Anti-il36r antibodies
a technology of anti-il36r and antibodies, applied in the field of antibodies, can solve the problems that the property of spesolimab is not ideal for chronic long-term treatment, and achieve the effects of less potency, greater exposure, and superior properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of Human Antibodies that Specifically Bind to IL-36R
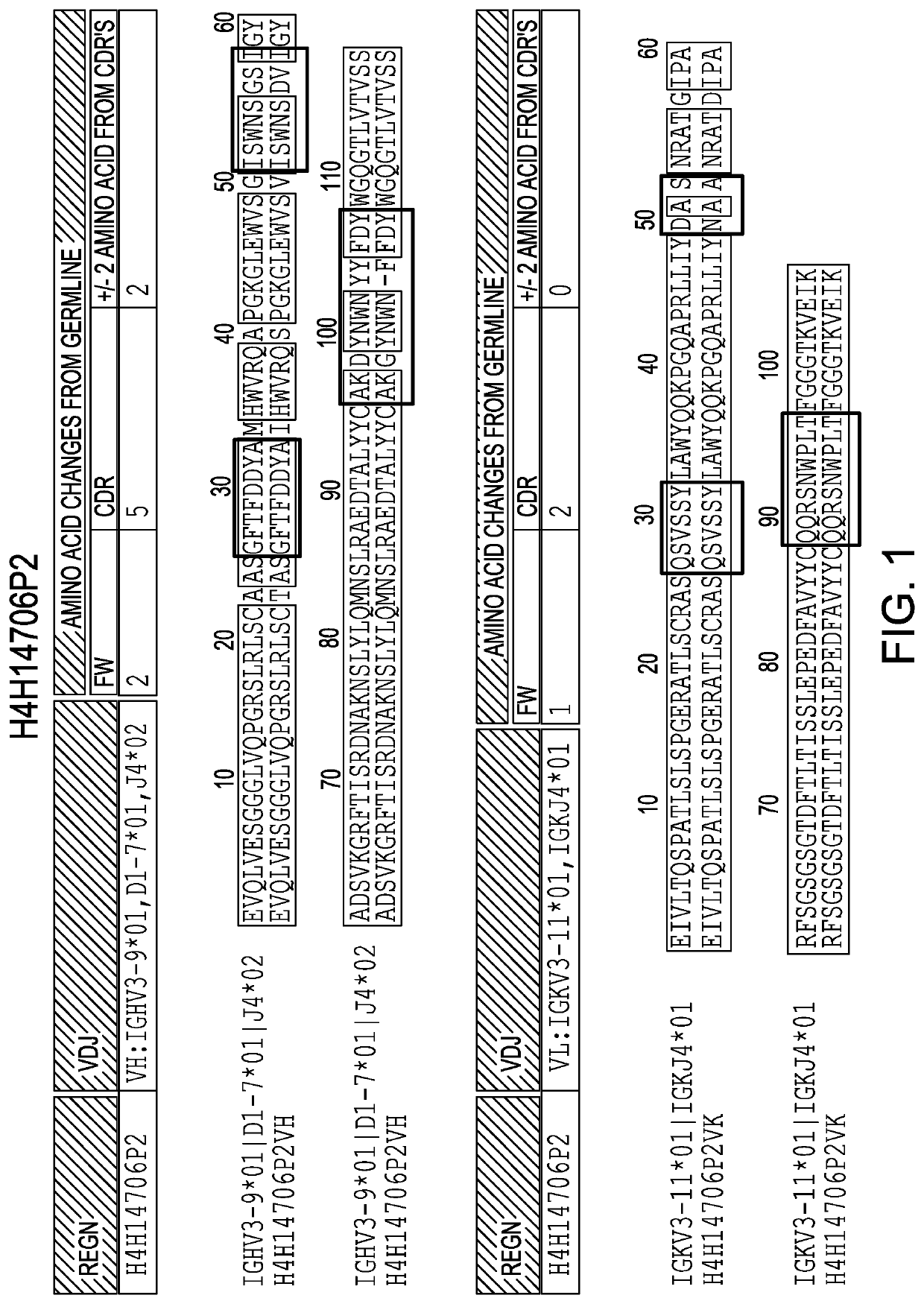
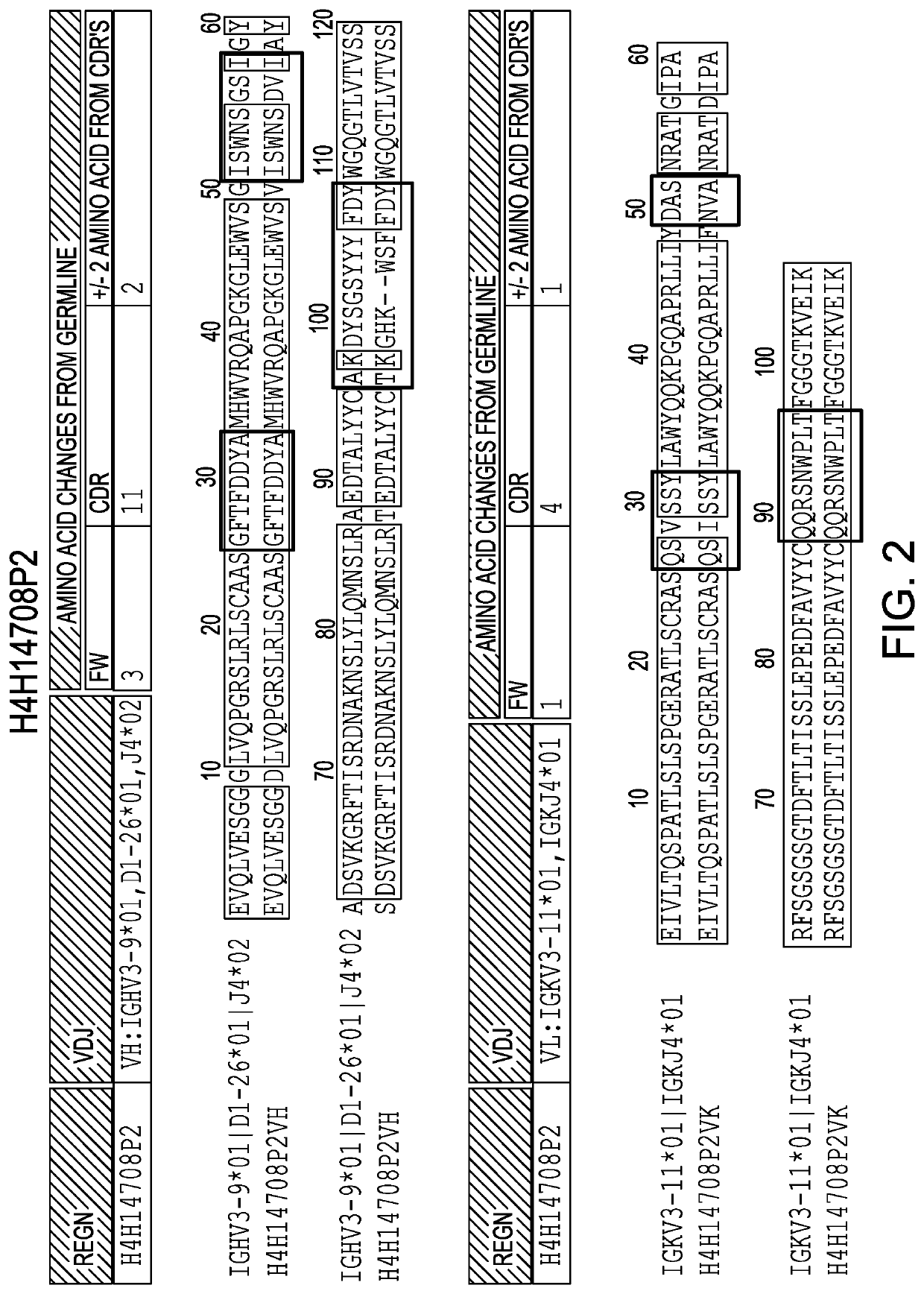
[0168]Anti-IL36R antibodies were obtained by immunizing a VELOCIMMUNE mouse (i.e., an engineered mouse comprising DNA encoding human immunoglobulin heavy and kappa light chain variable regions) with a DNA immunogen comprising the full length IL36R (IL-1RL2) sequence. The antibody immune response was monitored by an IL36R-specific immunoassay and fully human anti-IL36R antibodies were isolated and purified. Two exemplary comparisons between the VH and VL of antibodies generated as set forth herein and their respective germlines are set forth in FIG. 1 and FIG. 2.
TABLE 1Immunoglobulin chain sequences of the present invention*AntibodyVHCDR1CDR2CDR3#NameDNAPEPDNAPEPDNAPEPDNAPEP1H4H14699P2123456782H4H14700P217181920212223243H4H14706P233343536373839404H4H14708P249505152535455565H4H14709P65666768697071726H4H14728P81828384858687887H4H14731P9798991001011021031048H4H14732P21131141151161171181191209H4H14734P212913013113213313413513610H4H14757P
example 2
with HEK293 / D9(NFκB-luciferase) / hIL-36R and HEK293 / NFκB-luciferase / mfIL-36R Cells
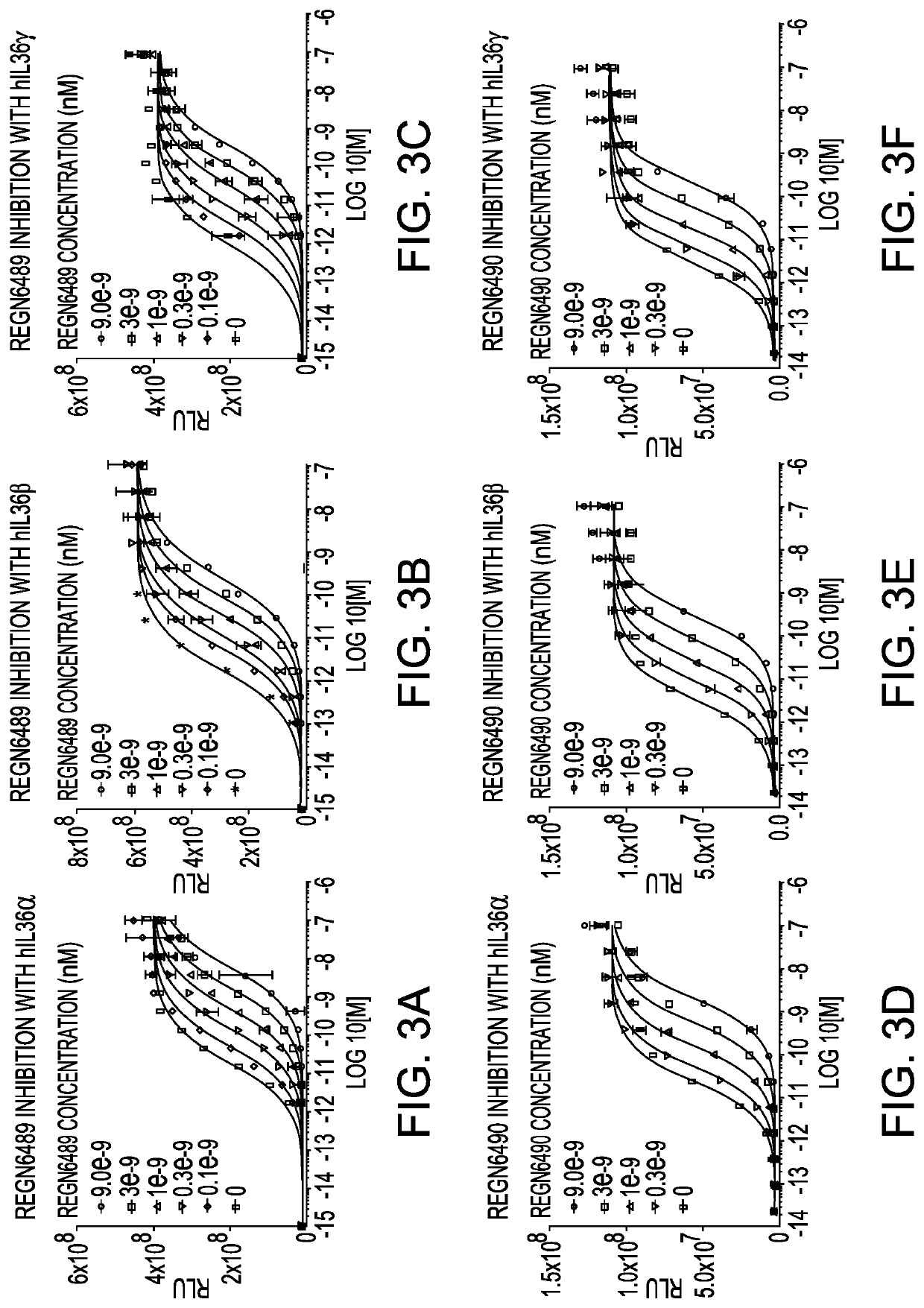
[0170]IL-36 receptor (IL-36R) is a single-pass membrane receptor for a subset of members of the IL-1 family of cytokines, IL-36α, IL-36β, and IL-36γ, and upon binding to these ligands, there is recruitment of its co-receptor, the IL-1R accessory protein (IL-1RAcP), which induces a signaling cascade that involves NFκB and mitogen-activated kinase pathways (Sims et al, 2010). A bioassay was developed to detect the transcriptional activation by NFκB via IL-36R activation using reporter cell lines that stably express either full-length human IL-36R (hIL-36R; amino acids 1 through 575 of accession number NP_003845.2) or Macaca fascicularis IL-36R (MfIL-36R) along with a luciferase reporter [NFκB response element (5×)-luciferase-IRES-GFP] in HEK293 cells. IL-1RAcP is endogenously expressed in the HEK293 cell line. The resulting stable cell lines, referred to as HEK293 / NFκB-luc / hIL-36R and HEK293 / NF
example 3
tet Cross-Competition
[0175]Binding competition between a panel of different anti-IL-36R antibodies was determined using a real time, label-free bio-layer interferometry assay on an Octet® HTX biosensor (ForteBio, A Division of Pall Life Sciences). The entire experiment was performed at 25° C. in 0.01M HEPES pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.05% v / v Surfactant Tween-20, 0.002% NaN3 and 1 mg / mL BSA (HBS-ET kinetics buffer) with the plate shaking at the speed of 1000 rpm. To assess whether two antibodies are able to compete with one another for binding to their respective epitopes on the recombinant human IL-36R extracellular domain expressed with a C-terminal myc-myc-hexahistidine tag (hIL-36R-MMH: mROR1 signal sequence (M1-A29)-human IL36R(D20-Y337)-mycmycHis6), around 0.3 nM of hIL-36R-MMH was first captured onto anti-His antibody coated Octet biosensors (Fortebio Inc, #18-5079) by submerging the biosensors for 3 minutes into wells containing 30 μg / mL of hIL-36R-MMH. The antigen-
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap