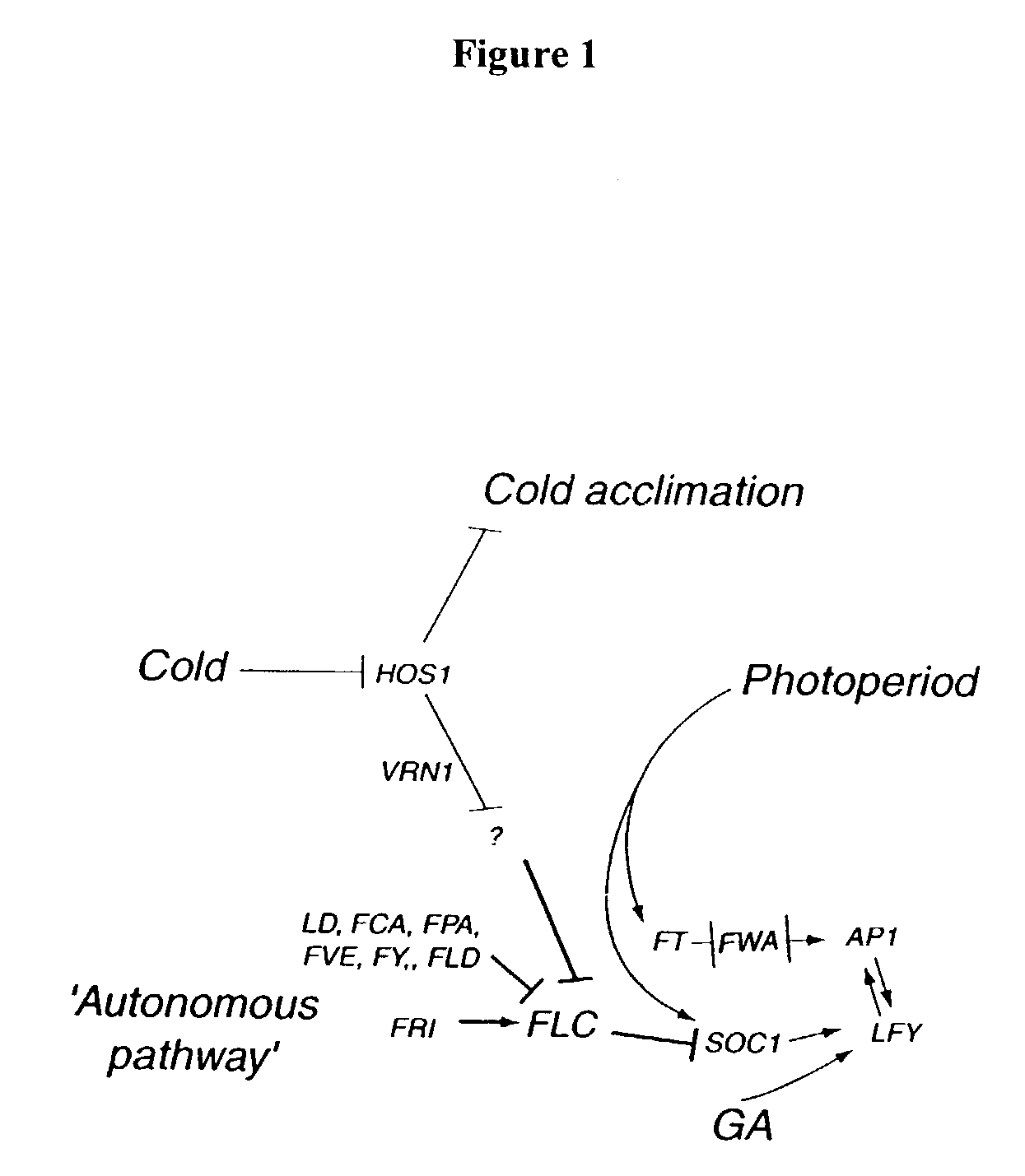
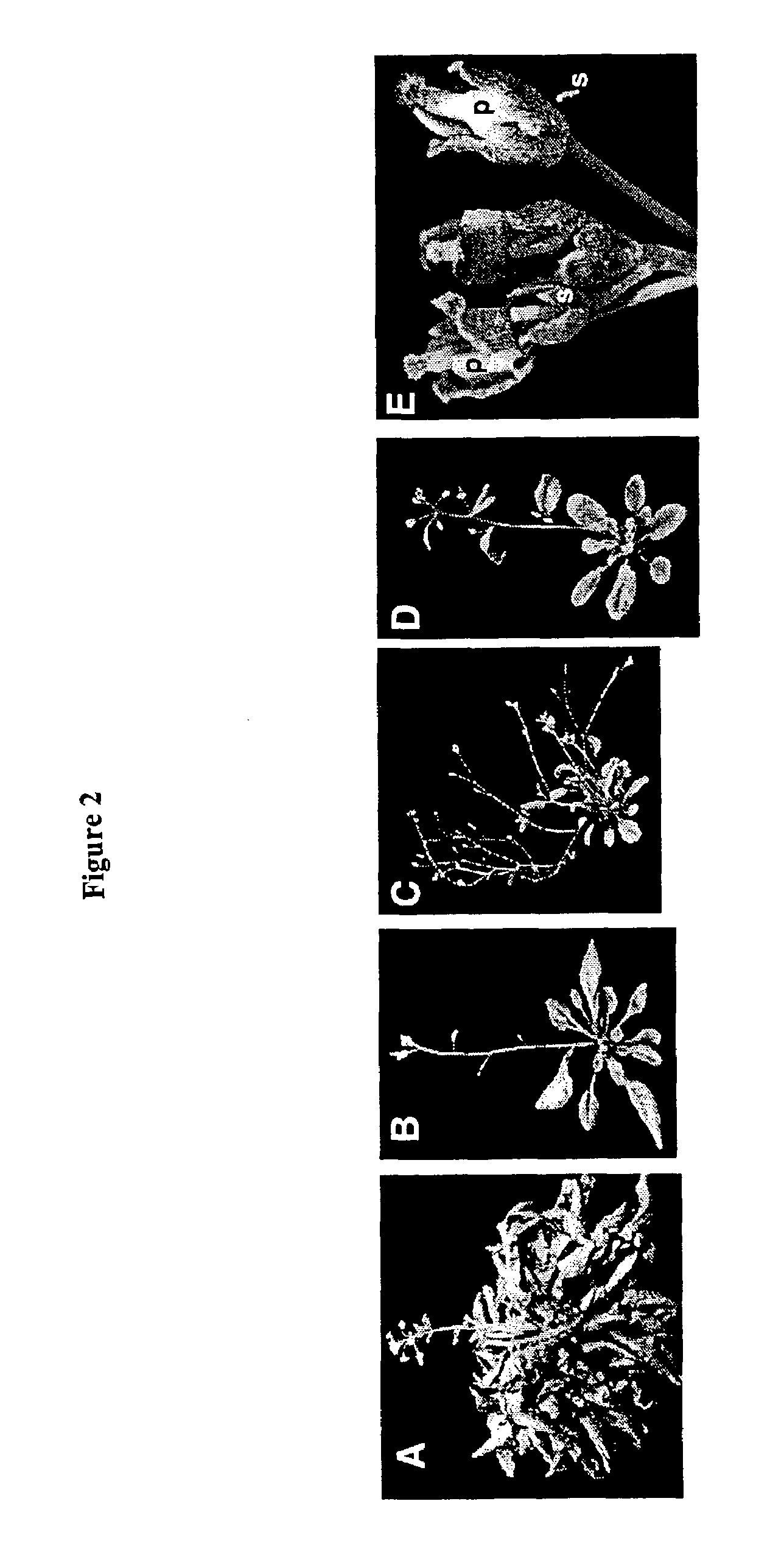
Plant vernalization independence (VIP) genes, proteins, and methods of use
InactiveUS7365182B2Decreased FLC RNA expressionEarly floweringSugar derivativesDepsipeptidesMutantVernalization
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Method of Manufacturing Reference Material Using Plant Cultured Cell Lines
ActiveUS20120282620A1Improve reliabilitySugar derivativesMicrobiological testing/measurementGenetic traitsUniform - quality
Owner:KOREA RES INST OF STANDARDS & SCI
Obesity gene
The invention describes a previously unknown gene, termed 5′OT-EST, which is responsible for inducing an obesity and / or infertility phenotype in transgenic animals, and transgenic animals comprising mutants of 5′OT-EST which are useful for assaying compounds for the treatment of obesity and / or infertility.
Owner:MEDICAL RESEARCH COUNCIL
Neurotrophic factor NNT-1
Disclosed are nucleic acids encoding novel neurotrophic factors, designated NNT-1. Also disclosed are amino acid sequences for NNT-1 polypeptides, methods for preparing NNT-1 polypeptides, and other related aspects. Such polypeptides are active in stimulating B-cell and / or T cell production, as well as reducing inflammatory responses.
Owner:AMGEN INC
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap