Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3 results about "Patient population" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Patient population meaning, patient population definition | English Cobuild dictionary. patient. 1 n-count A patient is a person who is receiving medical treatment from a doctor or hospital. A patient is also someone who is registered with a particular doctor.
Uses for and article of manufacture including her2 dimerization inhibitor pertuzumab
InactiveUS20130095172A1Prolong progression-free survivalReduce riskOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMetastatic gastric cancerHER2 Positive Breast Cancer
The present application describes uses for and articles of manufacture including Pertuzumab, a first-in-class HER2 dimerization inhibitor. In particular, the application describes methods for extending progression free survival in a HER2-positive breast cancer patient population; combining two HER2 antibodies to treat HER2-positive cancer without increasing cardiac toxicity; treating early-stage HER2-positive breast cancer; treating HER2-positive cancer by co-administering a mixture of Pertuzumab and Trastuzumab from the same intravenous bag; treating HER2-positive metastatic gastric cancer; treating HER2-positive breast cancer with Pertuzumab, Trastuzumab and Vinorelbine; treating HER2-positive breast cancer with Pertuzumab, Trastuzumab and aromatase inhibitor; and treating low HER3 ovarian, primary peritoneal, or fallopian tube cancer. It also describes an article of manufacture comprising a vial with Pertuzumab therein and a package insert providing safety and / or efficacy data thereon; a method of making the article of manufacture; and a method of ensuring safe and effective use of Pertuzumab related thereto. In addition the application describes an intravenous (IV) bag containing a stable mixture of Pertuzumab and Trastuzumab suitable for administration to a cancer patient.
Owner:GENENTECH INC
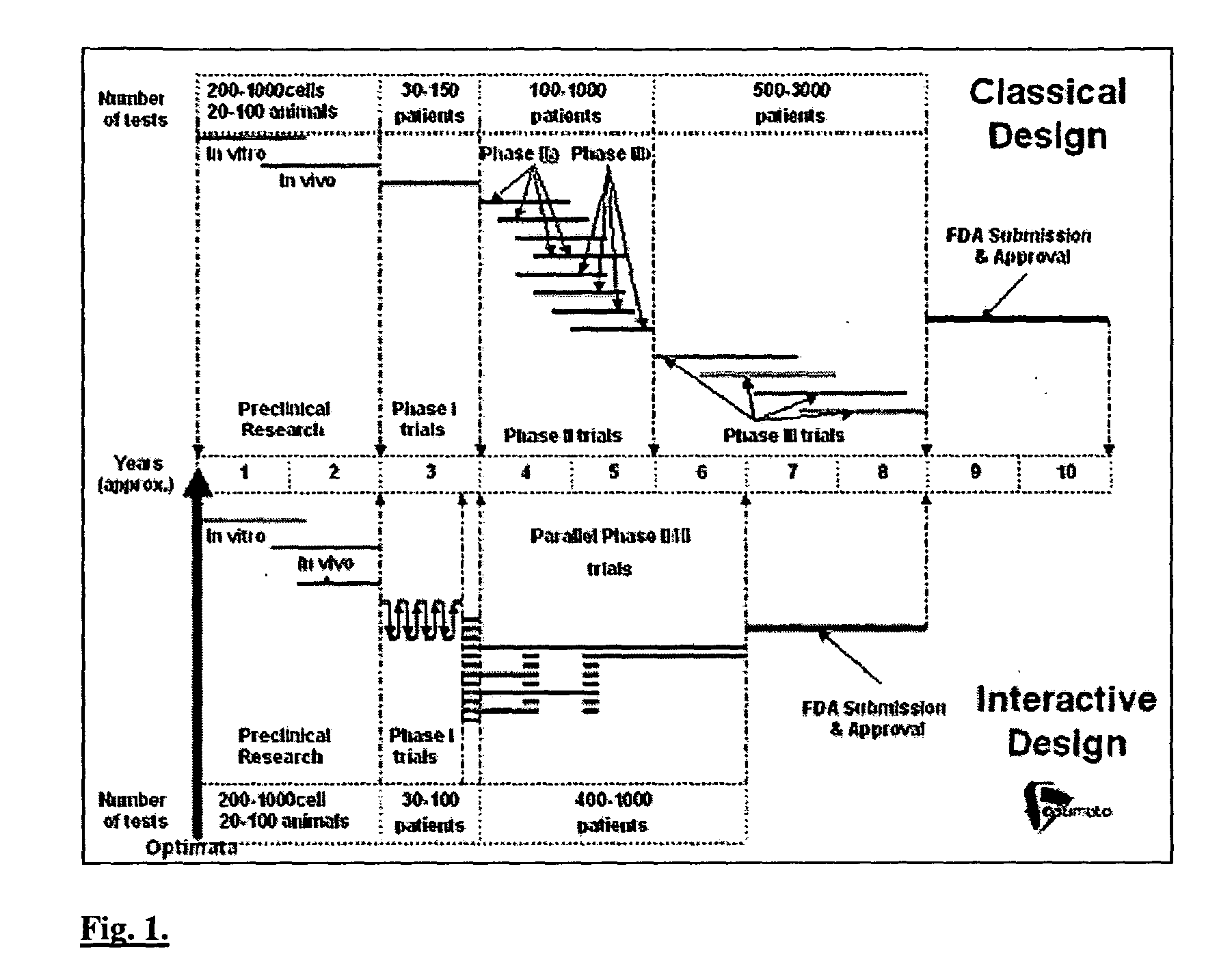
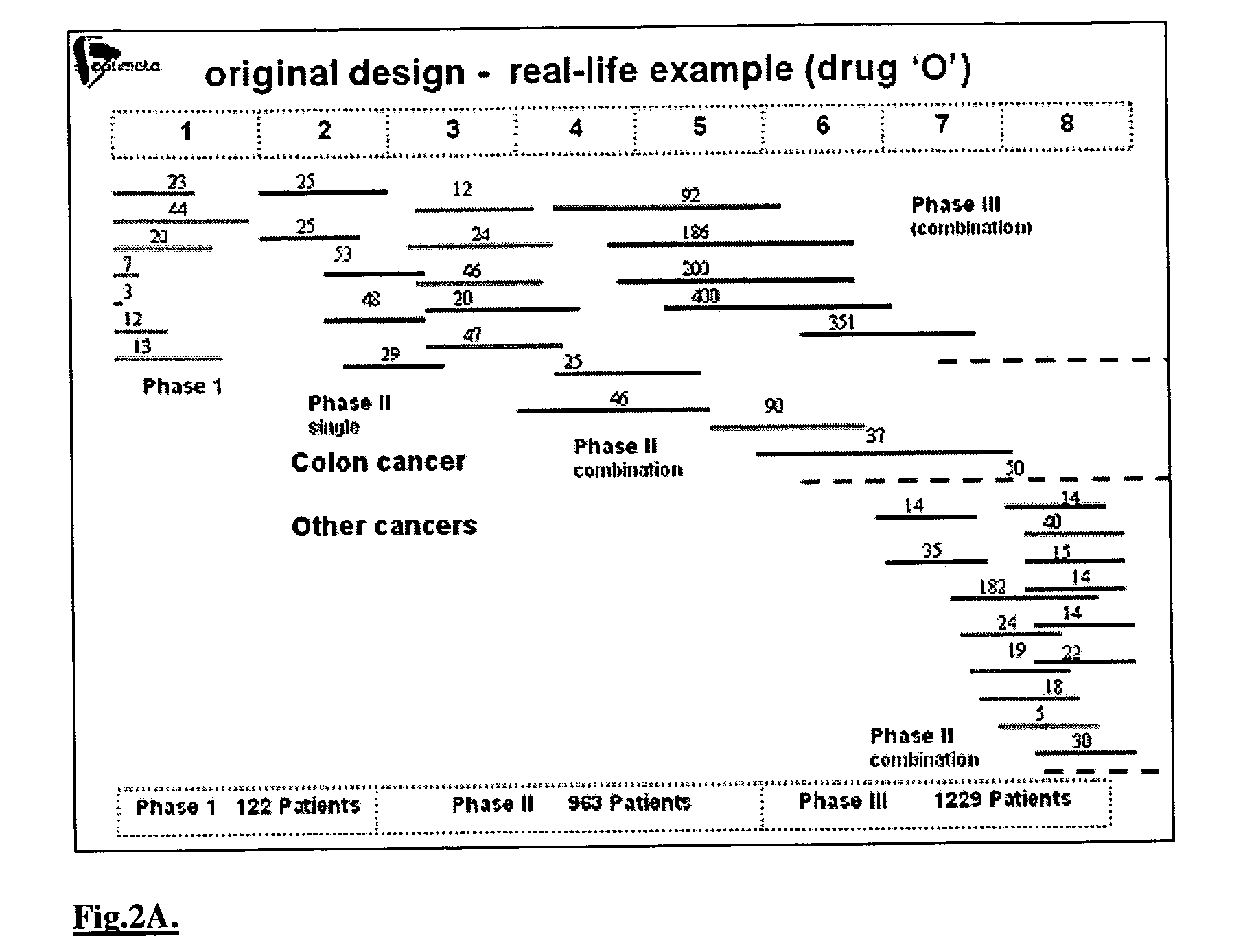
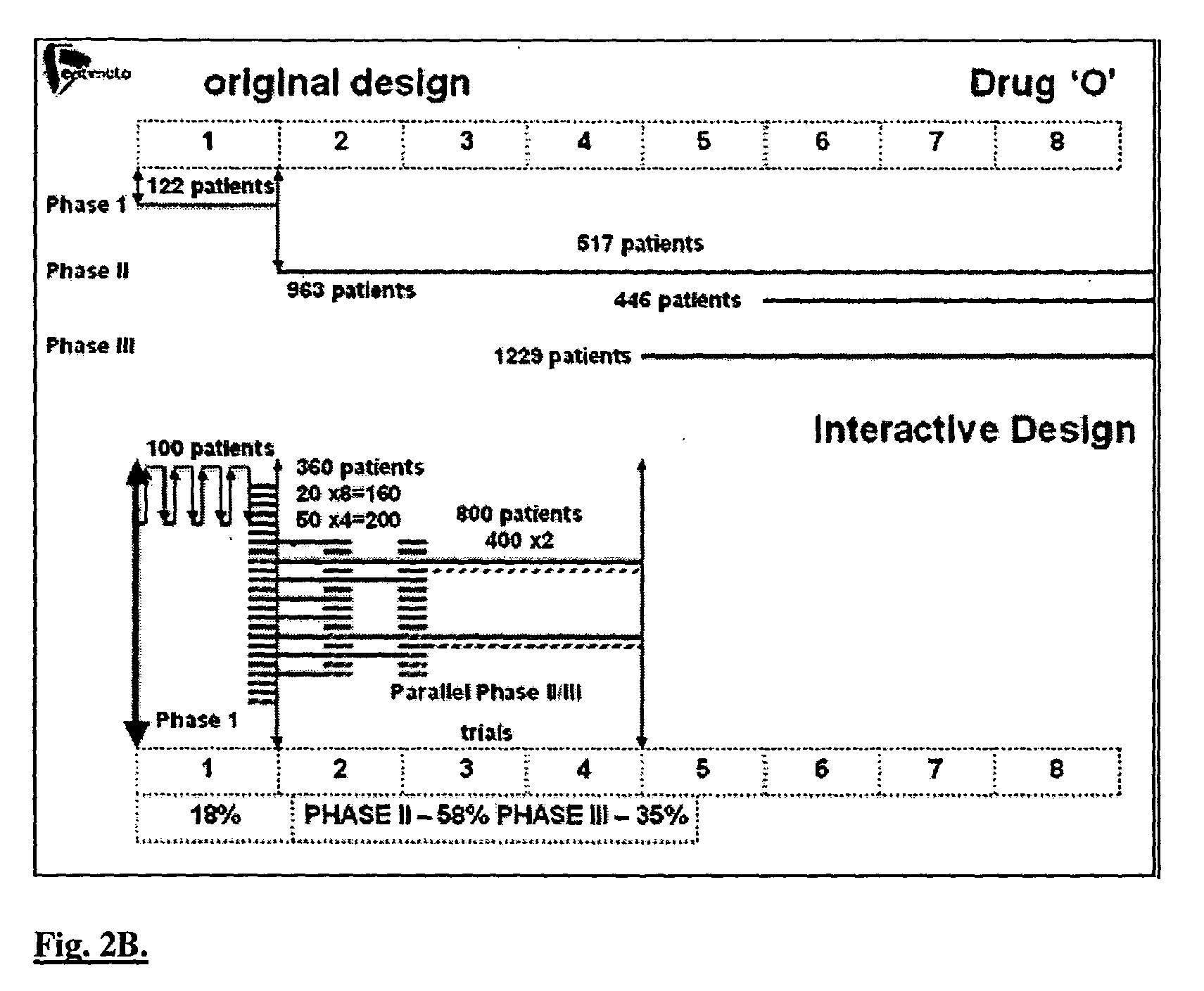
Interactive technique for optimizing drug development from the pre-clinical phases through phase-IV
InactiveUS20040107084A1Chemical property predictionMedical simulationPatient populationPhases of clinical research
Owner:OPTIMATA
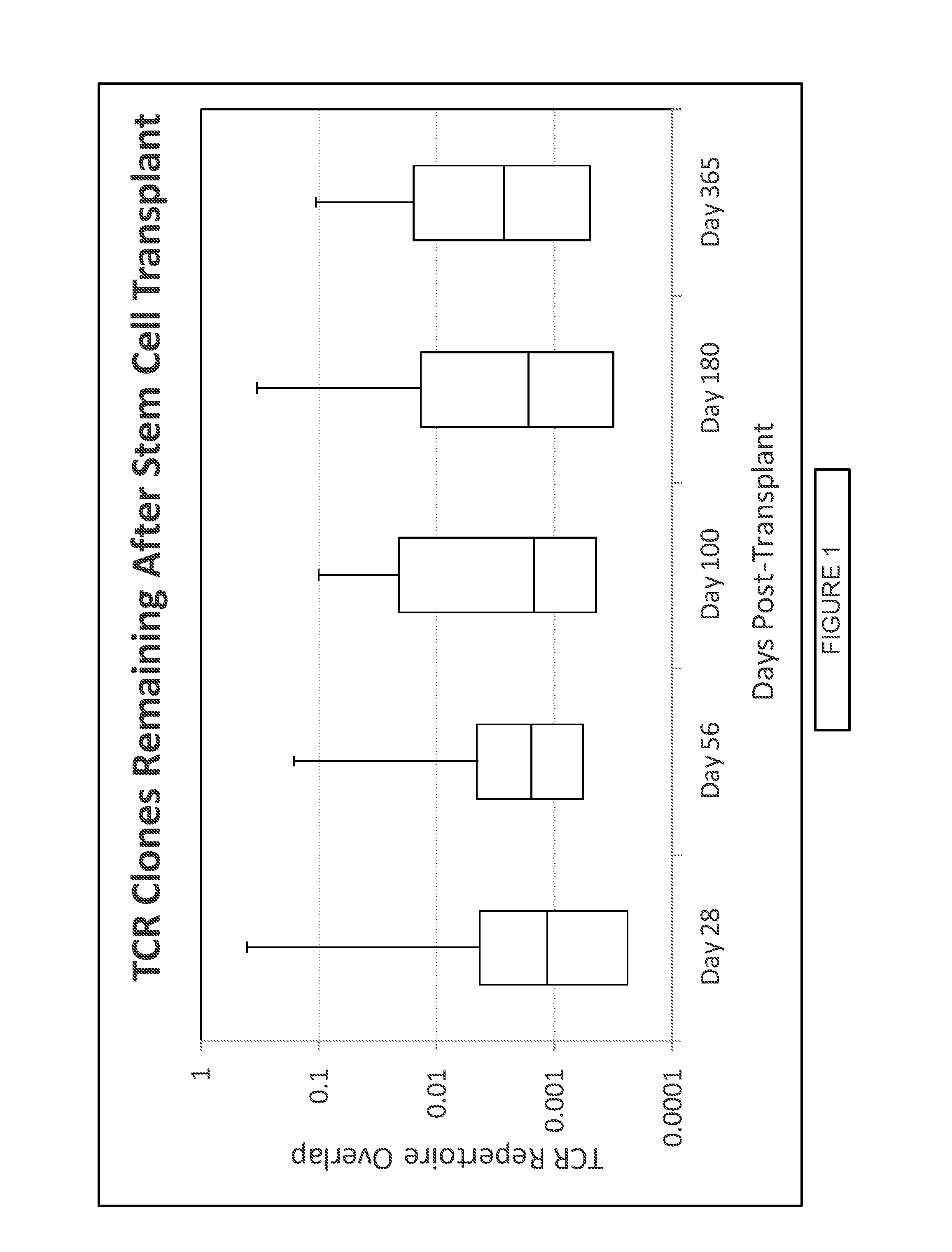
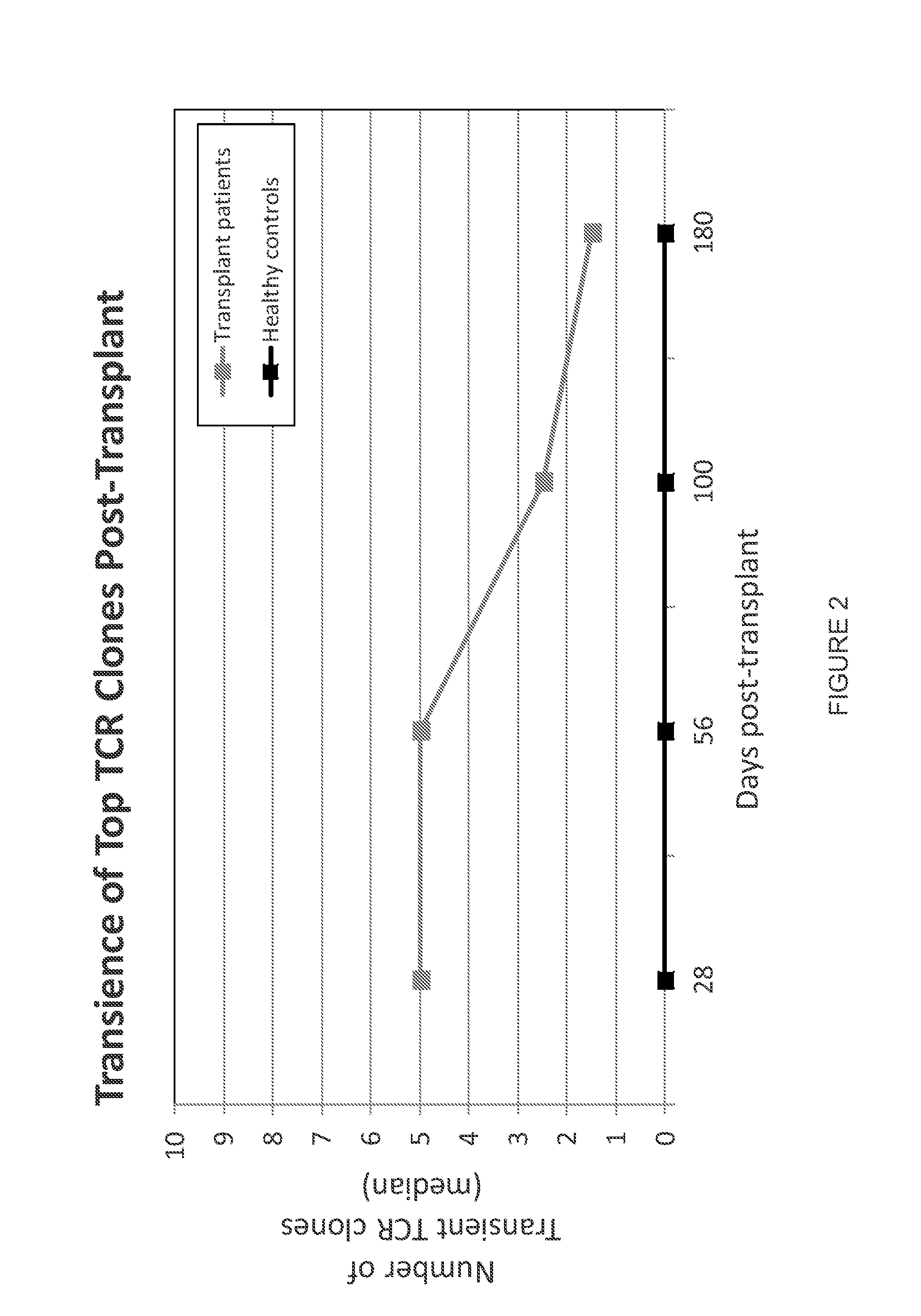
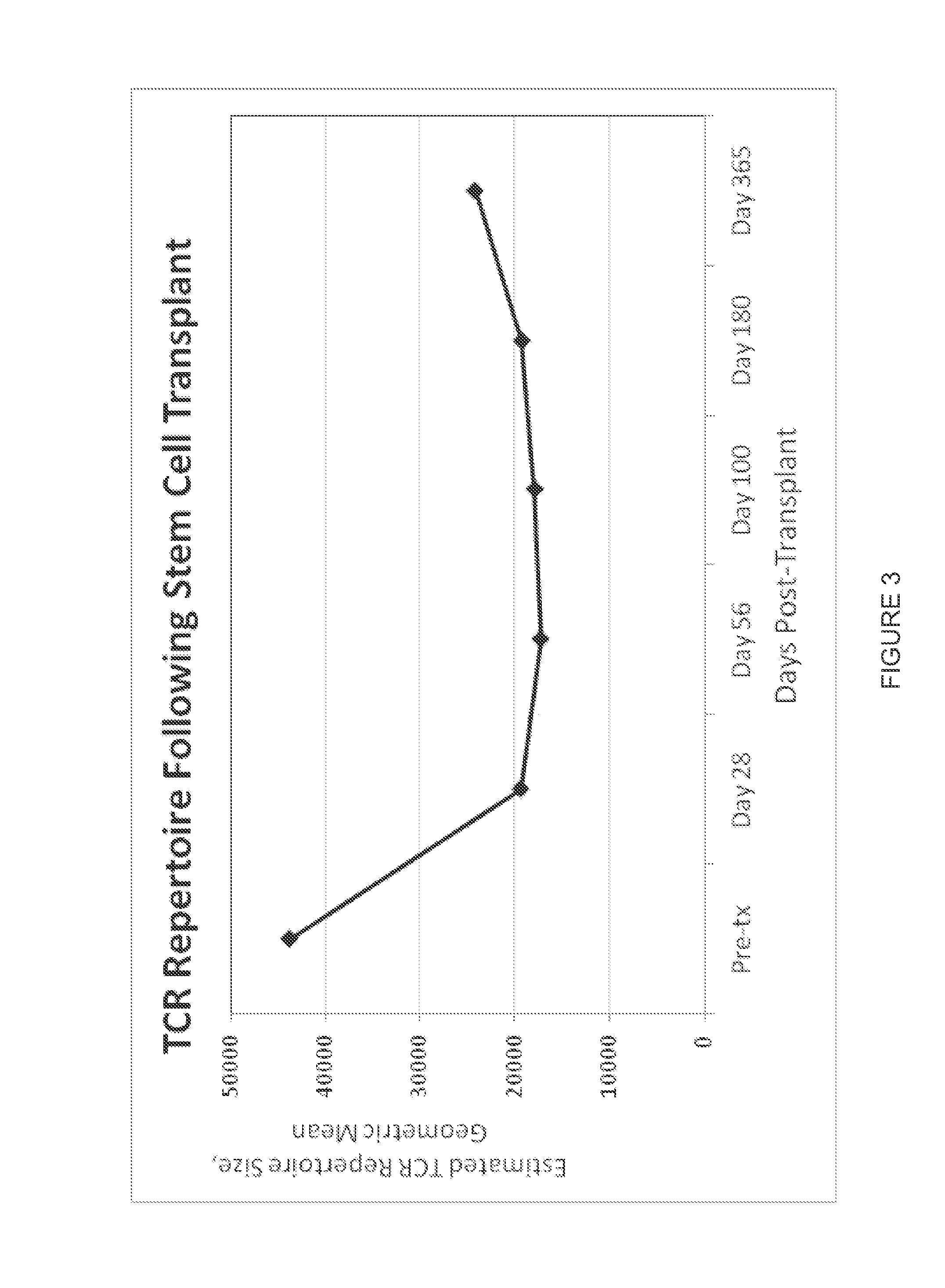
Immunocompetence assessment by adaptive immune receptor diversity and clonality characterization
InactiveUS20160002731A1Microbiological testing/measurementLibrary member identificationImmunocompetenceImmune receptor
Owner:ADAPTIVE BIOTECH +1
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap