Anti-human PD-1 monoclonal antibody preparation suitable for subcutaneous injection
A monoclonal antibody, PD-1 technology, applied in the direction of antibody, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-tumor drugs, etc., can solve the problems of aggregation, undesired immune response, etc. The effect of low aggregation and fragmentation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
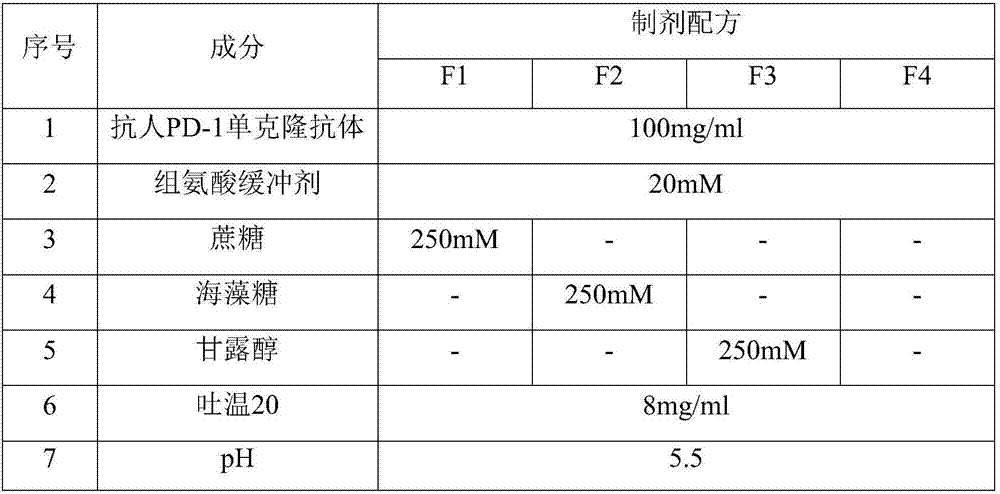
[0054] Example 1 Effect of sugar on the stability of liquid formulations
[0055] To study the stability of liquid anti-human PD-1 monoclonal antibody preparations containing different sugars at 50°C. In addition to sugar, the liquid formulation also contains other excipients as given in Table 1.
[0056] Table 1. Anti-human PD-1 monoclonal antibody liquid preparations containing different sugars
[0057]
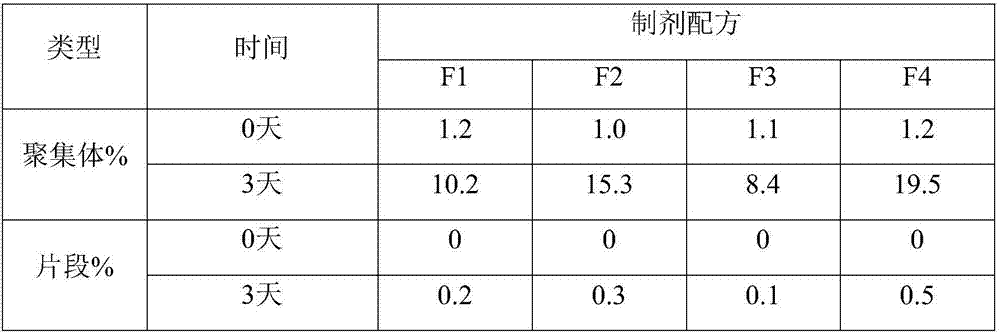
[0058] The above sample liquid preparation was incubated at 50°C for 3 days, and then analyzed by SDS-PAGE and SE-HPLC. The results are shown in Table 2.
[0059] Table 2. SE-HPLC results of liquid preparations of anti-human PD-1 monoclonal antibodies containing different sugars
[0060]
[0061] It can be seen from the results in Table 2 that, compared with sucrose and mannitol, the addition of trehalose resulted in an increase in aggregates. More preferred is mannitol.
Embodiment 2
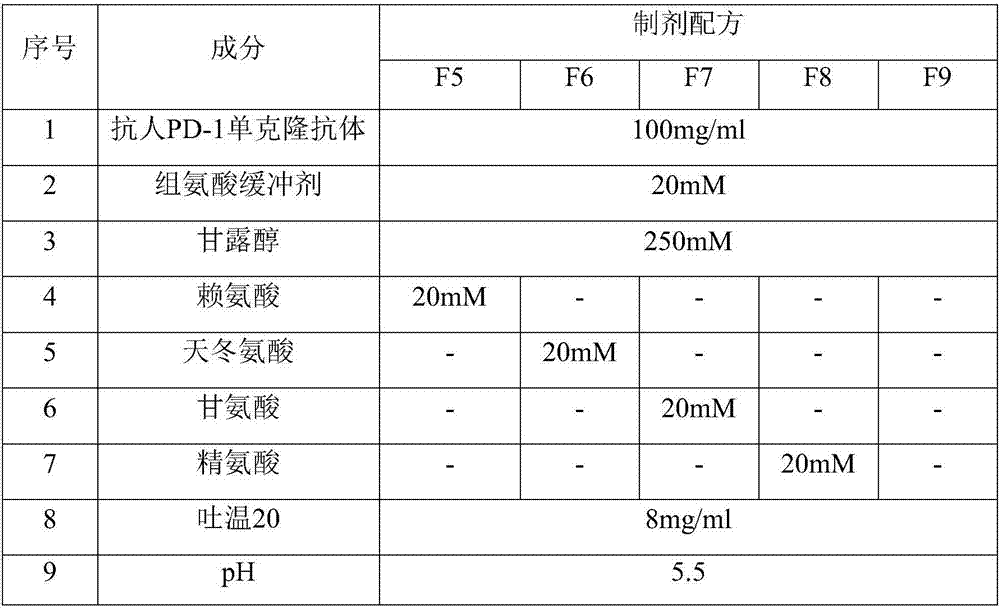
[0062] Example 2 Effect of aggregation inhibitor on the stability of liquid formulation
[0063] To study the effect of different aggregation inhibitors (lysine, aspartic acid, glycine and arginine) on the aggregation and fragmentation of liquid preparations of anti-human PD-1 monoclonal antibodies. The liquid formulation also contains other excipients as given in Table 3.
[0064] Table 3. Anti-human PD-1 monoclonal antibody liquid preparations containing different aggregation inhibitors
[0065]
[0066] The above-mentioned sample liquid preparations were charged and incubated at 50°C for 3 days, and then analyzed by SDS-PAGE and SE-HPLC. The results are shown in Table 4.
[0067] Table 4. SE-HPLC results of anti-human PD-1 monoclonal antibody liquid preparations containing different aggregation inhibitors
[0068]
[0069] It can be seen from the results in Table 4 that on the basis of the mannitol-added liquid formulation, compared with the further addition of glycine (F7) and argini
Embodiment 3
[0070] Example 3 Effect of double buffer on the stability of liquid formulation
[0071] Study the aggregation and fragmentation of anti-human PD-1 monoclonal antibody liquid formulations with different buffers (histidine buffer, phosphate buffer, citrate buffer, phosphate and citrate double buffer) Impact. The liquid formulation also contains other excipients as given in Table 5.
[0072] Table 5. Anti-human PD-1 monoclonal antibody liquid preparations containing different buffers
[0073]
[0074] The above-mentioned sample liquid preparations were charged and incubated at 50°C for 3 days, and then analyzed by SDS-PAGE and SE-HPLC. The results are shown in Table 6.
[0075] Table 6. Anti-human PD-1 monoclonal antibody liquid preparations containing different buffers
[0076]
[0077] It can be seen from the results in Table 6 that compared with the formulations containing histidine, phosphate and citrate single buffers, the composition with phosphate and citrate double buffers as buff
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap